Abstract
Purpose of Review
Familial predisposition to bipolar disorder is associated with increased risk of affective morbidity in the first-degree relatives of patients. Nevertheless, a substantial proportion of relatives remain free of psychopathology throughout their lifetime. A series of studies reviewed here were designed to test whether resilience in these high-risk individuals is associated with adaptive brain plasticity.
Recent Findings
The findings presented here derive from structural and functional magnetic resonance imaging data obtained from patients, their resilient first-degree relatives, and healthy individuals. Patients and relatives showed similar abnormalities in activation and connectivity while performing tasks of interference control and facial affect recognition and in the resting-state connectivity of sensory and motor regions. Resilient relatives manifested unique neuroimaging features that differentiated them from patients and healthy individuals. Specifically, they had larger cerebellar vermis volume, enhanced prefrontal connectivity during task performance, and enhanced functional integration of the default mode network in task-free conditions.
Summary
Resilience to bipolar disorder is not the reverse of risk but is associated with adaptive brain changes indicative of increased neural reserve. This line of research may open new avenues in preventing and treating bipolar disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive research has led to the identification of multiple risk factors for mental disorders, and particularly for schizophrenia, mood, and anxiety disorders. Familial psychopathology [1], adversity in childhood and adult life [2,3,4], and metabolic dysregulation [5,6,7] have emerged as key transdiagnostic risk factors; significant overlap across diagnostic categories has also been noted in risk-conferring genetic loci [8]. Other risk factors, mainly cannabis use for psychosis, appear more disorder-specific [2]. Thus far, neurobiological investigations have focused on identifying risk-associated “abnormalities” and have overlooked the fact that a significant proportion of risk-exposed individuals remain psychiatrically well. Investigation of the mechanisms that enable these “resilient” individuals to adapt successfully has the potential to yield new avenues for prevention of adverse mental health outcomes.
Concepts of Resilience to Adverse Mental Health Outcomes
The British psychiatrist, Sir Michael Rutter, is widely credited for introducing the concept of resilience in psychiatric research based on his seminal studies on children exposed social to adversity including family dysfunction, economic deprivation, and institutionalization [9,10,11,12,13,14,15,16]. He defined resilience as “an interactive concept that is concerned with the combination of serious risk experiences and a relatively positive psychological outcome despite those experiences” [11]. His work, along with that of other key figures in the field, identified psychological (e.g., sense of agency), family (e.g., close family bonds), and societal (e.g., community cohesion and support) attributes that promote resilience [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Collectively, current formulations of risk and resilience emphasize the role of external or environmental influences [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] but have not sought to specify associated biological mechanisms. In response, 10 years ago, we initiated a series of studies aiming to disambiguate neural mechanisms of risk, disease expression, and resilience, using bipolar disorder as an exemplar [26,27,28,29,30,31,32,33,34,35,36,37]. Bipolar disorder is a mood disorder characterized by episodes of depression and mania with variable inter-episode remission [38] which is ideally suited to this purpose for the following reasons: (i) extensive neuroimaging studies have established that disease expression in bipolar disorder is associated with alterations in brain structure and function, (ii) it is highly heritable with genetic influences explaining 60–85% of risk [39], and (iii) having a first-degree relative with bipolar disorder represents the most significant risk factor for affective morbidity, leading to approximately a fivefold increase in the likelihood of syndromal conversion to a mood disorder; up to 65% of these high-risk individuals convert, commonly during the second decade of life, while the remainder can be considered as resilient [39,40,41].
Resilience in our work refers to adaptive brain plasticity associated with avoidance of psychopathology despite genetic predisposition to bipolar disorder. Resilience is therefore predicated on the lifetime absence of adverse clinical outcomes (i.e., clinical-range symptoms) [42]. Other authors have developed scales (e.g., Connor-Davidson Resilience Scale) that commonly measure resilience in terms of individuals’ self-reported ability to cope with stress. This is not the approach that we used as we focus on the outcome of being resilient which is to maintain psychological well-being in the face of increased risk. At present, it is not possible to compute precise estimates of personalized risk for the first-degree relatives of patients with bipolar disorder and genetic proximity to an affected individual is the best measure of “genetic” burden. As this convention has been employed in all studies that have examined the impact of genetic risk and we consider it sufficient as a basis for beginning to examine resilience. We focus on the brain structural and functional correlates of resilience as assessed with magnetic resonance imaging (MRI) because we regard alterations in brain organization as the most proximal “cause” of affective morbidity. Since bipolar disorder is thought to arise from disruptive changes in brain systems, it is logical to test whether for the presence of adaptive brain changes may promote resilience.
In our work, we adopted the following operational definitions for disambiguating resilience-related brain changes from those associated with disease expression and risk: (i) brain structural and functional features that are common in resilient relatives and in patients, compared to healthy individuals, define a state of vulnerability but they are not sufficient for disease expression; (ii) brain structural and functional features that are unique to patients, compared to both to healthy individuals and relatives, relate to mechanisms involved in overt disease expression; and (iii) brain structural and functional features that are unique to resilient relatives, compared to both to healthy individuals and patients, are considered adaptive responses to genetic risk.
