Abstract
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is caused by mutations in the NOTCH3 gene located on chromosome 19p13. CADASIL causes a clinical syndrome of migraines (frequently with aura), progressive strokes, and cognitive decline in adults leading to severe functional impairment by the seventh decade of life. Genetic testing is the gold standard for diagnosing this condition, but the syndrome can be suspected clinically based on history and a characteristic pattern of confluent subcortical white matter disease in the anterior temporal poles and external capsule. Additional abnormalities include cerebral microbleeds and large vessel stenosis, particularly in Asian populations. Familiarity with radiologic findings in CADASIL is essential to the correct diagnosis and subsequent management of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common monogenetic cause of adult onset progressive cerebrovascular disease. The underlying gene has been mapped to NOTCH3 which encodes for a transmembrane receptor found in systemic and intracranial arterial smooth muscle cells. More than 200 mutations in the NOTCH3 gene have been associated with CADASIL with the majority being missense mutations at cysteine residues leading to disruption of tertiary protein structure and function [1•].
Although CADASIL can present acutely with stroke, other features of this disease such as migraine (without and without aura), cognitive decline, mood changes, and epilepsy, along with characteristic imaging findings, typically precede clinical strokes by years to decades [2]. For neurologists, particularly headache specialists, recognition of CADASIL through early clinical features and imaging is key to appropriate diagnosis and counseling. In addition to diagnosis, early recognition of CADASIL carries essential treatment implications as triptans and ergot-based medications are not generally recommended for use due to the theoretical risk of vasoconstrictors contributing to an even higher risk of stoke [3, 4].
We review the characteristic imaging findings associated with CADASIL in the context of its various clinical and diagnostic role.
Radiologic and Clinical Correlation
Diagnosis of CADASIL requires radiologic-clinical correlation. Although the severity and timing of symptom onset varies in CADASIL, it is a highly penetrant disease with clinical and radiologic manifestations present in the vast majority of patients by late middle age [2].
Migraine with aura is an early prominent feature of CADASIL with a prevalence of 20–50 % and with average age of onset of approximately 30 years [2, 5••, 6]. Associated auras are typically visual or sensory although more drastic presentations with hemiplegia, encephalopathy, confusion, or decreased level of consciousness have been reported [2, 7–9]. In a cohort of patients in Europe, 54.5 % had migraines overall with 84 % of those reporting migraine with aura in contrast to an estimated prevalence of 30 % for aura among those with migraine in the general population [10]. With increasing age, there was a decrease in the frequency of migraine (both with and without aura) such that in those >60 years of age, one third had no migraines over a two year period and one quarter experienced migraine at most once every 3 months. The presence of migraine did not appear to be a risk factor for disease progression in this population [5••].
The prevalence of transient ischemic attack (TIA) and stroke is 60–85 % with onset in most patients by the fifth to sixth decade of life [2, 6, 11]. Stroke and TIA presentations are mainly lacunar syndromes, reflecting the predominance of subcortical ischemic disease in CADASIL [2].
Onset of radiologic manifestations closely mirrors the onset of migraine with mean age of imaging abnormalities starting at 30 years. By age 35, essentially all patients with CADASIL have abnormal MRI findings, which occasionally may occur in the absence of clear clinical correlates [9, 12]. Early in the disease course, a nonspecific pattern of subcortical white matter disease may be present with characteristic progression over the course of several years to confluent white matter disease that preferentially affects the anterior temporal poles and the external capsules [2, 13, 14]. Subcortical strokes are a defining feature though large vessel disease with intracranial stenosis may also be present, particularly in patients of East Asian descent [1•, 15–17].
Cognitive impairment is a common and relatively early presentation of CADASIL which tends to emerge with the onset of stroke/TIA [2, 11]. In later stages, typical manifestations include mood disturbance, apathy, and gait impairment. Epilepsy is also more common in those with CADASIL at 5–10 % [2, 6, 11].
Computed Tomography
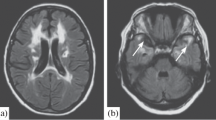
In early stages of CADASIL, nonspecific, periventricular, and other subcortical white matter hypodensities may be seen. The characteristic white matter disease affecting the anterior temporal poles would not be evident on computer tomography (CT) until more advanced stages of the disease. In the appropriate clinical context (eg, migraines, strokes, or TIA), these findings should prompt further imaging with MRI for clarification. Figure 1 demonstrates these findings on CT scan.
