Abstract
Quantitative sensory testing (QST) is a widely accepted tool to investigate somatosensory changes in pain patients. Many different protocols have been developed in clinical pain research within recent years. In this review, we provide an overview of QST and tested neuroanatomical pathways, including peripheral and central structures. Based on research studies using animal and human surrogate models of neuropathic pain, possible underlying mechanisms of chronic pain are discussed. Clinically, QST may be useful for 1) the identification of subgroups of patients with different underlying pain mechanisms; 2) prediction of therapeutic outcomes; and 3) quantification of therapeutic interventions in pain therapy. Combined with sensory mapping, QST may provide useful information on the site of neural damage and on mechanisms of positive and negative somatosensory abnormalities. The use of QST in individual patients for diagnostic purposes leading to individualized therapy is an interesting concept, but needs further validation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
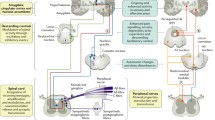
Assessing the sensory symptoms is an essential part in neurological examination. Sensory examination helps to determine the diagnosis and assess the function of the different submodalities of the somatosensory system (eg, mechanoreception, proprioception, thermoreception, nociception, and visceroception). These different submodalities are conveyed via several peripheral and central nervous system pathways (Fig. 1). In the periphery, large fibers mediate mechanoreception (Aβ) and proprioception (Aβ and Aα: Ia, Ib) and small fibers mediate thermoreception, nociception, and visceroception (all Aδ and C). For all qualities, the first-order neuron is in the dorsal root ganglion of the spinal cord (or in the trigeminal ganglion, the midbrain trigeminal nucleus, and the vagal ganglion). Pathways for the transmission of somatosensory qualities separate after entering the spinal cord dorsal horn. Axons of the postsynaptic second-order neuron (the projection neurons) for thermoreception, nociception, and visceroception cross the midline of the spinal cord at the same segment level and enter the ventrolateral spinothalamic tract (STT). Impulses from mechanoreceptors and proprioceptive neurons are conveyed to the brain via the dorsal column on the same side and cross to the contralateral side in the lower brainstem (cuneate and gracile nucleus [Fig. 1]). Axons of these neurons in medial lemniscus then project via the ventrobasal thalamus (third-order neuron) to the primary and secondary somatosensory cortex (SI/SII), to the posterior parietal cortex, to the posterior and mid-insula, and to the mid-cingulate cortex [1•, 2].
Schematic drawing of the somatosensory system, illustrating its two divisions. Somatosensory information is mediated by the spinothalamic tract (nociception, thermoreception, visceroreception) and the medial lemniscus (mechanoreception and proprioception). Visceroreception is also mediated by the vagus nerve (not shown). (Modified from Treede [77], with permission)
Due to the complex structure of the somatosensory system, a comprehensive investigation of both the area and the quality of somatosensory changes (Table 1) is necessary. Mapping of sensory signs (hypo- as well as hyperphenomena) combined with the appropriate determination of the neuroanatomical distribution provides important clues for the location of a lesion or dysfunction that may be responsible for the pain symptoms. It is necessary to examine at least one submodality processed by the medial lemniscus (touch, proprioception), and in addition, one submodality processed by the STT (temperature, pain).
According to the revised definition of neuropathic pain proposed by the Special Interest Group of Neuropathic Pain of the International Association for the Study of Pain (NeuPSIG), neuropathic pain is defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” [3••, 4•]. This implies an important role of sensory testing in classifying a pain syndrome as neuropathic. This is also reflected by the grading system for neuropathic pain that has additionally been developed. It is based on four criteria: pain distribution (criterion 1), the link between pain distribution and the patient’s history (criterion 2), confirmatory tests of neurologic status demonstrating positive or negative sensory signs confined to the innervation territory of the lesioned nervous structure (criterion 3), and further confirmatory diagnostic tests to identify the lesion or disease entity underlying the neuropathic pain (criterion 4). Criteria 1 and 2 must be met to initiate the working hypothesis of possible neuropathic pain. Either criterion 3 or 4 must be met in addition to reach the grade of probable neuropathic pain, while the grade of definite neuropathic pain is achieved only when both criteria 3 and 4 are satisfied. Regarding those criteria, a reasonable QST result is, together with electrophysiological test procedures and assessment of intraepidermal nerve fiber density (Table 1), considered a confirmatory diagnostic test to verify sensory signs in addition to sensory mapping [3••].
