Abstract
Although the survival rate for systemic lupus erythematosus (SLE) has improved dramatically during the past 50 years, the quality of life of patients afflicted with this disease remains poor. Currently existent measures of disease activity and damage in SLE do not capture the patient’s perspective and health-related quality of life (HRQoL). Most studies in SLE pertaining to HRQoL are from developed Western societies, with only a few from others. These studies have been conducted predominantly in women and using the Medical Outcomes Survey Short Form 36, a generic HRQoL instrument that has been shown not to be sensitive to change in lupus. Existent lupus-specific HRQoL measures have not yet been used in SLE clinical trials. New HRQoL research tools are currently undergoing validation in different countries, languages, and cultural settings, which may help dissect the underlying role of socioeconomic status and specific disease-related features that impact SLE-related quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a multisystemic chronic autoimmune disease with an unpredictable waxing and waning course. Although survival has improved dramatically during the past few decades, life expectancy remains shorter and quality of life poorer than in the general population.
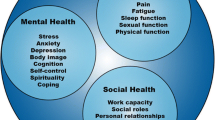
SLE may profoundly affect not only the physical aspects of a person’s life but also his or her mental, social, psychological, and sexual well-being, none of which are captured by current measures of disease activity or damage. Disease activity is assessed by different validated instruments: the Systemic Lupus Activity Measure-Revised (SLAM-R); the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and its revised version, the SLEDAI-2000; and the British Isles Lupus Assessment Group Index. Accrued damage, on the other hand, is ascertained by the Systemic Lupus International Collaborating Clinics Damage Index (SDI) [1–4]. The application of these instruments requires skillful and trained physicians; only the SLAM-R incorporates the patient’s perspective on disease activity.
In the recent past, the Outcome Measures in Rheumatology Clinical Trials (OMERACT) group and the US Food and Drug Administration have recommended also assessing the patient’s experience with the disease through health-related quality of life (HRQoL) or patient-reported outcome measures; this applies to both clinical trials and longitudinal observational studies [5, 6]. These measures reflect the individual’s perspective on the impact of a disease and its treatments on his or her function and perceived well-being, including the physical, mental, and social domains of life at a single time point [7]. These measures also provide an important facet of the treatment response in clinical trials and daily clinical practice and may aid in the formulation of specific treatment recommendations.
Health-Related Quality of Life
Generic Measures
Generic measures are commonly used among patients with different diseases or conditions and in various populations and clinical settings. They are useful to compare populations with different disorders of varied clinical severity as well as to compare patients with a given condition with healthy individuals. They lack specificity and are not useful to identify certain domains that may be especially important to SLE patients, such as sexual functioning and body image. The Medical Outcomes Survey Short Form 36 and 20 (SF-36 and SF-20) have been the most commonly used in multiethnic, multicenter, and single-center studies [8••, 9–14••]. Other generic measures, such as the European-QoL scale (EQ-5D) and the World Health Organization-QoL Scale (WHOQoL-Bref), the Health Assessment Questionnaire (HAQ), the Arthritis Impact Measurement Scale (AIMS), the Sickness Impact Profile, and the quality-of-life scale, have been used in SLE, but not in multiethnic SLE cohorts [15–19]. The HAQ and AIMS, as measures of functional status, have not been universally accepted in SLE research despite the frequency of neurological and musculoskeletal manifestations in SLE patients.
SLE-Specific Measures
These include domains not addressed by generic measures and identify some patient characteristics that are not usually captured by the current biomedical model, permitting the distinction between useful and useless therapeutic interventions in SLE clinical trials and in clinical care settings. Disease-specific measures were developed to capture the patient’s perspective on the impact of SLE and its treatments over time in a sensitive and reliable manner. These measures are the following: the Systemic Lupus Erythematosus–Specific QoL (SLE-QoL), the SLE-QoL questionnaire (L-QoL), the Lupus-QoL Scale (LupusQoL), the LupusPRO, the Lupus-QoL (LUP-QoL), and the Simple Measure of Impact of Lupus Erythematosus in Youngsters (SMILEY) [20, 21•, 22•, 23••, 24•, 25, 26••, 27].
To adapt a new quality-of-life (QoL) instrument across cultures and preserve the equivalence of what it measures, these steps should be followed: preparation, forward translation, reconciliation, back translation and its review, harmonization, cognitive debriefing and its review, results, finalization (expert judgment), proofreading, pretesting (probe technique or testing the instrument in bilingual laypeople), weighting of scores (ie, adapting the weights of scores to the cultural setting), and final report of the QoL instrument [28]. Given that most clinical studies are conducted in Western European or North American cultural environments (at least until recently), the cultural adaptation of HRQoL measures is very important to avoid selection bias associated with studies that might selectively exclude non-English-speaking patients [28]. In addition, the measurement properties of the adapted HRQoL instrument should be known and demonstrated by its use in the target population. The preservation of the metric properties of the HRQoL instrument must take into account internal consistency (measures the correlation between answers to different questions about the same concept) and reliability or repeatability. A measure is reliable at the group level in terms of internal consistency if Cronbach’s α is greater than 0.7. The test–retest reliability of the measure assesses its stability over time and should be satisfactory.