Magnetic Resonance Brain and Vascular Imaging
Brain magnetic resonance imaging (MRI) is the most clinically relevant imaging modality in CADASIL. In early stages, nonspecific periventricular and other subcortical hyperintensities seen on fluid attenuated inversion recovery (FLAIR) sequences may be indistinguishable from changes related to small vessel disease in association with uncontrolled hypertension and other risk factors. However, the typical course of CADASIL is marked by clinical deterioration and radiologic progression with confluent white matter FLAIR changes within several years, particularly in the anterior temporal poles and external capsules [2, 13]. Figure 2 shows characteristic findings of white matter changes in CADASIL.
Images a–e are MRI FLAIR sequences. There is abundant subcortical white matter and periventricular white matter disease (a, b) with notable involvement of the external capsule (c) and confluent anterior temporal pole white matter involvement (d, e). A subcortical microbleed is seen as a punctate, rounded hypointensity on fast field echo sequence (f)
Although patients with CADASIL may experience cortical strokes associated with the disease itself or other comorbid risk factors, the presence of multiple or predominantly cortical pattern of strokes should raise the clinician’s suspicion for alternative underlying diagnoses. Mitochondrial encephalomyopathy lactic acidosis (MELAS) is a maternally inherited disease with features that overlap with CADASIL, such as migraines, strokes, and encephalopathy. Distinguishing features for MELAS include earlier age of onset (childhood or potentially infancy) with additional features of myopathy, vision loss, hearing loss, and more prominent, early onset encephalopathy. On imaging, MELAS may be associated with subcortical white matter disease, but key differentiating features are large areas of cortical involvement with vasogenic edema and mass effect in acute and subacute phases [18].
Cerebral Bleeds in CADASIL
In case series, cerebral microbleeds (CMBs) were present in approximately one quarter to one half of symptomatic patients with CADASIL [17, 19, 20], most commonly affecting the thalami, basal ganglia, and brain stem. In a series of Asian patients with CADASIL, 55 % (11 of 20) had CMBs and 25 % (5 of 20) had larger intracerebral hemorrhages (ICH) [17]. The patients with ICH all had multiple CMBs, and the location of ICH was predominantly deep subcortical white matter, mirroring the locations of CMBs. In another series, the presence and number of cerebral microbleeds was comorbid with hypertension and diabetes and was associated with greater disability on modified Rankin scale [19]. Figure 2, panel F shows a subcortical microbleed on MRI on fast field echo sequence.
Intracranial Arterial Stenosis in CADASIL
CADASIL may be associated with the presence of intracranial arterial stenosis, particularly in Asian patients. In a series of patients in Korea with genetically confirmed CADASIL, 14 % (7/49) had territorial infarcts associated with large artery disease, and an additional 10 % (5/49) had evidence of large vessel stenosis without associated territorial strokes [16]. In this series, there were no significant differences in cerebral vascular disease risk factors such as hypertension or diabetes, suggesting that at least in Asian populations, large vessel disease may be associated with CADASIL. Another study of patients in mainland China also showed significant intracranial arterial disease in 26 % (5/19) of those with NOTCH3 mutations [21].
As almost all studies of large vessel intracranial arterial disease were described in Asian populations, generalizability is limited for other patients. Additionally, intracranial stenosis is more prevalent in Asian populations as a whole, and the findings in the CADASIL series may be a reflection of this separate, unrelated phenomenon.
Population Differences
Genetic sequencing shows significant variations in NOTCH3 mutations in different ethnic populations. While NOTCH3 mutations in exons 4 and 3 were the most common sites of mutation in Caucasian and mainland Chinese populations [1•], mutations in exon 11 were the most common in Korean and Taiwanese populations [1•, 15], possibly due to founder effects. Compared to CADASIL in Caucasian populations, East Asians had significantly less radiologic involvement of the anterior temporal lobe, more involvement of brain stem white matter, lower prevalence of migraine, a later age of clinical onset, and higher rate of ICH [1•, 22].
