Abstract
Neuromodulation is based on the revolutionary concept that paresthesia-inducing electrical stimulation could be analgesic. Its historical basis emanates from Melzack and Wall’s gate control theory of pain proposed in 1965. Neuromodulation has given us ready access to the systems of pain modulation and helped mature the understanding of the pathophysiology of pain. Physiologic studies highlight the complex ascending influence of neurostimulation on sensory processing. However, the present understanding of pain is rudimentary and evidence that neuromodulation works is modest. This paper emphasizes the historical antecedents, present state, and emerging future of 3 commonly applied neuromodulatory techniques—spinal cord stimulation, peripheral nerve and field stimulation, and deep brain stimulation—for chronic pain. It is hoped this article will enhance the understanding of neuromodulation and its role in pain management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The modern history of neuromodulation spans over half a century, during which the transition from nascent offering to mature, evidence-based medical subspecialty was made. Neuromodulation continues to evolve, shaped by rapid innovation in science and technology. Its strengths are its reversibility, programmability, low risk, and specificity. Its benefits are evidenced by improved pain relief, functional status, health-related quality of life, and reduced demand for health-care resources [1, 2, 3••, 4–6].
Neuromodulation has demonstrated efficacy in the treatment of pain pathologies resistant to conventional medical management (CMM) or surgery; in doing so it has become the approach of choice for improving quality of life at minimal risk. The key to success with neuromodulation is careful patient selection and recognizing pathologies that yield a higher success rate. The challenge for neuromodulatory therapies is 2-fold: (1) justifying the value of technological innovation, and associated expenditures, in the form of robust randomized controlled trials (RCTs) and physiological models that adequately explain pain relief; and (2) generating awareness and uptake amongst patients, payers, and health-care professionals in a financial landscape characterized by cost-cutting.
Spinal Cord Stimulation (SCS)
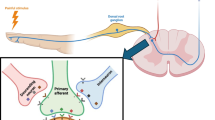
The earliest recorded use of neuromodulation for the treatment of pain dates back to 15 AD when Scribonius, after observing that gout pain had been relieved by the accidental contact with torpedo fish, recommended this treatment for pain in general [7]. The present conception of neuromodulation took shape in the 1965 with the publication of Melzack and Wall’s gate control theory of pain. It proposed the existence of a gate involved in pain perception that could be opened or closed depending on the differential activation of small and large neural fibers [8]. Two years later, Shealy and Mortimer designed an electrode and successfully implanted it in a patient to alleviate cancer-related pain [9]. These leads consisted of plate-style, platinum electrodes and were placed in the spinal subarachnoid space. These leads required an external power supply connected by needles passed through the skin. Implantation techniques were fraught with complications such as cerebrospinal fluid leak, arachnoiditis, and infection. The first SCS systems were modifications of pre-existing stimulators used for cardiovascular conditions. Medtronic Inc. (Minneapolis, MN) introduced the first commercially available SCS system in 1968, which used radiofrequency coupled with dorsal-column stimulators.
The architecture of the present-day SCS originated in the 1970s‒1980s with the creation of permanently implantable percutaneous leads for epidural placement [10, 11]. In 1981, Medtronic provided the first fully implantable SCS system and the first rechargeable implantable pulse generator (IPG) was released by Boston Scientific in 2004 [12]. The ability to capture dermatomal pain distributions and maximize paresthesia coverage has been further augmented by the development of multicontact leads and ability to implant multilead arrays. More recently, innovative lead delivery systems, such as the Epiducer (St. Jude Medical Inc, Plano, TX) [13] have enabled percutaneous placement of narrow paddle leads (S-Series paddle leads; St. Jude Medical Inc) [14].
Present State
SCS is the dominant segment of the neuromodulation sector. Its wide-spread acceptance is attributable to low morbidity and simplicity of implantation, burgeoning clinical indications for use, and robust evidence of efficacy [2, 4] and cost-effectiveness [3••]. By the end of this year, it is estimated that over 50,000 SCS units will be sold globally [12].