Validation studies are required when implementing a new HRQoL measure. In addition to feasibility, all HRQoL instruments should demonstrate content validity (measures what is intended to measure), discriminant validity (distinguishes disease severity, captured by disease activity or damage), criterion validity (measures accurately the same phenomenon), construct validity (measures accurately the underlying construct), convergent validity (measures the extent of correlation between observed relationships of the concepts and hypothesized concepts), sensitivity to change or responsiveness (detects changes individually or in groups of people), and finally the minimal clinically important difference (clinically important/relevant change in disease status) [28, 29••]. Compliance with these steps allows the comparison of dissimilar health care systems across and within countries and maintains internal and external validity of the HRQoL instrument.
Validated SLE-Specific Measures
The salient characteristics of SLE-specific QoL measures are depicted in Table 1. The SLE-QoL has been developed and validated by Leong et al. [20] in SLE patients from Singapore; it was found to be more sensitive, but not specific, to change than the SF-36, although its concurrent validity remains an issue and the sensitivity to change in other populations remains to be determined [20]. The instrument was recently cross-culturally adapted for Chinese-speaking SLE patients from Singapore [30].
The L-QoL instrument, developed by Doward et al. [21•], was derived from in-depth interviews with SLE patients and measures the impact of the disease and its treatments; however, most patients were in fair to excellent health. It has good face and content validities as per cognitive debriefing conducted with United Kingdom SLE patients, but it requires larger validation studies. Hungarian and Turkish versions of the L-QoL are currently available. Sensitivity to change remains to be proven [21•].
The LupusQoL, developed by McElhone et al. [22•], was derived from semistructured interviews with predominantly Caucasian SLE women from the United Kingdom. The relevance of the items included in the instrument was confirmed by cognitive debriefing [22•]. The LupusQoL has been assessed in United Kingdom SLE patients and demonstrated good internal consistency, test–retest reliability, and concurrent validity with the SF-36. It has also shown good discriminant validity for different levels of disease activity and damage. The LupusQoL was recently adapted for cross-cultural use and adapted, validated, and evaluated for use in the United States (LupusQoL-US), but its sensitivity to change has yet to be determined [23••]. It has been adapted to Canadian-English and translated into other languages, including Spanish, Dutch, French, Greek, Hungarian, Italian, Portuguese, Swedish, and Chinese. Cross-cultural validation of this tool in these other languages is under way.
The LupusPRO is a disease-targeted, patient-reported outcome measure that has been developed in SLE patients (female and male) of heterogeneous ethnicity within the United States. It has HRQoL and non-HRQoL items and may be used with a generic HRQoL tool in clinical research. It has good content validity, internal consistency reliability, test–retest reliability, and construct and convergent validity against the SF-36, EQ-5D, and LupusQoL-US [31]. It is responsive to changes over time. A Spanish version is being validated in Hispanic SLE patients from the continental United States, Puerto Rico, Mexico, and South America. Adaptation/translation and cross-cultural validation for Canadian-English, Canadian-French, Turkish, and other languages as well as responsiveness are currently being evaluated [24•].
The LUP-QOL measure developed by Yu et al. [25] was found to be a valid and reliable instrument in 121 SLE patients with mild to moderate disease, in whom it showed high test–retest reliability and validity.
Moorthy et al. [26••, 27] developed the SMILEY, a brief and valid English-US HRQoL instrument for children and adolescents with SLE (≤19 years of age) that is currently being cross-culturally adapted, validated, and having its responsiveness determined in many other languages and countries, including Danish, Dutch, French (France), German (Germany), Hebrew, Italian, Portuguese (Brazil), Slovene, Spanish (Spain, Argentina, Mexico, United States, and Puerto Rico), and Turkish.