The clinical need for widespread use of sensory testing is increasing due to the high incidence of neuropathic pain and small-fiber neuropathies associated with diabetes, HIV, and chemotherapies. Thus, comprehensive but brief protocols for QST are needed not only in neurology, but also in endocrinology, oncology, and general practice. In this review, we report recent developments to optimize the clinical and experimental use of standardized sensory examination, collectively labelled as quantitative sensory testing (QST).
Procedures of Quantitative Sensory Testing and Its Importance for Diagnosis
The term quantitative sensory testing refers to diagnostic procedures in which perceived stimulus intensities are referenced to test stimuli applied with defined intensities [5•]. Hence, QST is a “semi-objective” method, and therefore, a high grade of standardization is needed. QST protocols deliver important information about the clinical features of patients with pain symptoms. However, it is difficult to compare the results assessed by different test protocols (eg, between different research groups). The need to set up specific age- and sex-controlled normative values for each test protocol and the variety of sensory tests have hampered the routine use of QST [6•, 7, 8]. The German Research Network on Neuropathic Pain (DFNS) has taken efforts to standardize test procedures to set up a protocol with a good comparability of data across different laboratories. This standardization includes the complete test procedure, from the written test instructions to the application of the individual stimuli, and the data analyses [9••]. Z-transformation of each single QST parameter allows reporting of complete QST profiles independent of age and sex, and of units of raw data (Fig. 2). Reproducibility and interobserver reliability in patients and healthy control patients for QST, including an intraoral QST test, were recently confirmed [10, 11•]. A certification procedure was developed to increase the comparability of QST data between centers [12].
QST profile of a single 46-year-old female patient with chronic regional pain syndrome of the left hand (black symbols; contralateral site: white symbols), using a multimodal QST test protocol according to the German Research Network on Neuropathic Pain (DFNS) [12]. Data are presented as z-scores, using the following expression: \( {\text{Z}} - {\text{value}} = \left( {{\text{Valu}}{{{\text{e}}}_{{{\text{single}}\,{\text{patient}}}}} - {\text{Mea}}{{{\text{n}}}_{{{\text{controls}}}}}} \right)/{\text{S}}{{{\text{D}}}_{{{\text{controls}}}}} \). Data of healthy control patients are represented by a z-score of ”0“; patients’ data are presented as positive or negative z-values. The grey area represents the confidence interval of healthy control patients. Values are defined as pathological when deviating more than two SD from the respective control sample (age- and sex-matched). This profile presents signs of sensory loss by increased thermal detection thresholds (CDT, WDT) and increased mechanical and vibration detection thresholds (MDT, VDT). Signs of sensory gain are presented by pinprick hyperalgesia (MPT, MPS). QST quantitative sensory testing; SD standard deviation; CDT cold detection threshold; WDT warm detection threshold; TSL thermal sensory limen; CPT cold pain threshold; HPT heat pain threshold; PPT pressure pain threshold; MPT mechanical pain threshold; MPS mechanical pain sensitivity; WUR wind-up ratio; MDT mechanical detection threshold; VDT vibration detection threshold; NRS numerical rating scale; DMA dynamic mechanical allodynia; PHS paradoxical heat sensation. (With kind permission from Springer Science+Business Media: Der Schmerz, Zertifizierungsrichtlinien für QST-Labore, 23, 2009, 65–69, C. Geber, A. Scherens, D. Pfau, et al., Figure 2. [12])
There is no simple correlation between threshold testing of the different pain modalities [13•]. This indicates that a unique “pain phenotype” does not exist and underpins the importance of multimodal pain assessment by QST. Different QST protocols most often comprise thermal or mechanical detection, but less frequently a combination of both [3••]. Clinically, QST might serve as a useful diagnostic tool to confirm neuropathy as a prerequisite for neuropathic pain [4•, 14–16]. Examination of sensitivity to thermal stimuli is highly recommended for the assessment of small-fiber neuropathies [17, 18], but touch and vibration also should be tested [19•]. A recent study has shown that thermal detection thresholds have a high positive predictive value (93%) for decreased intraepidermal nerve fiber density, indicating that QST can noninvasively detect small-fiber neuropathies [20]. In addition, small-fiber assessment is considered to be useful in the diagnosis of carpal tunnel syndrome, especially in the early stages when patients report pain, but electrophysiologic studies fail to detect significant abnormalities [21]. Nociceptive function is assessed by determination of pain thresholds and the responses to suprathreshold stimuli [3••, 22•] and should be analyzed not only for an increased sensitivity to painful stimuli (gain of function for pain), but also for a decreased sensitivity (loss of function for pain), and thus, provides a complete somatosensory phenotype of tested patients. In addition to physiological stimuli, the sensitivity to electrical stimuli is used in some protocols [23••]. Thermal sensory testing has been shown to be a sensitive tool in combination with blink reflexes in the diagnosis of trigeminal neuropathy and neuropathic orofacial pain [24]. QST also may complete findings by laser-evoked potentials [25].