Initially conceived of as a therapy of last resort [15•, 16••], SCS has gradually acquired first-line status for the treatment of chronic neuropathic pain [17, 18]. SCS buttressed its legitimacy in the scientific and public arenas in the 2007, when Kumar and associates published results from the multicenter PROCESS RCT, providing Class I evidence of efficacy for the use of SCS in failed back surgery syndrome (FBSS) [1]. There is widespread agreement among experts that patients presenting with neuropathic pain who do not respond to CMM by 12 to 16 weeks should be offered a trial of SCS [17, 18].
SCS is Food and Drug Administration (FDA) approved for chronic pain of the trunk and limbs. Several RCTs support its use in chronic neuropathic pain pathologies such as FBSS and complex regional pain syndrome (CRPS), which constitute the overwhelming majority of implants. In the United States, it is also used off-label for refractory angina pectoris and peripheral vascular disease.
Current systems tend to employ multilead arrays—each lead may contain up to 20 contact points— connected to a rechargeable IPG. Multicolumn leads facilitate improved current steering and paresthesia coverage. The advent of multicontact leads (≥8 contacts) has lowered surgical revision rates from 22% to ≤5% [6, 16••]. The incidence of lead fracture has also declined from 6% [6] to <3% [2] with improved manufacturing methods.
Contact points per lead continue to increase in conjunction with price, in the notable absence of evidence of superiority. Commonly used cylindrical leads have 4, 8, or 16 contacts while paddle leads are available in configuration of 2, 3, and 5 columns with contacts ranging from 4, 8, 16–20 [16••].
Kumar et al previously demonstrated that efficacy of SCS treatment is time dependent with success rates exceeding 85% if implantation occurs within 2 years of symptom onset.
The long-term success rate of SCS is inversely proportional to the time delay between the onset of the chronic pain syndrome to SCS implantation. Present mean wait-times of 65.4 months roughly translate into a long-term success rate of 47%. This implies that the key to optimizing success is to employ strict patient selection criteria and to offer SCS earlier [6, 19••].
Emerging Stimulation Targets
Capitalizing on the recognition of dorsal root ganglion hyper-excitability in pain transduction and maintenance of chronic pain, Spinal Modulation (Menlo Park, CA) has released its Axium neurostimulator in Europe and Australia [20]. Advantages are durable pain, technical ease of lead placement, and ability to provide sub-dermatomal specificity while retaining multidermatome coverage.
Novel Modes of Stimulation
A departure from low-frequency stimulation parameters—a staple of SCS since its conception—is underway. High-frequency SCS (HFS) operates at 5–10 kHz. The notable advantage is absence of paresthesia, which at times can be annoying to some patients when using conventional SCS. In a recent study, HFS provided significant low back and leg pain relief to more than 70% of patients [21•]. A phase III pivotal trial of HFS vs conventional SCS recently completed enrollment, results are anticipated shortly (SENZA-RCT; Nevro Corp, Menlo Park CA) [16••].
De Ridder et al have demonstrated that burst stimulation (BrS) delivering square waves (40 Hz bursts with 5 spikes at 500 Hz per burst) can produce equivalent or better pain relief than conventional tonic stimulation. BrS appears to suppress pain without the induction of paresthesia, possibly because significantly more charge per second is delivered over tonic stimulation but at lower amplitudes, resulting in sub-threshold stimulation of Aβ fibers [22].
Technology: Perils and Promise
MRI Computability
MRI incompatibility is a significant concern for patients and clinicians [23]. Through its SureScan program, Medtronic Inc. has launched the first implantable SCS solution compatible with full-body 1.5 Tesla MRI. These leads incorporate a braided body that acts as radio-frequency shield and dissipation surface. Filtered feed-through capacitors built into the neurostimulator output channels shunt high frequency energy to the neurostimulator case, preventing damage to internal circuitry. The use of ferrous materials is also minimized. Other manufacturers are racing to produce MRI-compatible equipment [24, 25].
IPG Design
Just as dual-channel IPGs replaced single-channel stimulators, 4-, 8-, and 16-channel devices have followed. Boston Scientific (Valencia, CA) recently introduced 16-contact leads and is expected to soon release a 32-channel IPG in the United States. It is not yet known, however, if the increased number of lead contacts and IPG channels potentiate analgesic effect [16••].