Disease-Related Brain Alterations in Patients with Bipolar Disorder
Patients show subtle but measurable reductions in cortical thickness in lateral and medial prefrontal regions, in the insula and in the fusiform gyrus, and in the volume of the hippocampus and thalamus [43,44,45]. Functional magnetic resonance imaging (fMRI) studies provide a richer source of information about brain organization as they assess regional mean signal changes (activation) and inter-regional interactions (connectivity) across distinct situational demands (i.e., in response to task demands and at rest). In bipolar disorder, task-dependent activation and connectivity have been examined mostly in terms of affect processing and executive control, based on behavioral data that implicate dysfunction in these domains [46, 47]. During affective and cognitive control tasks, patients exhibit exaggerated activation in the amygdala (AMG), insula, and the anterior cingulate cortex (ACC) and reduced ventrolateral prefrontal cortical (vlPFC) engagement [48, 49]. Additional abnormalities have been noted in inter-regional connectivity in tasks and resting-state fMRI studies; specifically, patients show abnormal connectivity between affect processing subcortical regions and in their forward connections to ventral PFC regions and reduced regulatory input from the PFC to subcortical and posterior cortical regions [50, 51]. Other studies have reported abnormal resting-state connectivity of sensorimotor networks and of the default mode network (DMN) [52,53,54], which consists of posterior and midline regions that become more active during internally generated cognition [55]. Thus, disease expression for bipolar disorder appears to be associated with (i) hyperactivation and hyperconnectivity between affect processing regions, (ii) reduced regulatory input from the PFC regions involved in cognitive control, and (iii) reduced connectivity of the DMN and sensorimotor networks.
Brain Alterations Associated with Genetic Risk for Bipolar Disorder
Studies in first-degree relatives of patients have found little or no evidence of reductions in either global measures or in specific subcortical regions-of-interest involving the amygdala, hippocampus, and striatum [56,57,58,59,60,61,62]. Task-related functional abnormalities have been observed in high-risk individuals in the prefrontal-subcortical reward circuitry [63] and in prefrontal-amygdala connectivity during affect processing [64,65,66,67]. Further, resting-state functional studies have reported reduced connectivity of the vlPFC [68] and in striatal-thalamo-cortical [69], in prefrontal-visual cortical, and in sensorimotor networks [70]. Thus, genetic risk for bipolar disorder appears to be associated mostly with abnormalities in the connectivity between prefrontal regulatory regions and affect processing and sensorimotor networks.
Brain Alteration Associated with Resilience to Bipolar Disorder
In our first foray into characterizing neuroimaging phenotypes of resilience to bipolar disorder, we obtained structural MRI data from patients with bipolar disorder (n = 47), their resilient first-degree relatives, and demographically matched healthy individuals (n = 71) [27]. At the time, a previous study had reported that the intracranial volume (ICV) of unaffected relatives of patients was about 3% larger than that of healthy individuals [71]. In addition to replicating the ICV increase [29], we also found that resilient relatives had larger cerebellar vermal volumes [27]. Both findings have been subsequently confirmed by other primary [60, 72] and meta-analytic studies [62, 73]. The identification of the cerebellum as a correlate of resilience in relatives was unexpected as this region is traditionally considered in terms of its contribution to motor control. However, there is compelling evidence implicating the vermis in the “homeostatic” regulation of autonomic function and cognition [74,75,76,77] and affective processing [78]. Cerebellar lesions, although relatively rare, give rise to a wide array of cognitive and affective abnormalities that constitute the cerebellar cognitive affective syndrome [79]. The anterior cerebellum has been linked to higher-order cognitive functions [80, 81]. The posterior cerebellum, including the vermis, is thought to form representations of affective and somatic states [82,83,84] but does not influence the conscious experience of emotions [85, 86]. Instead, it participates in contextual emotional learning and contextually appropriate response selection [82,83,84]. The cerebellar vermis in particular is involved in the integrative processing of somatosensory information (through its connections with the brainstem and thalamus), emotional states (via the amygdala, septum, and locus coeruleus), and motor responses (through its connections with the motor cortex) [82,83,84]. Accordingly, increased vermal volume in relatives could assist in maintaining affective equilibrium and thus likely to represent an aspect of resilient adaptation to genetic risk.