To assess all somatosensory modalities, the comprehensive protocol implemented by the DFNS includes 11 tests for 13 test parameters for both detection and pain thresholds, and also tests for suprathreshold painful stimuli. Aβ-fiber–related sensitivity is characterized by the assessment of the mechanical detection threshold (von Frey filaments) and the vibration detection threshold (64-Hz tuning fork); small-fiber function (C-, Aδ) is assessed by thermal detection and pain thresholds. Only natural stimuli are used for this protocol so that it can even be used in children with an adaption of the numerical analog scale to a facial expression scale [26•]. A strong argument for the use of this protocol is the existence of age- and sex-matched normative data for the face, hand, and foot, ranging from 6 [26•] to 75 years [6•, 27].
Possible Pathophysiological Mechanisms
If used properly, QST is likely to play a more important role in future pain research [28]. It could bridge a gap between basic researches, experimental studies using models of neuropathic pain and pain patients, using the same test stimuli in all three contexts. This could facilitate learning about the underlying mechanisms in neuropathic pain syndromes [23••].
A comprehensive literature review that links abnormal somatosensory findings to pathophysiological mechanisms is not yet available, but from the literature so far, the following implications are proposed.
Loss of Aβ-fiber–related sensitivity (eg, deafferentation) is reflected by increased vibration and mechanical detection thresholds. This was confirmed by nerve block studies [29, 30]. Sensitivity related to small-fiber function can be assessed by testing thermal detection thresholds. Warm detection threshold tests C-fiber function, while cold detection threshold predominantly tests Aδ-fiber function. These assumptions were confirmed by so-called A-fiber block studies [31].
Positive sensory signs, in contrast, point to sensitization in the peripheral or central parts of the somatosensory system. As a first approximation, increased sensitivity to heat stimuli hints to a peripheral disturbance of nociceptive processing, and increased sensitivity to mechanical stimuli hints to a disturbance in central parts of the nervous system [32]. In addition, multiple positive sensory signs can hint to a decreased function of descending pain control mechanisms.
Heat hyperalgesia within an area of injured skin (primary hyperalgesia) is regarded as a cardinal sign of peripheral sensitization [33, 34]. Pressure pain thresholds test the sensitivity of deeper tissues [35] and may either hint to peripheral [36•] or central sensitization [37•]. Disinhibition of the central processing of thermal stimuli is a further mechanism that may be uncovered by QST. Paradoxical heat sensations to cold stimuli point to such a central disintegration of input from heat-pinch-cold (HPC) neurons and cold neurons. This disintegration was shown by nerve block studies where cold stimuli turn into C-fiber-mediated heat sensations when Aδ cold fibers are blocked [31, 38, 39] and also in patients with multiple sclerosis [40].
Central sensitization may be represented by dynamical-mechanical allodynia (pain perception following non-noxious stimuli, such as stroking with a cotton wool tip) mediated by low-threshold Aβ fibers [41]. This is considered as a “shunt” of transmission to central pain-mediating neurons (eg, in the spinal cord) [42, 43]. It can be accompanied by pinprick hyperalgesia as a sign of increased central processing of high-threshold Aδ nociceptor–derived activity [30]. In human pain models, pinprick hyperalgesia is predominantly observed in an area surrounding the zone of a primary injury (secondary hyperalgesia) [44].