Additional Imaging Modalities
Other imaging modalities such as fludeoxyglucose positron emission tomography (FDG-PET) and diffusion tensor imaging (DTI) have been used to study cerebral functional changes associated with CADASIL.
Arterial spin labeling in one case report showed unilateral hyperperfusion to the occipital/temporal/parietal lobes during an attack of migrainous encephalopathy; the location of the affected lobe temporally and anatomically correlated with right-sided aura symptoms [23]. The finding of hyperperfusion associated with migraine is not specific to those with CADASIL as hyperperfusion has been demonstrated in those with hemiplegic migraine attacks as well as in otherwise healthy migraineurs during attacks [24, 25, 26••]. DTI in CADASIL may be abnormal due to subcortical white matter damage [27]. FDG-PET in CADASIL has shown subcortical and cortical hypometabolism, but the pattern was not specific for CADASIL; features were similar to those seen in other causes of vascular dementia [28].
The diagnostic value of the above techniques is questionable as data are limited to case reports, and findings may be nonspecific for CADASIL.
Discussion
CADASIL is an uncommon cause of two common problems: migraine and stroke. Recognition of CADASIL as an underlying diagnosis is essential to appropriate treatment and counseling. The diagnosis should be considered based on the clinical history and brain imaging, and confirmatory testing can be done via genetic testing for NOTCH3 mutations.
Migraine, particularly migraine with aura, is often the earliest manifestation of CADASIL symptoms and can begin as early as adolescence although on average it often manifests at the fourth decade of life. Visual aura is most common, but unusual aura with sensory/motor deficits and altered mental status is also associated. Following onset of migraine, by middle age, most patients with CADASIL have already begun to experience ischemic consequences (TIA/stroke). Brain MRI may be normal early in the disease course, but by the fifth decade, significant white matter changes are the rule rather than the exception.
Early MRI abnormalities in CADASIL include nonspecific periventricular subcortical white matter T2/FLAIR hyperintensities and possibly lacunar strokes. However, by the fifth decade, MRIs almost always show confluent white matter changes in the anterior temporal poles and external capsule, areas that tend to be less affected in other causes of small vessel ischemic disease (e.g., uncontrolled diabetes or hypertension). In the appropriate clinical context with symptoms suggestive of CADASIL, confluent anterior temporal pole white matter changes have sensitivity and specificity of 89 and 86 %, respectively, based on case series [14].
There are significant population differences in the clinical and radiologic manifestation of CADASIL, potentially due to differences in underlying genetic mutations. In particular, East Asian populations tend to have less migraine and anterior temporal pole MRI abnormalities but more brain stem white matter changes and intracerebral hemorrhage [1•, 22]. As such, the patient’s ethnic background may be important when considering a diagnosis of CADASIL.
Although many other imaging modalities such as FDG-PET, DTI, and arterial spin labeling have been shown to be abnormal in CADASIL, the findings appear to be nonspecific and have limited value in diagnosis.
Conclusion
A thorough understanding of the natural history and imaging findings of CADASIL is necessary for appropriate diagnosis and management. Migraine and early onset stroke/TIA are the most frequent early manifestations that bring patients to clinical attention and subsequent imaging. Subcortical ischemic changes occur earlier in CADASIL than in the general population, and by middle age, typically this progresses to involve the anterior temporal poles, helping differentiate it from other causes of small vessel ischemic disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Liao YC, Hsiao CS, Fuh JL. Characterization of CADASIL among the Han Chinese in Taiwai: distinct genotypic and phenotypic profiles. PLoS. 2015;10:e0136501. Reviews some distinctive genetic and clinical features of CADASIL in the Asian population.
Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol. 2009;8:643–53.
Vahedi K, Bousser MG. The patient with an unusual familial headache disorder. In: Rapoport AM, Sheftell FD, Purdy A, editors. Advanced therapy of headache. London: BC Decker; 1999. p. 293–301.
Roberts KA, O’Rourke K, Ross OA. Clinicogenetic and pathologic characteristics of CADASIL. In: Sharma P, Meschia JF, editors. Stroke genetics. London: Springer; 2012. p. 81–95.
Guey S, Mawet J, Herve D, et al. Prevalence and characteristics of migraine in CADASIL. Cephalagia. 2015;0:1–10. Traces the characteristics and natural history of migraine in CADASIL patients.