Functional activation and connectivity in the same sample were examined using the Stroop Color Word Test (SCWT) [87], the 3-back working memory task [88], and the facial affect recognition task [89, 90]. These tasks are respectively thought to capture core aspects of interference control, working memory, and affect processing. The SCWT and the 3-back task typically engage overlapping networks [91]. Within these networks, the dorsolateral prefrontal cortex (dlPFC) and dorsal parietal cortex (dPAR) act in concert to update and maintain representations of task-relevant information and to bias activity in other regions toward task-appropriate responses [88, 91,92,93]; the ACC is involved in performance monitoring while ventral parietal regions (vPAR) integrate task relevance and stimulus features in the service of interference control of attention [91, 93]. Response execution is primarily implemented through the action of the vlPFC [88, 91,92,93] and the striatum [93,94,95]; the engagement of these regions is more pronounced when implementing contextually appropriate responses over habitual choices [88, 91,92,93,94,95]. Facial affect processing involves a different set of functionally and anatomically connected cortical and subcortical brain structures [89,90,91], that principally include the inferior occipital gyrus (IOG) [96, 97], the fusiform gyrus (FG) [97, 98], the AMG, and the ventral prefrontal cortex (vPFC) [99, 100]. Within this network, the AMG implements rapid detection of facial affect and biases behavioral responses accordingly [101, 102] while the vPFC is involved in a more detailed evaluation of the contextual significance of emotional stimuli [103, 104].
The availability of all three tasks in the same sample of patients, resilient relatives, and healthy individuals enabled us to characterize brain mechanisms of resilience in different situational demands. In all cases, we examined both task-related activation and connectivity; for the latter, we investigated effective connectivity that reflects directed interactions between brain regions [105]. In the section below, the normative values from the healthy individuals was used as reference.
During the SCWT, decreased activation in the right vlPFC and the caudate was observed only in patients while both patients and resilient relatives demonstrated quantitatively similar signal reduction in the parietal cortex [30] (Fig. 1). We infer that genetic predisposition to bipolar disorder disrupts in the engagement of brain regions involved in interference control of attention while vlPFC and striatal activation during inhibitory control is preserved in resilient relatives. The functional connectivity of the task-related network was also altered. Specifically, patients showed abnormally increased connectivity between the right vlPFC and limbic regions (vACC and insula) and abnormally reduced vlPFC-striatal connectivity. Enhanced connectivity between the dlPFC and vlPFC was uniquely observed in resilient relatives [31]. These findings suggest that resilient relatives manifest brain changes associated with genetic risk which allows us to propose that the greater conjunctive activity within the PFC represents an adaptive response to functional dysregulation in other areas of the network (Fig. 1).
Connectivity associated with risk, disease expression and resilience during interference control. Study participants performed the stroop color word test that required them to name the font color of incongruent color names. CN caudate nucleus, DLPFC dorsolateral prefrontal cortex, GP globus pallidus, INS insula, SPL superior parietal cortex, vACC ventral anterior cingulate cortex, VLPFC ventrolateral prefrontal cortex. Green line indicates enhanced task-related connectivity. Blue lines indicate increased task-related connectivity. Red lines indicate decreased task-related connectivity. Dotted lines indicate loss of task-related connectivity. Details in reference [31]
During the 3-back task, patients showed bilateral hypoactivation in the dlPFC, vlPFC, and hyperactivation in the ACC and widespread hypoconnectivity within the working memory network; resilient relatives showed hyperactivation in all these areas with preserved connectivity [35]. This pattern suggests that genetic risk for bipolar disorder influences the activation of the ACC, which we interpret as reflecting increased demand for performance monitoring. Prefrontal dysfunction and hypoconnectivity were noted again in relation to disease expression while the function and connectivity of the working memory network remained intact in resilient relatives.
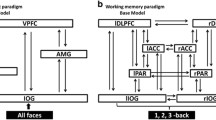
During the facial recognition task, abnormal hyper- and hypoactivation in limbic and prefrontal areas, respectively, were only noted in patients [35] (Fig. 2). The connectivity analyses revealed that genetic risk was associated with increased connectivity from the AMG to the vlPFC during the processing of emotional faces, as this features was common in patients and resilient relatives. The connectivity from the IOG to the vlPFC was preserved in resilient relatives but reduced in patients; additionally, enhanced connectivity that was unique to resilient relatives was observed between the vlPFC and the IOG and AMG [35]. It would therefore appear that resilience in relatives is associated with additional recruitment throughout the affect processing network.