A generalized hypersensitivity to pain for different modalities may suggest a disturbance of descending pain control mechanisms as observed for example in fibromyalgia syndrome [45]. Quite the opposite, the co-occurrence of spontaneous pain and increased detection thresholds (deafferentation) for different qualities may be explained by ectopic activity in lesioned nerve fibers [46].
Differentiation of Subgroups of Patients Based on Underlying Pathological Mechanisms
Future directions might lead to the development of a classification of neuropathic pain on the basis of symptoms and signs irrespective of the underlying disease (eg, diabetic polyneuropathy) [9••, 47•, 48•, 49]. Recently, a first step toward a mechanism-based approach was taken by Maier et al. [47•]. In this study, 1236 patients with neuropathic pain of different origin were investigated and the somatosensory profiles of all patients were analyzed according to the etiology. It could be shown that every single sensory abnormality occurred in each neuropathic pain syndrome, but the frequencies differed significantly. For example, patients with peripheral polyneuropathy present most frequently as positive for paradoxical heat sensations (37%), and negative for pallhypoesthesia (46%), while patients with postherpetic neuralgia present most frequently as positive for dynamic mechanical allodynia (49%) and negative for thermal hypoesthesias and a hypoesthesia to tactile stimuli (all 53%–63%; for detailed profiles see [47•]). This indicates the first assessment of a differentiated phenotype of neuropathic pain [50•]. Sensory heterogeneity (loss and gain of function) [47•] was found not only among different pain syndromes but also within a single disease entity [51]. For example, in patients with postoperative pain, this heterogeneity might be explained by the structural damage and subsequent sprouting of nerve fibers [52], or by a functional inhibition of non-noxious input as sensory loss. These phenotypes have been observed in human surrogate models of neuropathic pain without nerve lesion [53].
As additional examples, warm and cold phenotype complex regional pain syndrome patients differed not only in skin temperature but also in QST pattern [54], and patients with primary or secondary restless legs syndrome (RLS) with small-fiber neuropathy could be differentiated according to their thermal detection thresholds [55]. Subgroups also may be differentiated according to localized or generalized patterns of increased pain sensitivity. For example, subgroups of patients with temporomandibular disorders, originally suggested being a localized facial pain syndrome, present with similarly increased pain sensitivity over the back and the hand [56] pointing to a generalized rather than localized nociceptive changes. In subgroups of patients with peripheral arterial disease, different patterns of somatosensory changes indicate also central sensitization in addition to degeneration of peripheral nerve fiber types [57].
Prediction of Treatment Responders and Monitoring of Therapeutic Interventions
QST may be a valuable tool to predict the development of pain, such as postoperative pain [58], or to detect (subclinical) sensory deficits after surgery and their convalescence [58, 59]. Severe oxaliplatin-induced neuropathy [60] can be predicted by early (third treatment cycle) cold hyperalgesia. Correlations of different patterns of brain activity with different subtypes of allodynia (cold vs mechanically evoked) were found in patients with syringomyelia [61], and the extent of somatosensory deficits was correlated with the intensity of burning pain in these patients.
Single QST parameters have been found to be useful to differentiate responders from nonresponders in terms of a reduction of ongoing pain before pain therapy. This was shown in a study investigating patients with neuropathic pain in which the success of a spinal cord stimulator may be predicted by the vibration detection threshold and by the tolerance to electrical stimulation at different frequencies [62]. Duloxetine efficacy in painful diabetic neuropathy was predicted by conditioned pain modulation (modulation of contact heat pain by hot water immersion). Patients with less endogenous pain control benefitted more from this serotonin-noradrenaline reuptake inhibitor (SNRI), probably by enhancing endogenous pain control mechanisms [63].