Dichgans M, Mayer M, Uttner I, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neuro. 1998;44:731–9.
Feuerhake F, Volk B, Ostertag CB, et al. Reversible coma with raised intracranial pressure: an unusual clinical manifestation of CADASIL. Acta Neuropathol. 2002;103:188–92.
Schon F, Martin RJ, Prevett M, et al. “CADASIL coma”: an underdiagnosed acute encephalopathy. J Neurol Neurosurg Psychiatry. 2003;74:249–52.
Golomb MR, Sokol DK, Walsh LE, Christensen CK, Garg BP. Recurrent hemiplegia, normal MRI, and NOTCH3 mutation in a 14-year-old: is this early CADASIL? Neurology. 2004;62:2331–2.
Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–99.
Peters N, Herzog J, Opherk C, Dichgans M. A two-year clinical follow-up study in 80 CADASIL patients: progression patterns and implications for clinical trials. Stroke. 2004;35:1603–8.
Samoes R, Alves JE, Taipa R, et al. CADASIL: MRI may be normal in the fourth decade of life—a case report. Cephalalgia. 2014;0:1–4.
Singhal S, Rich P, Markus HS. The spatrial distribution of MR imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features. Am J Neuroradiol. 2005;26:2481–7.
Markus HS, Martin RJ, Simpson MA, et al. Diagnostic strategies in CADASIL. Neurology. 2002;59:1134–8.
Kim YE, Yoon CW, Seo SW, et al. Spectrum of NOTCH3 mutations in Korean patients with clinically suspicious cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurob Aging. 2014;35:726. e1-e6.
Kang HG, Kim JS. Intracranial arterial disease in CADASIL patients. J Neurol Sci. 2015;395:347–50.
Choi EJ, CHoi CG, Kim JS. Large cerebral artery involvement in CADASIL. Neurology. 2005;65:1322–4.
Pauli W, Zarzycki A, Krzysztalowski A, Walecka A. CT and MRI imaging of the brain in MELAS syndrome. Pol J Radiol. 2013;78:61–5.
Viswanathan A, Guichard JP, Gschwendtner A, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006;129:2375–83.
Lesnik Oberstein SAJ, van den Boom R, van Buchem MA, et al. Cerebral microbleeds in CADASIL. Neurology. 2001;57:1066–70.
Yin X, Wu D, Wan J, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and eukoencephalopathy: phenotypic and mutational spectrum in patients from mainland China. Int J Neurosci. 2015;125:585–92.
Liu X, Zuo Y, Sun W, et al. The genetic spectrum and the evaluation of CADASIL screening scale in Chinese patients with NOTCH3 mutations. J Neurol Sci. 2015;354:63–9.
Moreton FC, Santosh C, McArthur K, Muir KW. Cerebral hyperperfusion on arterial spin labeling MRI during CADASIL migrainous encephalopathy. Neurology. 2015;85:2177–9.
Lindahl AJ, Allder S, Jefferson D. Prolonged hemiplegic migraine associated with unilateral hyperperfusion on perfusion weighted magnetic resonance imaging. J Neurol Neurosurg Psych. 2002;73:2002–209.
Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. PNAS. 2001;98:4678–92.
Ayata C. Spreading depression and neurovascular coupling. Stroke. 2013;44 suppl 1:S87–9. Provides a review of the neurovascular changes during the course of a migraine from aura to pain phases.
Jang SH, Seo YS. Injuries of neural tracts in a patient with CADASIL: a diffusion tensor imaging study. BMC Neuro. 2015;15:176.
Tasch K, Koch W, Linke R, et al. Cortical hypometabolism and crossed cerebellar diaschiss suggest subcortically induced disconnection in CADASIL: An 18F-FDG PET study. J Nuclear Med. 2003;44:862–9.
Acknowledgments
We would like to thank the Jefferson Radiology Teaching Files for access to the images provided in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shuhan Zhu declares no conflict of interest.
Stephanie J. Nahas declares consultant fees from Supernus Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Zhu, S., Nahas, S.J. CADASIL: Imaging Characteristics and Clinical Correlation. Curr Pain Headache Rep 20, 57 (2016). https://doi.org/10.1007/s11916-016-0584-6
Published:
DOI: https://doi.org/10.1007/s11916-016-0584-6