Connectivity associated with risk, disease expression and resilience during facial affect recognition. Study participants performed a facial affect recognition task that required them to identify whether the faces presented showed happiness, anger, sadness, or fear. Right panel shows the connectivity of the facial affect recognition network in healthy participants. Left panel shows connectivity within this network in patients and relatives referenced to normative values. AMG amygdala, FG fusiform gyrus, IOG inferior occipital gyrus, VLPFC ventral prefrontal cortex. Blue line indicates reduced task-related connectivity in patients only. Red line indicates increased task-related connectivity in patients and relatives. Green line indicates enhanced task-related connectivity in relatives only. Details in reference (35)
We also examined resting-state functional connectivity in an independent sample of patients with bipolar disorder (n = 78), their unaffected siblings (n = 64), and unrelated healthy individuals (n = 41) [37] (Fig. 3). This investigation complemented our previous research as it enabled us to examine functional connectivity in task-free conditions. In line with advances in fMRI data analyses, we used graph theory to define and quantify the cohesion and integration of resting-state networks. The graph theory represents the brain as a graph in which regions and their connections are modeled as nodes and edges [106]. We used global efficiency and characteristic path length (both measures of network integration), clustering coefficient (a measure of network segregation), and small-worldness (a measure of the balance between segregation and integration) to assess global network organization [37]. Neither patients nor siblings showed any abnormalities in these measures. Regional connectivity was assessed using the nodal degree (the number of connections of a region to other regions within the network) and the participation coefficient (a measure of the connectivity of a given region to regions outside its own network) [37]. Relatives showed abnormally reduced intra-network cohesion and inter-network connectivity in primary motor and sensory regions (pre- and postcentral gyri, paracentral lobule) and in the visual cortex [37]. In patients, this pattern was coupled with abnormally increased intra-network connectivity of the secondary motor (supplementary motor area) and secondary somatosensory regions (supramarginal gyrus) involved in the perception, initiation, and production of ordered movement, including speech [107,108,109,110,111]. Further abnormalities that were specific to patients comprised increased intra-network cohesion and reduced inter-network integration of the anterior DMN regions, particularly the ventromedial PFC. By contrast, resilience was uniquely associated with enhanced inter-network integration of the core DMN regions (ventromedial PFC, angular gyrus, and the precuneus). Typically, sensory and motor networks show high intra-network connectivity and relatively low inter-network integration in line with their specialized function; DMN regions show high inter-network connectivity coupled with high between-network integration which allow the DMN to act as a “cohesive connector” within the brain functional connectome [112]. The findings from our studies identified dysconnectivity of sensorimotor regions as a correlate of genetic risk and disease expression. The dysconnectivity of the DMN was associated with disease expression while the enhanced integration of DMN within the brain functional connectome appeared to confer resilience.
Resting-state connectivity associated with risk, disease expression, and resilience. Regions with altered resting-state connectivity associated with genetic risk (left panel), disease expression (middle panel), and resilience (right panel). BD patients with bipolar disorder, SIB siblings, HV healthy volunteers. Details in reference (37)
Conclusions
Table 1 provides a summary of the brain features associated with risk, disease expression, and resilience identified in our studies. The findings regarding risk and disease expression support current models of bipolar disorder that propose abnormally increased activity and connectivity among affect processing regions coupled with reduced regulatory control from frontoparietal regions [113, 114]. Adaptive brain responses associated with resilience consisted mainly of enhanced connectivity between prefrontal regions and between core DMN regions and other brain networks. Of note, a study of individuals at risk for major depressive disorder found that resilience in that group was associated with enhanced connectivity within PFC-linked functional networks [115]. Enhanced connectivity may therefore emerge as a transdiagnostic feature of resilience although specific patterns may be both diagnosis and context related.
Such adaptive brain responses can be conceptualized in terms of neural reserve or neural compensation [116]. Neural reserve is the ability of brain networks to cope with pathology or higher demands as a function of their increased plasticity or recruitment of additional neural resources. Neural compensation refers to reallocation of processing to alternate brain regions. Accordingly, the features associated with resilience in bipolar disorder appear indicative of increased reserve. Clinical symptoms may arise because of failure to develop or maintain adaptive changes in response to genetically mediated brain pathology. Longitudinal studies of individuals at high risk for BD would be informative in this respect. Moreover, a more precise formulation of the nature of resilience-related brain mechanisms will require further studies to define its molecular and genetic mechanisms.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
McLaughlin KA, Gadermann AM, Hwang I, Sampson NA, Al-Hamzawi A, Andrade LH, et al. Parent psychopathology and offspring mental disorders: results from the WHO World Mental Health Surveys. Br J Psychiatry. 2012;200(4):290–9. https://doi.org/10.1192/bjp.bp.111.101253.
Belbasis L, Köhler CA, Stefanis N, Stubbs B, van Os J, Vieta E, et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137(2):88–97. https://doi.org/10.1111/acps.12847.
Bortolato B, Köhler CA, Evangelou E, León-Caballero J, Solmi M, Stubbs B, et al. Systematic assessment of environmental risk factors for bipolar disorder: an umbrella review of systematic reviews and meta-analyses. Bipolar Disord. 2017;19(2):84–96. https://doi.org/10.1111/bdi.12490.
Köhler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, et al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res. 2018;103:189–207. https://doi.org/10.1016/j.jpsychires.2018.05.020.
Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36(2):480–9. https://doi.org/10.2337/dc12-1442.
Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261–9. https://doi.org/10.1001/jamapsychiatry.2016.3803.
Perry BI, Upthegrove R, Thompson A, Marwaha S, Zammit S, Singh SP, et al. Dysglycaemia, inflammation and psychosis: findings from the UK ALSPAC Birth Cohort. Schizophr Bull. 2018. https://doi.org/10.1093/schbul/sby040.
Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–9. https://doi.org/10.1016/S0140-6736(12)62129-1.
Rutter M. Protective factors in children’s responses to stress and disadvantage. Ann Acad Med Singapore. 1979;8(3):324–38.
Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. English and Romanian Adoptees (ERA) Study Team. J Child Psychol Psychiatry. 1998;39(4):465–76.
Rutter M. Implications of resilience concepts for scientific understanding. Ann N Y Acad Sci. 2006;1094:1–12. https://doi.org/10.1196/annals.1376.002.
Rutter M. Resilience, competence, and coping. Child Abuse Negl. 2007;31(3):205–9. https://doi.org/10.1016/j.chiabu.2007.02.001.
Rutter M. Institutional effects on children: design issues and substantive findings. Monogr Soc Res Child Dev. 2008;73(3):271–8.
Rutter M. Resilience as a dynamic concept. Dev Psychopathol. 2012;24(2):335–44. https://doi.org/10.1017/S0954579412000028.
Rutter M. Annual research review: resilience—clinical implications. J Child Psychol Psychiatry. 2013;54(4):474–87. https://doi.org/10.1111/j.1469-7610.2012.02615.x.
Rutter M, Colvert E, Kreppner J, Beckett C, Castle J, Groothues C, et al. Early adolescent outcomes for institutionally-deprived and non-deprived adoptees. I: disinhibited attachment. J Child Psychol Psychiatry. 2007;48(1):17–30. https://doi.org/10.1111/j.1469-7610.2006.01688.x.
Ungar M. A constructionist discourse on resilience: multiple contexts, multiple realities among at-risk children and youth. Youth & Society. 2004;35(3):341–65. https://doi.org/10.1177/0044118X03257030.
Ungar M, Brown M, Liebenberg l CM, Levine K. Distinguishing differences in pathways to resilience among Canadian youth. CJCMH. 2008;27:1):1–13. https://doi.org/10.7870/cjcmh-2008-0001.
Ungar M, Brown M, Liebenberg L, Othman R, Kwong WM, Armstrong M, et al. Unique pathways to resilience across cultures. Adolescence. 2007 Summer;42(166):287–310.
Werner EE, Smith RS. Vulnerable but invincible: a study of resilient children. New York: McGraw-Hill; 1982.
Werner EE. High-risk children in young adulthood: a longitudinal study from birth to 32 years. Am J Orthopsychiatry. 1989;59(1):72–81. https://doi.org/10.1111/j.1467-8624.1991.tb01555.x.
Garmezy N, Tellegen A. Studies of stress-resistant children: methods, variables and preliminary findings. In: Morrison FJ, Lord C, Keating DP, editors. Applied developmental psychology, vol. 1. New York: Academic Press; 1984.
Garmezy N, Masten AS, Tellegen A. The study of stress and competence in children: a building block for developmental psychopathology. Child Dev. 1984;55(1):97–111. https://doi.org/10.2307/1129837.
Masten AS, Garmezy N, Tellegen A, Pellegrini DS, Larkin K, Larsen A. Competence and stress in school children: the moderating effects of individual and family qualities. J Child Psychol Psychiatry. 1988;29(6):745–64. https://doi.org/10.1111/j.1469-7610.1988.tb00751.x.
Luthar SS. Vulnerability and resilience: a study of high-risk adolescents. Child Dev. 1991;62(3):600–16. https://doi.org/10.1111/j.1467-8624.1991.tb01555.x.
Frangou S. Risk and resilience in bipolar disorder: rationale and design of the Vulnerability to Bipolar Disorders Study (VIBES). Biochem Soc Trans. 2009;37(Pt 5:1085–9. https://doi.org/10.1042/BST0371085.
Kempton MJ, Haldane M, Jogia J, Grasby PM, Collier D, Frangou S. Dissociable brain structural changes associated with predisposition, resilience, and disease expression in bipolar disorder. J Neurosci. 2009;29(35):10863–10,868. https://doi.org/10.1523/JNEUROSCI.2204-09.2009.
Walterfang M, Wood AG, Barton S, Velakoulis D, Chen J, Reutens DC, et al. Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(6):1050–7. https://doi.org/10.1016/j.pnpbp.2009.05.019.
Takahashi T, Walterfang M, Wood SJ, Kempton MJ, Jogia J, Lorenzetti V, et al. Pituitary volume in patients with bipolar disorder and their first-degree relatives. J Affect Disord. 2010;124(3):256–61. https://doi.org/10.1016/j.jad.2009.12.002.
Pompei F, Jogia J, Tatarelli R, Girardi P, Rubia K, Kumari V, et al. Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. Neuroimage. 2011;56(3):1677–84. https://doi.org/10.1016/j.neuroimage.2011.02.052.
Pompei F, Dima D, Rubia K, Kumari V, Frangou S. Dissociable functional connectivity changes during the Stroop task relating to risk, resilience and disease expression in bipolar disorder. Neuroimage. 2011;57(2):576–82. https://doi.org/10.1016/j.neuroimage.2011.04.055.