QST parameters investigating hyperalgesia and allodynia also may be used as end points to confirm therapeutic benefits [64]. A significant reduction of evoked pinprick pain could be demonstrated in RLS patients after long-term treatment with levodopa [65]. A reduction of brush-evoked dysesthesia, but not of cold allodynia and increased pinprick sensitivity, was demonstrated in a placebo-controlled study investigating the effects of intravenous lidocaine application in patients with neuropathic pain following spinal cord injury [66]. As with lidocaine, an AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor antagonist (NS1209) was able to reduce dynamic mechanical allodynia and sensitivity to cold sensation, but failed to change sensitivity to pinprick and heat [67]. A subclinical positive treatment effect of the antidepressant venlafaxine in patients with neuropathic pain has been reported [68]. Treatment over 2 weeks with venlafaxine in different doses (75 mg/150 mg per day) significantly increased electrical and heat pain thresholds and decreased areas of pinprick hyperalgesia and dynamic mechanical allodynia without exceeding placebo effects on ongoing pain [68]. In addition, QST may be useful to follow sensory changes of individual patients with secondary neuropathies induced by alcohol abuse, renal failure, HIV infection, immune-mediated disorders, hereditary neuropathy, vitamin B12 deficiency, toxin exposure, leprosy, and connective tissue disease to detect any worsening or response to therapy [69].
Limitations of Quantitative Sensory Testing
QST should not be considered as a stand-alone procedure providing all information needed for a complete characterization of sensory changes (and neuropathic pain). It is recommended as a supplement to mapping of the area of interest in terms of a standard bedside sensory testing [22•]. The mapping should be performed for the same modalities as in the complete QST protocol as may be the sensitivity for cool, warm, pricking sensations and touch (Table 1). Distributions of sensory changes should be documented using a pain mannequin.
Conceptual Factors
A coexistence of pain and sensory signs found by QST does not necessarily confirm the clinical diagnosis of neuropathic pain. Findings by QST can hint to a neuropathy, but not to neuropathic pain. An additional causality is needed for this diagnosis [70]. Because psychophysical test procedures may be influenced by malingering or other nonorganic factors, it is not appropriate as a stand-alone procedure for pain assessment for medicolegal purposes [5•]. The results of QST should be interpreted along with the patient’s clinical presentation [71]. However, accurate sensory testing is time consuming, especially when performed within a standardized and reliable protocol such as QST. The sensory findings depend on both the skills of the examiner and how sensory disturbances are communicated by the patient. Furthermore, in contrast to electrophysiology [1•], for example, sensory examination is not an objective test. However, these limitations are common to many clinical examinations such as muscle strength, auditory tests, visual acuity, and even some seemingly “objective” clinical signs like tendon reflexes. All vary with the clinical skills of the examiner.
Patient Factors
Because QST needs cooperative patients who are able to react and make appropriate decisions, it is of limited value in the assessment of patients with hearing impairments, excessive sedation from medications, or language deficits (eg, aphasia). The DFNS has made efforts to provide validated translations of the standardized test instructions into multiple languages to avoid bias. It is not useful to assess very young children (< 6 years), severely mentally disabled patients, or acutely psychotic patients [72•]. To minimize distraction, the investigation should be performed in a quiet room with the investigator and the patient as the only attendees. Additional factors that may influence somatosensory function like shaving of the skin must be considered and avoided.
Investigator Factors
A highly standardized test procedure is recommended to maintain reproducible results and to ensure the comparability of data between different laboratories and normative data. Similar problems are encountered in other tests, such as clinical neurophysiology and skin biopsies.
QST cannot be seen as replacement for electrophysiological methods investigating somatosensory function, but it can complement them because it assesses different parameters. QST also does not replace neurological examination because it is not possible to localize the distribution of a lesion by QST because it is performed only at selected skin sites in the painful area. In addition, it has been shown that not all clinical findings of bedside examination could be confirmed by QST [73]. However, standardized QST protocols performed by trained examiners will ensure a high interobserver reliability and a comparability of results across laboratories [6•, 10].
In summary, information delivered by QST must be interpreted in the appropriate clinical context. Pain involves three different levels of classification: pain symptoms (as assessed by QST), pain mechanisms (as assumed in a patient), and pain syndromes (the diagnosis), the latter including knowledge about etiology, genetics, history, and previous treatment responses [74, 75].
A promising approach to overcome these limitations is to combine and correlate QST findings with further functional (eg, electrophysiological, functional imaging), structural (ie, nerve/skin biopsies, imaging studies), and therapeutic data through multiple assessments. [76].