Cross-Cultural Adaptation of QoL Instruments
Patients from some ethnic and racial groups tend to be younger at disease onset, to have a more severe disease course, and to have worse outcomes (eg, more acute onset or more severe lupus, including lupus nephritis [LN], in African Americans and Hispanics) [32]. The cultural features of SLE patients from different ethnic backgrounds affect the course of the disease; health behaviors, beliefs, and medical care systems vary by ethnicity, cultures, and nations. For example, in developing nations, SLE patients and those with other chronic diseases tend to seek medical care only when life-threatening symptoms occur or when disability is present. This phenomenon also has been reported among minority groups in the United States. In addition, it is well-known that socioeconomic status (SES; poverty is an SES marker) influences profoundly the course of the disease and its outcomes [33•]. These issues may contribute to unique QoL concerns and health outcomes among SLE patients based on ethnic/cultural differences. Thus, SLE is a disease with protean manifestations that leads to different responses in patients (biologically, psychosocially, and culturally), health professionals, and society itself, giving rise to different health policies and health care provisions. As SLE predominantly affects younger patients, they inherently have a greater number of years of productive life at risk as compared with patients with other chronic diseases. Also, they may not have acquired significant social or economic resources due to their relative young age. The social, emotional, and economic burdens imposed by SLE may in addition become significant QoL concerns when the cost of long-term medical care is primarily a patient’s own responsibility. These issues affect the generalizability of various SLE-specific QoL tools in various ethnic groups or medical care settings.
The emergence of multinational, multicenter studies in SLE underscores the need for validated HRQoL measures to compare patients in an equivalent manner across and within nations, taking into account not only different languages and dialects but also cultural differences among these patients. Obviously, spoken languages and dialects and how they are used vary within and among countries, affecting the adaptation/validation and the subsequent understanding of HRQoL instruments. To make studies comparable, equivalent use of HRQoL instruments is essential. To achieve this goal, the cross-cultural use of HRQoL outcome measures requires careful cross-cultural adaptation (to capture the same concept in another cultural setting equivalently), as well as validation of the transformed instrument [28]. Despite the cross-cultural validation of an existent tool, whether a tool developed in a particular ethnic or cultural background will be contextually comprehensive in content validity for another ethnicity/culture/medical care system remains to be determined.
Observational Studies Assessing the Relationship Between Clinical and Nonclinical Variables with HRQoL in SLE Populations with Different Sociodemographic Features, Ethnic Background, and Disease Status
The most relevant characteristics of selected observational studies using HRQoL measures in SLE are shown in Table 2.
The SF-36 has been used successfully among SLE patients from diverse Western societies (United States, Canada, Norway, and Spain) and in other settings, such as Singapore, where it has been shown to have satisfactory psychometric properties and construct validity. The instrument has been found to be suitable for different sociocultural environments and has been used in cross-sectional and longitudinal studies. Furthermore, it has been shown that reductions in disease activity are associated with better HRQoL [8••]. However, in a longitudinal study of 146 University of Toronto Lupus Clinic patients (predominantly Caucasian [75%] and female [90%]), disease features (disease activity, steroids, or damage accrual) were not associated with changes in physical functioning, except for the presence of fibromyalgia [34••]. It is likely that the ethnic composition (Caucasian predominance), longstanding disease, the presence of mild to moderate disease activity, and these patients’ low SDI explain the lack of sensitivity to change of the SF-36 over an 8-year period. This study also highlights the potential usefulness of documenting fibromyalgia and/or pain/tenderness in SLE patients.
In another predominantly Caucasian SLE cohort (n = 386) from Montreal, Canada, the relationship between renal activity and HRQoL (assessed by the SF-36) was studied because it has been reported that irreversible renal damage does not influence HRQoL [35]. SLE patients with concurrent active renal disease, as defined by the SLEDAI renal items, experienced a slightly poorer HRQoL—especially in the physical domains—than those without renal disease; however, in this study, changes in renal activity associated with changes in QoL could not be estimated [36••].
In contrast to the above studies, those that have included SLE patients with an ethnic composition associated with a potentially more serious disease phenotype have shown discrepant results. For example, the effects of disease activity and damage on the SF-36 were assessed in age- and sex-matched Chinese SLE patients (n = 155) observed longitudinally for 2 years with repeated HRQoL measurements; previous organ damage was associated with poorer HRQoL, and new damage predicted a further decline in HRQoL, whereas persistent disease activity was associated with deterioration in some subscales of the SF-36 [37•].
Demographic and HRQoL variables in two different African American patient groups, one urban from Chicago (n = 137 with high genetic admixture [20–30%]) and the other rural from South Carolina (n = 35 with low genetic admixture [9.8–12%]) were compared using the SF-36. In this predominantly female study, HRQoL was significantly worse in urban African Americans than in the age- and gender-matched rural patients [38•].
In the LUMINA (Lupus in Minorities, Nature Versus Nurture Multiethnic Cohort; 19% Hispanic-Texans, 17% Hispanic-Puerto Ricans, 35% African Americans, 29% Caucasians), the SF-36–derived utility measure or SF-6D was assessed in 2662 visits from 588 SLE patients, and all variables from the enrollment or preceding visits were examined to predict HRQoL. A single number represents health status in the SF-6D; the lower the value, the poorer the QoL is. Within this multiethnic cohort, Hispanic-Texan ethnicity and higher levels of social support predicted HRQoL, whereas other variables known to affect disease outcomes (older age, poverty, greater disease activity and damage), as well as fatigue, helplessness, and abnormal illness-related behaviors were negative predictors, with prior SF-6D being the strongest predictor of subsequent HRQoL [39••].