Forcada I, Papachristou E, Mur M, Christodoulou T, Jogia J, Reichenberg A, et al. The impact of general intellectual ability and white matter volume on the functional outcome of patients with bipolar disorder and their relatives. J Affect Disord. 2011;130(3):413–20. https://doi.org/10.1016/j.neuroimage.2016.08.066.
Lelli-Chiesa G, Kempton MJ, Jogia J, Tatarelli R, Girardi P, Powell J, et al. The impact of the Val158Met catechol-O-methyltransferase genotype on neural correlates of sad facial affect processing in patients with bipolar disorder and their relatives. Psychol Med. 2011;41(4):779–88. https://doi.org/10.1017/S0033291710001431.
Frangou S. Brain structural and functional correlates of resilience to Bipolar Disorder. Front Hum Neurosci. 2012;5:184. https://doi.org/10.3389/fnhum.2011.00184.
Dima D, Roberts RE, Frangou S. Connectomic markers of disease expression, genetic risk and resilience in bipolar disorder. Transl Psychiatry. 2016;6:e706. https://doi.org/10.1038/tp.2015.193 This study provided first evidence for task-specific aspects of resilience in healthy relatives of patients with bipolar disorder by showing dissociation between working memory and specific aspects of facial affect processing.
Frangou S, Dima D, Jogia J. Toward person-centered neuroimaging markers for resilience and vulnerability in Bipolar Disorder. Neuroimage. 2017;145(Pt B):230–7. https://doi.org/10.1016/j.neuroimage.2016.08.066.
Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S. The role of intrinsic brain functional connectivity in vulnerability and resilience to bipolar disorder. Am J Psychiatry. 2017;174(12):1214–22. https://doi.org/10.1176/appi.ajp.2017.17010095 This is the first study to show that the functional integration of the default mode network plays an important role in differentiating resilient from affected siblings.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C(1):48–58. https://doi.org/10.1002/ajmg.c.20013.
Mesman E, Nolen WA, Reichart CG, Wals M, Hillegers MH. The Dutch bipolar offspring study: 12-year follow-up. Am J Psychiatry. 2013;170:542–9. https://doi.org/10.1176/appi.ajp.2012.12030401.
Loftus J, Etain B, Scott J. What can we learn from offspring studies in bipolar disorder? BJPsych Advances. 2016;22(3):176–85. https://doi.org/10.1192/apt.bp.114.013086.
Carpenter SR, Walker B, Anderies JM, Abel N. From metaphor to measurement: resilience of what to what? Ecosystems. 2001;4(8):765–81. https://doi.org/10.1007/s10021-001-0045-9.
Hibar DP, Westlye LT, van Erp TG, Rasmussen J, Leonardo CD, Faskowitz J, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21(12):1710–6. https://doi.org/10.1038/mp.2015.227.
Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932–42. https://doi.org/10.1038/mp.2017.73.
Pezzoli S, Emsell L, Yip SW, Dima D, Giannakopoulos P, Zarei M, et al. Meta-analysis of regional white matter volume in bipolar disorder with replication in an independent sample using coordinates, T-maps, and individual MRI data. Neurosci Biobehav Rev. 2018;84:162–70. https://doi.org/10.1016/j.neubiorev.2017.11.005.
Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38(6):771–85. https://doi.org/10.1017/S0033291707001675.
Glahn DC, Almasy L, Barguil M, Hare E, Peralta JM, Kent JW Jr, et al. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry. 2010;67(2):168–77. https://doi.org/10.1001/archgenpsychiatry.2009.184.
Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13(1):1–15. https://doi.org/10.1111/j.1399-5618.2011.00893.x.
Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22(2):100–13. https://doi.org/10.1016/j.euroneuro.2011.07.003.
Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder—a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr. 2017;3:15. https://doi.org/10.1038/s41537-017-0013-9.
Vargas C, López-Jaramillo C, Vieta E. A systematic literature review of resting state network—functional MRI in bipolar disorder. J Affect Disord. 2013;150(3):727–35. https://doi.org/10.1016/j.jad.2013.05.083.
Ongür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. https://doi.org/10.1016/j.pscychresns.2010.04.008.
Calhoun VD, Sui J, Kiehl K, Turner JA, Allen EA, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2012;2:75. https://doi.org/10.3389/fpsyt.2011.00075.
Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, et al. Is aberrant functional connectivity psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74(6):458–66. https://doi.org/10.1016/j.biopsych.2013.04.024.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. https://doi.org/10.1073/pnas.98.2.676.
Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Nau S, Kalas C, et al. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry. 2008;47(5):532–9. https://doi.org/10.1097/CHI.0b013e318167656e.
Hajek T, Gunde E, Slaney C, Propper L, MacQueen G, Duffy A, et al. Amygdala and hippocampal volumes in relatives of patients with bipolar disorder: a high-risk study. Can J Psychiatry. 2009;54(11):726–33. https://doi.org/10.1177/070674370905401102.