Medtronic Inc. (Minneapolis MN) has recently integrated accelerometers (AdaptivStim; RestoreSensor) to allow the IPG to sense whether the patient is sitting or lying and to automatically adjust programs that have been pre-selected for each position or activity [16••]. Ethical considerations include security, privacy, and conditions of data release to third parties.
The hunt for alternative power sources continues; a more radical approach involves radio-frequency and microwave technology [24].
Programming
With increasing contact points per lead and utilization of multiple lead arrays, the number of possible configurations is nearly infinite, making manual combination determination time-consuming and tiring to both clinician and patient. Advanced automated programming algorithms have been developed with the goal of reducing programming effort (Precision Spectra Boston Scientific; Valencia, CA) [16••].
Axial Back Pain and Multicolumn Stimulation
To date, axial back pain has evaded the grasp of SCS. This reality may be altered by multicolumn stimulation, which effectively harnesses axial low back pain. The Specify 5-6-5 lead (Medtronic Inc., Minneapolis, MN) and 5-column Penta lead (St. Jude Medical Inc., Plano, TX) enable SCS programming in both medial/lateral and rostral/caudal orientations. The rationale for this approach is based on computer modeling by Holsheimer et al, which proposes that transverse tripolar stimulation provides superior current steering [26].
Unresolved Questions
While the technological SCS revolution continues unabated, fundamental questions of biophysical and clinical relevance have not been satisfactorily addressed. For example, the relationship between number of contact points and patient outcomes is unclear, as is the relevance of contact spacing and multiple independent current controls or the advantages of constant current vs constant voltage strategies. The ideal number or configuration of leads required for harnessing back pain with uni- or bilateral leg pain requires elaboration [27].
Peripheral Nerve Stimulation (PNS)
Despite considerable development, PNS continues to suffer from underutilization, limited commercial support, paucity of clinical and basic research, reimbursement challenges, and a lack of regulatory approval for existing devices [28••].
The genesis of the Electreat in the early 20th century, a consumer electrical device, represents the historical antecedent of peripheral electrical stimulation. This device subsequently morphed into the technology for transcutaneous electrical nerve stimulation. PNS originated in the 1960s, when Wall and Sweet inserted an electrode into their own infraorbital foramina and obtained analgesia during the period of electrical stimulation. The first PNS surgery was performed on a 26- year- old woman with CRPS on October 9, 1965. During this operation, Wall and Sweet implanted a pair of silastic split-ring platinum electrodes around the ulnar and another pair around the median nerve in the arm with externalization of the electrodes at the mid- forearm [29]. In 1967, the duo published the first article documenting the idea of PNS with implantable devices [30].
In 1976, Campbell et al demonstrated a tentative role of PNS in the treatment of chronic pain in a study of 23 patients. However, they documented that patients treated with a sciatic implant for low back pain had suboptimal outcomes [31]. Presently, for PNS the targets are greater occipital, trunk, and extremity peripheral nerves. Common cranial nerve targets are the trigeminal and vagus nerves [32].
PNS was initially achieved using cuff-type electrodes, which were later superseded by button-type (paddle) electrodes. The major limitations of these techniques included surgical nerve exposure, difficulty in achieving and maintaining adequate positioning of contacts for optimal paresthesia coverage, and declining pain relief over time. Multiple reports of nerve injury from electrode insertion and electrode-related fibrosis dampened the appeal of PNS [28••, 29].
Paddle electrodes are suitable for stimulation of large peripheral nerves, and occipital nerve stimulation (ONS) for relief occipital neuralgia (ON) or migraine. As is the case with SCS and other neuromodulating methods, the utility of cylindrical vs paddle leads for PNS is debated. The benefits of paddle design include the unidirectional stimulation, greater electrical efficiency, and lowered incidence of lead migration [28••].
In the 1980s, the advent of IPGs enabled neuromodulators to forgo radiofrequency-coupled devices, thus making long- term stimulation easier. To help maintain contact between the paddle electrode and peripheral nerve, a dedicated PNS paddle electrode with integrated-mesh was developed in the early 1990s (On-Point, Medtronic Inc., Minneapolis, MN). This lead has been approved by FDA for PNS. However, despite increased clinical use there was a lack of interest in obtaining FDA approval for IPGs [28••]. Eventually manufacturing of RF generators was discontinued and the whole field of PNS became off-labeled.