Whether SF-6D measured early in the disease course is associated with damage accrual and mortality was also examined in 552 patients from the same cohort. SF-6D was associated with damage accrual, but not with mortality. The physical component summary scale (PCS) and mental component summary scale (MCS) of the SF-36 were also examined; the PCS, but not the MCS, was found to be associated with damage, but not with mortality [40••]. The SF-6D originally had been derived for economic evaluations as a utility measure and has been shown to be a reliable instrument to assess HRQoL. Especially during early disease, the SF-6D seems more sensitive than the SF-36 to detect subtle changes in HRQoL and more likely to predict damage accrual. In SLE, mortality is related to SES factors such as age, ethnicity, and poverty, as well as to disease activity and damage; in the LUMINA study, neither the SF-6D nor the PCS and MCS scores of the SF-36 predicted mortality. However, it is possible that the influence of SF-6D in mortality is mediated by damage accrual. Until now, the role of HRQoL measures as a predictor of mortality in SLE has not been demonstrated in large cohort studies. Nevertheless, a small study conducted in 63 Brazilian SLE patients suggested that the most important predictor of mortality at 5 years is a mean low score in the role emotional subscale of the SF-36. As expected, SDI scores were higher in those who died [41].
Wolfe et al. [42••] have shown that HRQoL was predicted by disease damage, comorbidity, and SES features (income, education, and age), and that overall EQ-5D and PCS scores were at the same levels in SLE as in RA and noninflammatory rheumatic diseases, but the MCS and mental health subscale were more abnormal in SLE.
Satisfactory psychometric properties of the EQ-5D and SF-6D were recently reported by Aggarwal et al. [14••] in a multiethnic US SLE cohort from Chicago; however, these measures were weakly correlated with disease activity and damage [14••].
Khanna et al. [43] assessed QoL using the WHOQoL-Bref instrument in 73 mostly female Indian (n = 70) patients with short disease duration and showed that higher disease activity scores were also associated with lower QoL scores in the physical and psychological domains.
It is well-established that cutaneous lupus erythematosus (CLE) may have a negative impact on SLE patients’ QoL, particularly when it affects body image. Recently, the relationship between CLE and QoL was studied in 157 SLE patients in whom the QoL of patients with CLE was compared with the QoL of patients with other skin diseases. QoL was assessed using the Skindex-29 (symptoms, emotions, and functioning, with higher scores indicating worse QoL) and disease severity with the Cutaneous Lupus Erythematosus Disease Area and Severity Index. In this study, CLE had a negative impact on QoL, especially in women with more severe CLE [44]. Similar findings have been reported for body image and HRQoL in SLE by Jolly et al. [45].
The findings from the above-mentioned studies stress the role and importance of ethnic composition (including genetic admixture and gene expression), disease duration (early vs late disease), the underlying biology of SLE patients (disease phenotype), the setting in which the HRQoL instruments are administered, as well as the importance of SES, including behavioral factors (eg, smoking, compliance), and emotional aspects, including fibromyalgia, in patients’ self-perception of QoL. Several studies have also shown a lack of association between disease activity and generic HRQoL measures, especially with SF-36, which suggests that generic HRQoL assessment tools may not be the most sensitive to assess HRQoL over time [13]. Lack of correlation between self-perceived patient health status and clinical symptoms is well-known (eg, patients with active LN tend to underestimate their overall health in visual analogue scales [VAS], and fibromyalgia patients tend to overestimate it). Thus, it is conceivable that HRQoL measures are more useful and sensitive to signals of clinical improvement rather than to those pointing out clinical deterioration. Even though in cross-sectional studies, poor correlation may be noted between HRQoL and disease activity, it is plausible that in longitudinal studies (eg, clinical intervention trials or larger observational studies), evaluation of within- and between-patient variations in health status and disease activity over time in response to the intervention or natural waxing/waning disease course may be better appreciated
Therapeutic and Nontherapeutic Interventions Influencing HRQoL in SLE
In the lupus community, the ability to assess interventions aimed at controlling not only potentially life-threatening complications (eg, severe LN, neuropsychiatric involvement) but also mild to moderate SLE manifestations should not be underestimated. The role and impact of several therapeutic interventions on self-perceived HRQoL have been examined in open-label studies and in randomized controlled clinical trials of pharmacologic and nonpharmacologic interventions.