Hajek T, Gunde E, Slaney C, Propper L, MacQueen G, Duffy A, et al. Striatal volumes in affected and unaffected relatives of bipolar patients—high-risk study. J Psychiatr Res. 2009;43(7):724–9. https://doi.org/10.1016/j.jpsychires.2008.10.008.
Hajek T, Gunde E, Bernier D, Slaney C, Propper L, Grof P, et al. Subgenual cingulate volumes in affected and unaffected offspring of bipolar parents. J Affect Disord. 2008;108:263–9. https://doi.org/10.1016/j.jad.2007.10.024.
Hajek T, Novak T, Kopecek M, et al. Subgenual cingulate volumes in offspring of bipolar parents and in sporadic bipolar patients. Eur Arch Psychiatry Clin Neurosci. 2010;260:297–304. https://doi.org/10.1007/s00406-009-0077-2.
Singh MK, Delbello MP, Adler CM, Kopecek M, Gunde E, Alda M, et al. Neuroanatomical characterization of child offspring of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2008;47(4):526–31. https://doi.org/10.1097/CHI.0b013e318167655a.
Fusar-Poli P, Howes O, Bechdolf A, Borgwardt S. Mapping vulnerability to bipolar disorder: a systematic review and meta-analysis of neuroimaging studies. J Psychiatry Neurosci. 2012;37(3):170–84. https://doi.org/10.1503/jpn.110061.
Bertocci MA, Bebko G, Versace A, Fournier JC, Iyengar S, Olino T, et al. Predicting clinical outcome from reward circuitry function and white matter structure in behaviorally and emotionally dysregulated youth. Mol Psychiatry. 2016;21(9):1194–201. https://doi.org/10.1038/mp.2016.5.
Hafeman D, Bebko G, Bertocci MA, Fournier JC, Chase HW, Bonar L, et al. Amygdala-prefrontal cortical functional connectivity during implicit emotion processing differentiates youth with bipolar spectrum from youth with externalizing disorders. J Affect Disord. 2017;208:94–100. https://doi.org/10.1016/j.jad.2016.09.064.
Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, et al. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain. 2015;138(Pt 9):2777–90. https://doi.org/10.1093/brain/awv176.
Ladouceur CD, Diwadkar VA, White R, Bass J, Birmaher B, Axelson DA, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev Cogn Neurosci. 2013;5:185–96. https://doi.org/10.1016/j.dcn.2013.03.004.
Breakspear M, Roberts G, Green MJ, Nguyen VT, Frankland A, Levy F, et al. Network dysfunction of emotional and cognitive processes in those at genetic risk of bipolar disorder. Brain. 2015;138(Pt 11:3427–39. https://doi.org/10.1093/brain/awv261.
Roberts G, Lord A, Frankland A, Wright A, Lau P, Levy F, et al. Functional dysconnection of the inferior frontal gyrus in young people with bipolar disorder or at genetic high risk. Biol Psychiatry. 2017;81(8):718–27. https://doi.org/10.1016/j.biopsych.2016.08.018.
Lui S, Yao L, Xiao Y, Keedy SK, Reilly JL, Keefe RS, et al. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med. 2015;45(1):97–108. https://doi.org/10.1017/S003329171400110X.
Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71(10):881–9. https://doi.org/10.1016/j.biopsych.2012.01.025.
McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56(8):544–52. https://doi.org/10.1016/j.biopsych.2004.07.020.
van der Schot AC, Vonk R, Brans RG, van Haren NE, Koolschijn PC, Nuboer V, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry. 2009;66(2):142–51. https://doi.org/10.1001/archgenpsychiatry.2008.541.
de Zwarte S, Brouwer R, Ching C, van Erp T, Thompson P, Andreassen O, Turner J, van Haren N, ENIGMA Relatives Group. Biol Psychiatry. 2018; 83 (9): S220.
Reis DJ, Golanov EV. Autonomic and vasomotor regulation. Int Rev. Neurobiol. 1997;41:121–49.
Parsons LM, Denton D, Egan G, McKinley M, Shade R, Lancaster J, et al. Neuroimaging evidence implicating cerebellum in support of sensory/cognitive processes associated with thirst. Proc Natl Acad Sci U S A. 2000;97(5):2332–6. https://doi.org/10.1073/pnas.040555497.
Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev. Neurosci. 2002;3(8):655–66. https://doi.org/10.1038/nrn894.
Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. https://doi.org/10.1126/science.1093535.
Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129(Pt 2):290–2. https://doi.org/10.1093/brain/awh729.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121 (Pt 4:561–79.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. https://doi.org/10.1016/j.neuroimage.2008.08.039.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44. https://doi.org/10.1016/j.cortex.2009.11.008.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004 Summer;16(3):367–78. https://doi.org/10.1176/jnp.16.3.367.
Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev. Neurosci. 2006;7(7):511–22. https://doi.org/10.1038/nrn1953.
Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neuroscience. 2009;162(3):756–62. https://doi.org/10.1016/j.neuroscience.2009.01.064.