The use of PNS for craniofacial neuropathic pain was resurrected in 1999 by a report from Weiner and Reed that cited durable pain relief in 12 patients undergoing percutaneous electrode insertion in the vicinity of the occipital nerves for treatment of ON [33]. Subsequently, Slavin et al extended this technique for craniofacial pain in the trigeminal distribution by placing electrodes over the supra and infra-orbital nerves [34].
In contrast to other forms of neurostimulation, the appeal of PNS is a direct stimulation of peripheral nerves and inhibition of primary afferents, thus producing nociceptive blockade. The recent introduction of the peripheral nerve field stimulation (PNFS; also known as subcutaneous stimulation) represents an evolution of PNS. As the name implies, small nerve endings within the subcutaneous tissue are stimulated. An advantage of PNFS over SCS or PNS is its technical ease and ability to place the lead directly in the affected painful area [29, 35].
Indications
Headache and Occipital Neuralgia
The strongest interest in PNS centers on the treatment of intractable headache. Current efforts have developed along 2 streams: cephalic neuralgia and primary headache. Several case series have reported impressive success rate in the range of 70%–100% for ONS in occipital neuralgia and cervicogenic headaches. More recently, investigators have successfully applied supraorbital nerve stimulation for neuropathic pain and postherpetic neuralgia in the V1 distribution of the trigeminal nerve. Yakolev et al have extended its use for the atypical facial pain. In our experience, however, the atypical facial pain responds poorly to PNS [36•].
Ultimately, the 3 device manufacturers undertook RCTs to evaluate the effectiveness of ONS in chronic migraine. Both the Boston Scientific [37] and St. Jude [38••] trials found no evidence for a significant therapeutic effect. The ONSTIM trial (Medtronic Inc., Minneapolis, MN) [39•] achieved its primary endpoint. However, a 30% improvement in pain was used to define a responder, rather than the standard 50%.
Traumatic and Postsurgical Neuropathies
Percutaneously inserted PNS electrodes were used for control of inguinal pain after herniorrhaphy, genitofemoral neuralgic pain, thoracic postherpetic or post-thoracotomy pain, and CRPS II. In addition, PNFS has been applied in the treatment of abdominal wall, low back, and neck pain [28••].
Chronic Low Back Pain
In November of 2012, Medtronic Inc. launched that the SubQStim II study, the first RCT comparing the clinical effectiveness of PNFS and CMM vs CMM alone for the management of the back pain following FBSS [36•]. This trial is in its recruitment phase.
In many ways, PNS is complementary to SCS. In cases where dorsal-column SCS fails to adequately relieve back pain, PNFS is a useful adjunct. Mironer and colleagues demonstrated that combination SCS-PNFS provided wider coverage of axial pain with an overall success of 90% [40].
Deep Brain Stimulation (DBS)
Horsley and Clarke invented animal stereotaxic techniques in 1908 and concurrently introduced stereotactically localized stimulation of deep brain structures [41]. The first stereotactic procedure in humans was reported by Spiegel and Wycis [42], and has become a staple of neurosurgery ever since. In 1969 Reynolds [43] observed that stimulation of the periventricular area of rats produced profound analgesia such that they could undergo surgery with no apparent pain. Similar stimulation was provided to patients with chronic pain by Richardson and Akil in 1977 [44, 45]. The following year, the duo linked this area to endorphin release [46].The genesis of modern DBS occurred in 1973, the result of a report by Hosobuchi [47] describing successful use of chronic thalamic stimulation for the treatment of anesthesia dolorosa.
The momentum behind neuromodulation prompted the FDA to sponsor a symposium on safety and efficacy in 1977 [48], which culminated in the determination that further studies were needed to prove the efficacy of DBS. Two industry-sponsored open-label multicenter trials of 246 enrollees from 1989 to 1995, in which investigators implanted electrodes within the somesthetic system (ventral posterior medial or ventral posterior lateral thalamic nuclei or internal capsule) and/or the periaqueductal/periventricular gray (PAG/PVG), failed to achieve their primary endpoints of ≥50% pain relief in ≥50% of patients [49]. On the basis of these trials, the FDA did not approve DBS for pain [50]. Over time, there has been a decline in the number of published studies and patients treated with DBS for pain. This is attributable to its off-label status and development of less invasive alternatives [50, 51].