The Dutch LN Study Group compared the treatment effect of cyclophosphamide pulses plus oral prednisone versus azathioprine combined with intravenous methylprednisolone during a 24-month period on HRQoL in 87 (predominantly Caucasian) patients with proliferative LN. HRQoL and disease activity were measured at baseline and at 12 and 24 months using patient VAS, SF-36, profile of mood states, and the SLE Symptom Check List [46]. The treatment burden was assessed at 24 months, and disease activity was measured with the SLEDAI and the physician’s VAS. Complete questionnaires were available in 47 patients. The study showed that aggressive treatment of LN with immunosuppressive drugs and concomitant glucocorticoids improved HRQoL, especially in the first year [47••]. In keeping with these results, the randomized controlled trial conducted by Dussán et al. [48••] compared high-dose cyclophosphamide (50 mg/kg for 4 days) with monthly intravenous cyclophosphamide in first-time cyclophosphamide users, demonstrating improvements on several components of the SF-36 measures at 6 months in patients receiving high-dose cyclophosphamide, but equal improvement in the two arms at 36 months.
Similarly, the effect of cyclophosphamide pulse therapy on HRQoL was examined in a Brazilian study that compared cyclophosphamide users with nonusers to ascertain treatment burden. In this study, cyclophosphamide pulse therapy did not worsen HRQoL; rather, psychological distress was the main explanatory variable of poor HRQoL in these patients [49].
A small open-label study of the biological agent infliximab at conventional doses and schedule showed that infliximab improves SLEDAI without altering any of the SF-36 subscales [50].
Hydroxychloroquine prevents lupus flares and accrual of disease damage while improving patient survival. Thus, its effects on HRQoL were studied cross-sectionally in 230 SLE patients (60% African American, 20% Caucasian, 14% Hispanic, 6% Asian) from one Chicago area center using the LupusQoL-US. As expected, hydroxychloroquine use was associated with decreased damage accrual; however, HRQoL status was apparently not affected when using the LupusQoL-US or the LupusPRO measure to ascertain it [51••].
These data suggest that aggressive interventions aimed at obtaining rapid disease control during active disease are not perceived by patients as a treatment burden. However, patient perception may change over time with subsequent treatment exposure when the long-term side effects become apparent and disease damage ensues. Given that hydroxychloroquine is not indicated for rapid, active disease control, it is therefore reasonable to expect its long-term benefits in retarding the occurrence of damage and on survival are not perceived by patients, as this was already corroborated in a well-conducted observational study [52].
Other therapeutic interventions also may improve the QoL of SLE patients. For example, hip arthroplasties contributed to improved HRQoL as measured by the SF-36 in a group of Japanese patients [53].
Not every therapeutic intervention may prove useful and safe; in fact, the use of complementary alternative medicine in SLE was associated with greater cumulative disease damage and worse QoL; however, some other nontherapeutic interventions, such as patient education, may be beneficial to patients’ QoL [54, 55].
Conclusions and Research Agenda
HRQoL has been assessed in SLE populations with different degrees of severity. Studies have included mostly patients from Western societies and very few from developing societies, with small sample sizes. It is still not known how different self-perceived HRQoL may be among men with SLE, as most studies have been conducted in women, who make up the majority of SLE patients. Overall, HRQoL is poor in SLE patients regardless of ethnicity, culture, geographic location, or SES; the factors that have emerged as being implicated in yielding a poor HRQoL experience in SLE are sociodemographic (age, poverty, education) and disease related (higher disease activity and damage, fibromyalgia, fatigue, helplessness, abnormal illness-related behaviors, and mental health domains).
SF-36 has been the most commonly used HRQoL assessment tool; however, it may not be the optimal instrument to ascertain HRQoL over time given its poor sensitivity to change. However lupus-specific HRQoL measures have not yet been used in any SLE longitudinal study.
The SF-6D independently predicts disease damage, but not mortality, especially in early disease.
A low SES may influence HRQoL perception and its ascertainment, and it may alter biological and nonbiological manifestations, such as pain perception. Thus, SES should be included in any new study of HRQoL and carefully ascertained using appropriate tools and statistical models.
Fibromyalgia, a common chronic comorbid condition that is present worldwide, may distort health perception in any sociocultural and geographic setting and contribute to poor HRQoL; it should be carefully documented, included in HRQoL studies, and addressed in the clinical setting.
Aggressive therapeutic interventions, either educational or pharmacologic, especially with immunosuppressive agents aimed to control active, severe lupus manifestations are well-perceived by patients and physicians.
Medical interventions directed at controlling grumbling disease and preventing known long-term deadly complications, such as atherosclerotic cardiovascular disease, may not be fully appreciated by SLE patients or even by some physicians.