Maschke M, Schugens M, Kindsvater K, Drepper J, Kolb FP, Diener HC, et al. Fear conditioned changes of heart rate in patients with medial cerebellar lesions. J Neurol Neurosurg Psychiatry. 2002;72(1):116–8. https://doi.org/10.1136/jnnp.72.1.116.
Turner BM, Paradiso S, Marvel CL, Pierson R, Boles Ponto LL, Hichwa RD, et al. The cerebellum and emotional experience. Neuropsychologia. 2007;45(6):1331–41. https://doi.org/10.1016/j.neuropsychologia.2006.09.023.
Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;XVIII:643–62.
Owen AM, McMillan KM, Laird AR, Bullmore ET. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. https://doi.org/10.1002/hbm.20131.
Said CP, Haxby JV, Todorov A. Brain systems for assessing the affective value of faces. Philos Trans R Soc Lond B Biol Sci. 2011;366(1571):1660–70. https://doi.org/10.1098/rstb.2010.0351.
Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–94. https://doi.org/10.1016/j.neuropsychologia.2006.06.003.
Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–68. https://doi.org/10.3758/s13415-011-0083-5.
Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–40. https://doi.org/10.1016/j.neuroimage.2005.01.054.
Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7(1):1–17. https://doi.org/10.3758/CABN.7.1.1.
Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):917–32. https://doi.org/10.1098/rstb.2007.2097.
Pochon JB, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. J Neurosci. 2008;28(13):3468–73. https://doi.org/10.1523/JNEUROSCI.4195-07.2008.
Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–33. https://doi.org/10.1016/S1364-6613(00)01482-0.
Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3(1):80–4. https://doi.org/10.1038/71152.
Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2000;51(1):59–67. https://doi.org/10.1016/S0006-3223(01)01330-0.
Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12(2):169–77. https://doi.org/10.1016/S0959-4388(02)00301-X.
Dima D, Stephan KE, Roiser JP, Friston KJ, Frangou S. Effective connectivity during processing of facial affect: evidence for multiple parallel pathways. J Neurosci. 2011;31(40):14378–14,385. https://doi.org/10.1523/JNEUROSCI.2400-11.2011.
LeDoux JE. The emotional brain: the mysterious underpinnings of emotional life. New York: Touchstone; 1998.
Rolls ET. The brain and emotion. Oxford: Oxford UP; 1999.
Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(9):242–9. https://doi.org/10.1016/j.tics.2005.03.010.
Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16(6):723–7. https://doi.org/10.1016/j.conb.2006.07.004.
Friston KJ, Harrison L, Penny W. Dynamic causal modeling. Neuroimage. 2003;19:1273–302. https://doi.org/10.1016/S1053-8119(03)00202-7.
Fornito A, Zalesky A, Bullmore ET. Network scaling effects in graph analytic studies of human resting-state FMRI data. Front Syst Neurosci. 2010;4:22. https://doi.org/10.3389/fnsys.2010.00022.
Reynaud E, Lesourd M, Navarro J, Osiurak F. On the neurocognitive origins of human tool use: a critical review of neuroimaging data. Neurosci Biobehav Rev. 2016;64:421–37. https://doi.org/10.1016/j.neubiorev.2016.03.009.
Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, et al. The role of the dorsal stream for gesture production. Neuroimage. 2006;29(2):417–28. https://doi.org/10.1016/j.neuroimage.2005.07.026.
Igelström KM, Graziano MSA. The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia. 2017;105:70–83. https://doi.org/10.1016/j.neuropsychologia.2017.01.001.
Cona G, Semenza C. Supplementary motor area as key structure for domain-general sequence processing: a unified account. Neurosci Biobehav Rev. 2017;72:28–42. https://doi.org/10.1016/j.neubiorev.2016.10.033.
Hertrich I, Dietrich S, Ackermann H. The role of the supplementary motor area for speech and language processing. Neurosci Biobehav Rev. 2016;68:602–10. https://doi.org/10.1016/j.neubiorev.2016.06.030.
Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC, et al. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci U S A. 2015;3;112(44):13,681–6. https://doi.org/10.1073/pnas.1502829112.
Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–28. https://doi.org/10.1016/S0006-3223(03)00171-9.
Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105–16. https://doi.org/10.1038/sj.mp.4001585.
Fischer AS, Camacho MC, Ho TC, Whitfield-Gabrieli S, Gotlib IH. Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry. 2018;75(5):493–502. https://doi.org/10.1001/jamapsychiatry.2017.4516 This study provided evidence of enhanced connectivity being associated with resilience to major affective disorder using a longitudinal design.
Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–60. https://doi.org/10.1017/S1355617702813248.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sophia Frangou declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Precision Medicine in Psychiatry
Rights and permissions
About this article
Cite this article
Frangou, S. Neuroimaging Markers of Risk, Disease Expression, and Resilience to Bipolar Disorder. Curr Psychiatry Rep 21, 52 (2019). https://doi.org/10.1007/s11920-019-1039-7
Published:
DOI: https://doi.org/10.1007/s11920-019-1039-7