DBS for pain, being an off-label indication in the United States, is practiced in a handful of specialized neurosurgical centers. Its present clinical use is best accomplished in the academic setting, within the construct of ongoing research. Salvaging DBS for pain is linked to bench and bedside efforts establishing biological models of plausibility and demonstration of efficacy in well-designed RCTs [28••]. Presently, DBS is gaining traction in other areas, notably in the movement disorders, neuropsychiatry, and epilepsy domains [51]. A recrudescence of DBS for pain could materialize if its usefulness in headache disorders such as cluster headache, hemicrania continua is substantiated [51].
DBS remains the subject of investigational interest. The benefit varies depending upon length of follow-up, condition treated, definition of adequate pain relief, and the site of stimulation [52]. DBS can be effective when combined with rigorous patient selection. A meta-analysis of 6 studies revealed that the long-term pain alleviation rate was highest with DBS of the PAG/PVG (79%), or the PAG/PVG plus sensory thalamus/internal capsule (87%). Stimulation of the sensory thalamus alone was less effective (58%). DBS was more effective for nociceptive than deafferentation pain (63% vs 47% long-term success; P < 0.01) [52]. However, in the review of 141 patients, Levy et al documented initial pain relief in only 83 (59%). With the mean follow-up of 80 months, only 42 patients (31%) continued to report significant pain relief. Some pain states, particularly anesthesia dolorosa and the spinal cord injury pain, did not seem to respond to DBS [53]. Much of the recent research has originated from Tipu Aziz of Oxford. Their latest findings describe 85 patients who underwent DBS for neuropathic pain, of which 66% gained benefit (average follow-up 19.6 months). On long-term follow-up of 42 months, an improvement of 30% in pain and quality of life was observed in 15 patients [54•].
Stimulation Targets
It is thought that stimulation of the PAG/PVG is efficacious for nociceptive pain, whereas DBS of the sensory thalamus is more advantageous for deafferentation pain such as thalamic pain and phantom-limb syndrome [52, 53]. It is suggested that DBS of the ipsilateral ventroposterior hypothalamus decreases attack frequency in cluster headache [51].
Conclusions
Neuropathic pain is complex and common. It is frequently resistant to conventional medical therapies and surgical approaches. A paradigm shift is underway, as neuromodulatory techniques begin to acquire first-line treatment status. The outlook for neuromodulation is optimistic and the field maintains a strong development pipeline, with novel solutions and new indications constantly emerging. Focused healthcare allocation coupled with growing disease burden and advances in translational and clinical research will drive future adoption.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–88.
Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the PROCESS trial. Neurosurgery. 2008;63:762–70.
Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013; [In press]. A comprehensive long-term cost-effective analysis, highlighting the advantages of SCS for 4 neuropathic pain pathologies over CMM.
Kemler MA, de Vet HC, Barendse GA, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: 5-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292–8.
Kumar K, Toth C, Nath RK, et al. Epidural spinal cord stimulation for treatment of chronic pain—some predictors of success. A 15-year experience. Surg Neurol. 1998;50:110–20.
Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58:481–96.
Gildenberg P. History of electrical neuromodulation for chronic pain. Pain Med. 2006;7 Suppl 1:S7–13.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9.
Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489–91.
Zumpano BJ, Saunders RL. Percutaneous epidural dorsal column stimulation. Technical note. J Neurosurg. 1976;45:459–60.
North RB, Fischell TA, Long DM. Chronic stimulation via percutaneously inserted epidural electrodes. Neurosurgery. 1977;1:215–8.
North American Neuromodulation Society Newsletter [Internet]. Glenview: North American Neuromodulation Society; 2008 [cited 2013 Sept 15]. Available from: http://www.neuromodulation.org/uploads/files/newsletter/nansnews08_fall.pdf.