The use of HRQoL research tools such as the LupusPRO and the SMILEY, which are currently undergoing validation studies in different countries, languages, and cultural settings, may help dissect the underlying role of SES and disease-related features not only in QoL but also in other intermediate and long-term outcomes (disease-related damage and mortality) and will help to overcome the limitations posed by ethnic heterogeneity within a given lupus cohort or between lupus cohorts with dissimilar ethnicities.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Liang MH, Socher SA, Larson MG, Schur PH: Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum 1989, 32:1107–1118.
Bombardier C, Gladman DD, Urowitz MB, et al.: Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992, 35:630–640.
Symmons DP, Coppock JS, Bacon PA, et al.: Development and assessment of a computerized index of clinical disease activity in systemic lupus erythematosus. Members of the British Isles Lupus Assessment Group (BILAG). Q J Med 1988, 69:927–937.
Gladman DD, Urowitz MB, Goldsmith CH, et al.: The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 1997, 40:809–813.
Strand V, Gladman D, Isenberg D, et al.: Endpoints: consensus recommendations from OMERACT IV. Outcome measures in rheumatology. Lupus 2000, 9:322–327.
Siegel JN: Development of an FDA guidance document for clinical trials in SLE. Lupus 1999, 8:581–585.
McHorney CA, Ware JE Jr, Raczek AE: The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993, 31:247–263.
•• Thumboo J, Strand V: Health-related quality of life in patients with systemic lupus erythematosus: an update. Ann Acad Med Singapore 2007, 36:115–122. This is an interesting review of the literature on HRQoL and of the measures on how to improve it in patients with SLE.
Alarcón GS, McGwin G Jr, Uribe A, et al.: Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum 2004, 51:465–474.
Gladman DD, Urowitz MB, Ong A, et al.: A comparison of five health status instruments in patients with systemic lupus erythematosus (SLE). Lupus 1996, 5:190–195.
Gladman DD, Urowitz MB, Ong A, et al.: Lack of correlation among the 3 outcomes describing SLE: disease activity, damage and quality of life. Clin Exp Rheumatol 1996, 14:305–308.
Jolly M: How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005, 32:1706–1708.
Jolly M, Utset TO: Can disease specific measures for systemic lupus erythematosus predict patients health related quality of life? Lupus 2004, 13:924–926.
•• Aggarwal R, Wilke CT, Pickard AS, et al.: Psychometric properties of the EuroQol-5D and Short Form-6D in patients with systemic lupus erythematosus. J Rheumatol 2009, 36:1209–1216. The psychometric properties of two preference-based generic HRQoL measures, the EQ-5D and SF-6D, among US patients with SLE were assessed and found to be satisfactory. Measures of disease activity and damage were found to be weakly correlated with HRQoL.
Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med 1998, 28:551–558.
Milligan SE, Hom DL, Ballou SP, et al.: An assessment of the Health Assessment Questionnaire functional ability index among women with systemic lupus erythematosus. J Rheumatol 1993, 20:972–976.
Burckhardt CS, Archenholtz B, Bjelle A: Quality of life of women with systemic lupus erythematosus: a comparison with women with rheumatoid arthritis. J Rheumatol 1993, 20:977–981.
Lash AA: Quality of life in systemic lupus erythematosus. Appl Nurs Res 1998, 11:130–137.
Archenholtz B, Burckhardt CS, Segesten K: Quality of life of women with systemic lupus erythematosus or rheumatoid arthritis: domains of importance and dissatisfaction. Qual Life Res 1999, 8:411–416.
Leong KP, Kong KO, Thong BY, et al.: Development and preliminary validation of a systemic lupus erythematosus-specific quality-of-life instrument (SLEQoL). Rheumatology (Oxford) 2005, 44:1267–1276.
• Doward LC, McKenna SP, Whalley D, et al.: The development of the L-QoL: a quality-of-life instrument specific to systemic lupus erythematosus. Ann Rheum Dis 2009, 68:196–200. The development and testing of a new instrument to measure QoL (L-QoL) in 93 adult SLE patients from the United Kingdom is described.
• McElhone K, Abbott J, Shelmerdine J, et al.: Development and validation of a disease-specific health-related quality of life measure, the LupusQoL, for adults with systemic lupus erythematosus. Arthritis Rheum 2007, 57:972–979. The development and validation process of a self-report (patient-derived items) lupus-specific measure (LupusQoL) in a predominantly female and Caucasian SLE population from the United Kingdom is described.
•• Jolly M, Pickard AS, Wilke C, et al.: Lupus-specific health outcome measure for US patients: the LupusQoL-US version. Ann Rheum Dis 2010, 69:29–33. The first SLE-specific HRQoL instrument modified and validated for use in an ethnically diverse US population is described. Satisfactory psychometric properties are presented.
• Jolly M, Pickard AS, Fogg L, et al.: US patient derived lupus-specific patient reported outcome measure (Lupus-PRO©): validity and reliability. Arthritis Rheum 2008, 58(9 S):S805. The validity and reliability of the LupusPRO in an ethnically heterogeneous US population are presented.
Yu EB, Shikiar R, Howard K, et al.: Validation of LUP-QOL: a lupus-specific measure of health-related quality of life (HRQL) [abstract 0499]. Ann Rheum Dis 2006, 65(Suppl II).
•• Moorthy LN, Peterson MG, Baratelli M, et al.: Multicenter validation of a new quality of life measure in pediatric lupus. Arthritis Rheum 2007, 57:1165–1173. The development and validation of SMILEY as a brief, easily understood, valid, and reliable pediatric, SLE-specific QoL scale are described.
Moorthy LN, Peterson MG, Baratelli MJ, et al.: Preliminary cross-cultural adaptation of a new pediatric health-related quality of life scale in children with systemic lupus erythematosus: an international effort. Lupus 2010, 19:83–88.
Beaton DE, Bombardier C, Guillemin F, Ferraz MB: Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000, 25:3186–3191.
•• Colangelo KJ, Pope JE, Peschken C: The minimally important difference for patient reported outcomes in systemic lupus erythematosus including the HAQ-DI, pain, fatigue, and SF-36. J Rheumatol 2009, 36:2231–2237. This was a Canadian study addressing the concept of the minimally important difference in patients with SLE. This minimally important difference may differ bidirectionally depending on the measured outcome (specific values for the MCS and PCS scores of the SF-36 are provided).
Kong KO, Ho HJ, Howe HS, et al.: Cross-cultural adaptation of the Systemic Lupus Erythematosus Quality of Life Questionnaire into Chinese. Arthritis Rheum 2007, 57:980–985.
Jolly M, Pickard AS, Wilke C, et al.: Development and validation of a US lupus specific patient reported outcome measure. Arthritis Rheum 2007, 56(9 S):S113.
Bastian HM, Roseman JM, McGwin G Jr, et al.: Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 2002, 11:152–160.
• Durán S, Apte M, Alarcón GS; LUMINA Study Group: poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007, 99:1196–1198. This carefully written review emphasizes the role of poor SES (poverty) in the increased mortality rates observed in patients with SLE.
•• Kuriya B, Gladman DD, Ibañez D, Urowitz MB: Quality of life over time in patients with systemic lupus erythematosus. Arthritis Rheum 2008, 59:181–185. In this study, SF-36 scores changed little over time (during an 8-year period); these changes were not affected by disease activity, glucocorticoid exposure, or accrued damage but were affected by the concomitant presence of fibromyalgia.
Clarke AE, Panopalis P, Petri M, et al.: SLE patients with renal damage incur higher health care costs. Rheumatology (Oxford) 2008, 47:329–333.
•• Appenzeller S, Clarke AE, Panopalis P, et al.: The relationship between renal activity and quality of life in systemic lupus erythematosus. J Rheumatol 2009, 36:947–952. SLE patients with active renal disease had a slightly poorer QoL (physical subscales) than those without renal disease; however, it was not possible to accurately estimate whether a longitudinal change in renal disease activity was associated with a change in QoL.
• Mok CC, Ho LY, Cheung MY, et al.: Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: a 2-year prospective study. Scand J Rheumatol 2009, 38:121–127. This study evaluated and demonstrated the effects of persistent active disease and previous and new damage on the deterioration of SF-36 scores.
• Dua A, Kamen DL, Barnado AL, et al.: Variable health-related quality of life among African American cohorts of patients with systemic lupus erythematosus. Arthritis Rheum 2009, 60(10 S):S112. This was a comparative study of demographic and HRQoL variables in two African American SLE patient groups of different genetic admixtures and geographic locations (urban and rural). Significantly worse HRQoL was found among the urban African Americans than among their rural counterparts.
•• Sanchez ML, McGwin G Jr, Durán S, et al.: Factors predictive of overall health over the course of the disease in patients with systemic lupus erythematosus from the LUMINA cohort (LXII): use of the SF-6D. Clin Exp Rheumatol 2009, 27:67–71. The SF-6D utility measure was used in this multiethnic study. Hispanic-Texan ethnicity and higher levels of social support were found to be positive predictors of HRQoL, whereas sociodemographic (older age, poverty) and disease-related (greater disease activity and damage, higher levels of fatigue, helplessness, and abnormal illness-related behaviors) variables were found to be negative predictors; prior SF-6D was found to be the strongest predictor of subsequent HRQoL.
•• Fernández M, Alarcón GS, McGwin G Jr, et al.: Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum 2007, 57:986–992. In this multiethnic SLE cohort, the SF-6D was found to be associated with damage accrual, but not with mortality.