Epiducer Lead Introduction System [Internet]. Plano: St. Jude Medical; 2013 [cited 2013 Sept 15]. Available from: http://professional.sjm.com/products/neuro/scs/steerable-leads-introducers/epiducer-lead-introduction-system#overview.
S-Series Steerable Paddle Leads [Internet]. Plano: St. Jude Medical; 2013. Available at: http://professional-intl.sjm.com/products/neuro/scs/steerable-leads-introducers/s-series-paddle-leads . Accessed September 15, 2013.
Levy RM. The politics of neuromodulation in the USA: spinal cord stimulation and the Washington State Department of Labor and Industry. Neuromodulation. 2010;13:249–52. This editorial illustrates the disparity between state of evidence and state of access to SCS. It provides a detailed description of the politics that curtail patient access to this important therapy in worker’s compensation board cases.
Levy RM. Spinal Cord Stimulation in 2020. Neuromodulation. 2013;16:93–6. A poignant essay on upcoming developments in spinal cord stimulation with emphasis on recurrent challenges..
Stanton-Hicks M. Complex regional pain syndrome: manifestations and the role of neurostimulation in its management. J Pain Symptom Manage. 2006;31:20–4.
National Institute for Health and Clinical Excellence: Spinal Cord Stimulation for Chronic Pain of Neuropathic or Ischaemic Origin. Available at: http://www.nice.org.uk/nicemedia/pdf/ta159guidance.pdf. Accessed September 2013.
Kumar K, Rizvi S, Nguyen R, et al. Impact of wait-times on spinal cord stimulation therapy outcomes. Pain Pract. 2013; [In press]. This paper illustrates how the long-term efficacy of SCS is inversely proportional to implantation delay. The key to success is early SCS implantation and adherence to rigorous patient selection.
da Vincilaan L, Brounstein D. Axium TM. Promising new data demonstrate that a novel pain therapy system manages patients with chronic neuropathic pain [Internet]. Menlo Park: Spinal Modulation; 2013 [cited 2013 Sept 15]. Available at: http://www.spinalmodulation.com/download/520bcaab-c5f0-402d-8cab-3056c0a82716/AXIUM%20Press%20Release.pdf. Accessed September 15, 2013.
Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multi-center European clinical study. Neuromodulation. 2013;16:59–66. This prospective trial describes the clinical efficacy of HF-SCS and sets the stage for future developments in this area.
De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery. 2010;66:986–90.
Desai MJ. Disparities in magnetic resonance imaging (MRI) utilization in the United States (US) general population, spinal cord stimulator-indicated, and implanted patients. [Lecture] International Neuromodulation Society. June 13, 2013.
Shellock FG, Audet-Griffin AJ. Evaluation of magnetic resonance imaging issues for a wirelessly powered lead used for epidural, spinal cord stimulation. Neuromodulation. 2013; [In press].
SureScan System | Radiology | MRI SureScan Spinal Cord Stimulators [Internet]. Minneapolis: Medtronic Inc; 2013. Available at: http://professional.medtronic.com/mri/surescan-mri-radiologists/scs/surescan-system/index.htm#.UjVWYH9GZHs. Accessed September 15, 2013.
Holsheimer J, Nuttin B, King GW, et al. Clinical evaluation of paresthesias steering with a new system for spinal cord stimulation. Neurosurgery. 1998;42:541–9.
Kumar K, Rizvi S. Spinal cord stimulation and other neuromodulation: in chronic pain. In: Toth C, Moulin D, editors. Neuropathic Pain: Causes, Management and Understanding. Cambridge: Cambridge University Press; 2013.
Slavin KV. History of peripheral nerve stimulation. Prog Neurol Surg. 2011;24:1–15. A historical account of the development of PNS.
White JC, Sweet WH. Pain and the Neurosurgeon: A Forty-Year Experience. Springfield: Thomas; 1969.
Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108–9.
Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976;45:692–9.
Stanton-Hicks M, Panourias IG, Sakasb D, Slavinc KV. The future of peripheral nerve stimulation. Prog Neurol Surg. 2011;24:210–7.
Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217–21.
Slavin KV, Colpan ME, Munawar N, Wess C, Nersesyan H. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurg Focus. 2006;21:E5.