Freire E, Bruscato A, Ciconelli R: Quality of life in systemic lupus erythematosus patients in northeastern Brazil: is health-related quality of life a predictor of survival for these patients? Acta Reumatol Port 2009, 34:207–211.
•• Wolfe F, Michaud K, Li T, Katz RS: EQ-5D and SF-36 quality of life measures in systemic lupus erythematosus: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, and fibromyalgia. J Rheumatol 2010, 37:296–304. This was a large comparison study of the EQ-5D and SF-36 (1316 SLE patients vs 13,722 rheumatoid arthritis, 3623 with noninflammatory rheumatic disorders, and 2733 with fibromyalgia). No differences in the EQ-5D and PCS scores among patients with these diseases were found. The MCS of the SF-36 was found to be lower in SLE than in rheumatoid arthritis and noninflammatory rheumatic disorders; poor QoL in SLE was predicted by disease damage and the number of comorbid events.
Khanna S, Pal H, Pandey RM, Handa R: The relationship between disease activity and quality of life in systemic lupus erythematosus. Rheumatology (Oxford) 2004, 43:1536–1540.
Klein R, Moghadam-Ki S, LoMonico J, et al.: Quality of life in cutaneous lupus erythematosus. Arthritis Rheum 2009, 60(10 S):S107.
Jolly M, Pickard AS, Mikolaitis R, et al.: Disease activity, body image and health status in Lupus. Arthritis Rheum 2007, 56:S454.
Grootscholten C, Ligtenberg G, Derksen RH, et al.: Health-related quality of life in patients with systemic lupus erythematosus: development and validation of a lupus specific symptom checklist. Qual Life Res 2003, 12:635–644.
•• Grootscholten C, Snoek FJ, Bijl M, et al.: Health-related quality of life and treatment burden in patients with proliferative lupus nephritis treated with cyclophosphamide or azathioprine/methylprednisolone in a randomized controlled trial. J Rheumatol 2007, 34:1699–1707. This small study compared the treatment effect of cyclophosphamide pulses plus oral prednisone versus azathioprine combined with intravenous methylprednisolone during a 24-month period on HRQoL in 87 predominantly Caucasian patients with proliferative LN. The study showed that aggressive treatment with immunosuppressive drugs and concomitant glucocorticoids improves HRQoL, especially in the first year.
•• Dussán KB, Magder L, Brodsky RA, et al.: High dose cyclophosphamide performs better than monthly dose cyclophosphamide in quality of life measures. Lupus 2008, 17:1079–1085. In this small randomized controlled trial, high-dose cyclophosphamide was compared with monthly intravenous cyclophosphamide in first-time cyclophosphamide users. Improvement in several components of the SF-36 was demonstrated at 6 months in the high-dose cyclophosphamide patient group, but equal improvement in the two arms was demonstrated at 36 months.
Medeiros MM, Menezes AP, Silveira VA, et al.: Health-related quality of life in patients with systemic lupus erythematosus and its relationship with cyclophosphamide pulse therapy. Eur J Intern Med 2008, 19:122–128.
Uppal SS, Hayat SJ, Raghupathy R: Efficacy and safety of infliximab in active SLE: a pilot study. Lupus 2009, 18:690–697.
•• Jolly M, Sandler D, Sequeira W, et al.: Hydroxy-chloroquine use and disease specific health related quality of life in systemic lupus erythematosus. Arthritis Rheum 2009, 60(10 S):S108. The beneficial effects of hydroxychloroquine in preventing major lupus flares, retarding damage accrual, and improving patient survival are well-known; however, no demonstrable effect of hydroxychloroquine on the LupusQoL-US or the LupusPRO could be demonstrated in this study.
Alarcón GS, McGwin G, Bertoli AM, et al.: Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort. Ann Rheum Dis 2007, 66:1168–1172.
Ito H, Matsuno T, Hirayama T, et al.: Health-related quality of life in patients with systemic lupus erythematosus after medium to long-term follow-up of hip arthroplasty. Lupus 2007, 16:318–323.
Alvarez-Nemegyei J, Bautista-Botello A, Dávila-Velázquez J: Association of complementary or alternative medicine use with quality of life, functional status or cumulated damage in chronic rheumatic diseases. Clin Rheumatol 2009, 28:547–551.
Karlson EW, Liang MH, Eaton H, et al.: A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum 2004, 50:1832–1841.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toloza, S.M.A., Jolly, M. & Alarcón, G.S. Quality-of-Life Measurements in Multiethnic Patients with Systemic Lupus Erythematosus: Cross-Cultural Issues. Curr Rheumatol Rep 12, 237–249 (2010). https://doi.org/10.1007/s11926-010-0110-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-010-0110-5