Eldabe S, Kern M, Peul W, Green C, Winterfeldt K, Taylor RS. Assessing the effectiveness and cost effectiveness of subcutaneous nerve stimulation in patients with predominant back pain due to failed back surgery syndrome (SubQStim study): study protocol for a multicenter randomized controlled trial. Trials. 2013;14:189.
Reed KL. Peripheral neuromodulation and headaches: history, clinical approach, and considerations on underlying mechanisms. Curr Pain Headache Rep. 2013;14:305. This paper details the ongoing interest for PNS as treatment for intractable headache disorders.
Lipton RB, Goadsby PJ, Cady RK, et al. PRISM study: occipital nerve stimulation for treatment-refractory migraine. Cephalalgia. 2009;29:30.
Silberstein SD, Dodick D, Saper J, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, prospective, multicenter double-blinded, controlled study. Cephalalgia. 2012;32:1165–79. A rigorously performed RCT. While the primary endpoint was not achieved, most patients who received ONS reported ≥50 % improvement in pain or HA days..
Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31:271–85. The only positive RCT on ONS for chronic migraine, citing a 40 % response rate with ONS.
Mironer YE, Hutcheson JK, Satterthwaite JR, LaTourette PC. Prospective, 2-part study of the interaction between spinal cord stimulation and peripheral nerve field stimulation in patients with low back pain: development of a new spinal-peripheral neurostimulation method. Neuromodulation. 2011;14:151–4.
Horsley V, Clarke R. The structure and functions of the cerebellum examined by a new method. Brain. 1908;31:45–124.
Spiegel EA, Wycis HT, Marks M, Lee AS. Stereotactic apparatus for operations on the human brain. Science. 1947;106:349–50.
Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–5.
Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47:178–83.
Richardson DE, Akil H. Long term results of periventricular gray self-stimulation. Neurosurgery. 1977;1:199–202.
Akil H, Richardson DE, Hughes J, Barchas JD. Enkephalin-like material elevated in ventricular cerebrospinal fluid of pain patients after analgetic focal stimulation. Science. 1978;201:463–5.
Hosobuchi Y, Adams JE, Rutkins B. Chronic thalamic stimulation for the control of facial anesthesia dolorosas. Arch Neurol. 1973;29:158–61.
Gildenberg PL. Symposium on the safety and clinical efficacy of implanted neuroaugmentive devices. Appl Neurophysiol. 1977;40:69–240.
Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001;2:183–92.
Schwalb JM, Hamani C. The history and future of deep brain stimulation. Neurotherapeutics. 2008;5:3–13.
Lyons MK. Deep brain stimulation: current and future clinical applications. Mayo Clin Proc. 2011;86:662–72.
Bittar RG, Kar-Purkayastha I, Owen SL, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515–9.
Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long-term follow-up and review of the literature. Neurosurgery. 1987;21:885–93.
Boccard SG, Pereira EA, Moir L, Aziz TZ, Green AL. Long-term outcomes of deep brain stimulation for neuropathic pain. Neurosurgery. 2013;72:221–31. The most recent case-series on the long-term outcomes of 85 patients who received DBS for treatment of neuropathic pain.
Compliance with Ethics Guidelines
Conflict of Interest
Kirshna Kumar is a consultant for Medtronic Inc. and Boston Scientific and has received research grants from Medtronic in the past. Syed Rizvi declares that he has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuromodulation
Rights and permissions
About this article
Cite this article
Kumar, K., Rizvi, S. Historical and Present State of Neuromodulation in Chronic Pain. Curr Pain Headache Rep 18, 387 (2014). https://doi.org/10.1007/s11916-013-0387-y
Published:
DOI: https://doi.org/10.1007/s11916-013-0387-y
Keywords
- Neuromodulation
- Spinal cord stimulation
- Peripheral nerve stimulation
- Peripheral field nerve stimulation
- Deep brain stimulation
- Historical overview
- Chronic pain
- Failed back surgery syndrome
- Complex regional pain syndrome
- Refractory angina pectoris
- Peripheral vascular disease
- Neuropathic pain
- Movement disorders
- Minimally invasive
- Effectiveness
- Quality of life
- Analgesia