Conclusions
QST is a scientifically accepted tool to investigate somatosensory changes in pain patients in addition to a clinical neurological examination. It consists of a comprehensive formalized examination that provides important information of the somatosensory system, but should not be used as a single test to confirm neuropathic pain [4•]. The goal to use QST as a diagnostic method to predict response to a pharmacological therapy needs further investigation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Cruccu G, Aminoff MJ, Curio G, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119:1705–19. This guideline by the International Federation of Clinical Neurophysiology (IFCN) provides standards for objective tests for the assessment of the somatosensory system.
Treede RD: Das somatosensorische System, in Physiologie des Menschen, Schmidt RF and F. L, Editors. 2007, Springer: Heidelberg. 297–323.
•• Haanpaa M, Attal N, Backonja M, et al.: NeuPSIG guidelines on neuropathic pain assessment. Pain 2011:152:14–27. This review includes recent recommendations and guidelines for the assessment of neuropathic pain, comparing QST with clinical examination, questionnaires, electrophysiology, and skin biopsy.
• Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–5. A new definition and a grading system of Neuropathic Pain are presented in this paper, emphasising the relevance of sensory signs in diagnosis.
• Shy ME, Frohman EM, So YT, et al. Quantitative sensory testing: report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 2003;60:898–904. This paper presents the use of QST in diagnosis of peripheral neuropathies, especially in small fiber neuropathies.
• Magerl W, Krumova EK, Baron R, et al.: Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain 2010:151:598–605. This paper presents reference data and statistical testing methods for the QST protocol developed by the DFNS.
Gruener G, Dyck PJ. Quantitative sensory testing: methodology, applications, and future directions. J Clin Neurophysiol. 1994;11:568–83.
O'Brien PC, Dyck PJ. Procedures for setting normal values. Neurology. 1995;45:17–23.
•• Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. This paper reports on the rationale and the methods of a widespread QST protocol developed by the German Research Network on Neuropathic Pain (DFNS).
Geber C, Klein T, Azad S, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain. 2011;152:548–56.
• Pigg M, Baad-Hansen L, Svensson P, et al.: Reliability of intraoral quantitative sensory testing (QST). Pain 2010:148:220–6. An intraoral version of the QST protocol according to the DFNS is presented in this paper.
Geber C, Scherens A, Pfau D, et al. Procedure for certification of QST laboratories. Schmerz. 2009;23:65–9.
• Neziri AY, Curatolo M, Nuesch E, et al.: Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain 2011:152:1146–55. This paper refers to the importance of multimodal testing as sensory testing assesses statistically independent the function of the nociceptive system.
Rader AJ. Surgical decompression in lower-extremity diabetic peripheral neuropathy. J Am Podiatr Med Assoc. 2005;95:446–50.
Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82:95–100.
Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–8.
Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20:198–204.
Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39:1071–5.
• Dyck PJ, O'Brien PC, Kosanke JL, et al. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43:1508–12. This paper compares speed and accuracy of several paradigms for threshold estimation and provides information on testing procedures and backgrounds of an earlier highly developed QST protocol (case IV system).
Scherens A, Maier C, Haussleiter IS, et al. Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain. 2009;13:711–8.
Tamburin S, Cacciatori C, Praitano ML, et al. Median nerve small- and large-fiber damage in carpal tunnel syndrome: a quantitative sensory testing study. J Pain. 2011;12:205–12.
• Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. 2007;129:256–9. This review provides an overview over QST procedures compared with bedside tests and focuses on clinical application possibilities.
•• Arendt-Nielsen L, Yarnitsky D: Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 2009:10:556–72. This review reports on QST methodology in different tissues, including skin, muscles, and viscera.
Jaaskelainen SK, Teerijoki-Oksa T, Forssell H. Neurophysiologic and quantitative sensory testing in the diagnosis of trigeminal neuropathy and neuropathic pain. Pain. 2005;117:349–57.
Geber C, Baumgartner U, Fechir M, et al. Comparison of LEP and QST and their contribution to standard sensory diagnostic assessment of spinal lesions: a pilot study. Neurol Sci. 2011;32:401–10.
• Blankenburg M, Boekens H, Hechler T, et al.: Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain 2010:149:76–88. This study demonstrates QST and presents normative values in children.
Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–43.
Backonja MM, Walk D, Edwards RR, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641–7.
Ziegler EA, Magerl W, Meyer RA, et al. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122(Pt 12):2245–57.
Magerl W, Fuchs PN, Meyer RA, et al. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–64.
Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20:355–61.
Treede RD, Meyer RA, Raja SN, et al. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421.
Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science. 1981;213:1527–9.
LaMotte RH, Thalhammer JG, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. J Neurophysiol. 1983;50:1–26.
Rolke R, Andrews Campbell K, Magerl W, et al. Deep pain thresholds in the distal limbs of healthy human subjects. Eur J Pain. 2005;9:39–48.
• Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. This review gives an overview over pathophysiological mechanisms of neuropathic pain and therapeutic targets and argues for mechanism-based rather than diagnosisbased classification of neuropathic pain.
• Woolf CJ: Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011:152:S2-15. This review reports on mechanisms of central sensitization and other mechanisms of persistent pain.
Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113(Pt 4):893–902.
Susser E, Sprecher E, Yarnitsky D. Paradoxical heat sensation in healthy subjects: peripherally conducted by A delta or C fibres? Brain. 1999;122(Pt 2):239–46.
Hansen C, Hopf HC, Treede RD. Paradoxical heat sensation in patients with multiple sclerosis. Evidence for a supraspinal integration of temperature sensation. Brain. 1996;119(Pt 5):1729–36.
Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–80.
Treede RD. Hyperalgesia and allodynia: taxonomy, assessment, and mechansims. In: Brune K, Handwerker HO, editors. Hyperalgesia: molecular mechanisms and clincal implications. Seattle: IASP; 2004.
Simone DA, Sorkin LS, Oh U, et al. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–46.
LaMotte RH, Shain CN, Simone DA, et al. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211.
Blumenstiel K, Gerhardt A, Rolke R, et al. Quantitative sensory testing profiles in chronic back pain are distinct from those in fibromyalgia. Clin J Pain. 2011;27:682–90.
Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992;48:261–8.
• Maier C, Baron R, Tolle TR, et al.: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010:150:439–50. QST profiles across different neuropathic pain diagnoses reveal a combination of signs of sensory gain and loss in most individuals.
• Scholz J, Mannion RJ, Hord DE, et al.: A novel tool for the assessment of pain: validation in low back pain. PLoS Med 2009:6:e1000047. This paper gives evidence of the interpretation of QST and other tests as predictive tool for low back pain.
Jensen TS, Baron R. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain. 2003;102:1–8.
• Serra J: Sensory profiles: the cliche and the challenge. Pain 2010:150:384–5. This commentary represents a critical statement on the future role of QST in clinical testing.
Aasvang EK, Brandsborg B, Jensen TS, et al. Heterogeneous sensory processing in persistent postherniotomy pain. Paint. 2010;150:237–42.
Jensen TS, Hansson PT. Chapter 34 Classification of neuropathic pain syndromes based on symptoms and signs. Handb Clin Neurol. 2006;81:517–26.
Geber C, Magerl W, Fondel R, et al. Numbness in clinical and experimental pain–a cross-sectional study exploring the mechanisms of reduced tactile function. Pain. 2008;139:73–81.
Eberle T, Doganci B, Kramer HH, et al. Warm and cold complex regional pain syndromes: differences beyond skin temperature? Neurology. 2009;72:505–12.
Bachmann CG, Rolke R, Scheidt U, et al. Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain. 2010;133:762–70.
Pfau DB, Rolke R, Nickel R, et al. Somatosensory profiles in subgroups of patients with myogenic temporomandibular disorders and Fibromyalgia Syndrome. Pain. 2009;147:72–83.
Lang PM, Schober GM, Rolke R, et al. Sensory neuropathy and signs of central sensitization in patients with peripheral arterial disease. Pain. 2006;124:190–200.
Aasvang EK, Gmaehle E, Hansen JB, et al. Predictive risk factors for persistent postherniotomy pain. Anesthesiology. 2010;112:957–69.
Said-Yekta S, Smeets R, Esteves-Oliveira M, et al.: Verification of Nerve Integrity After Surgical Intervention Using Quantitative Sensory Testing. J Oral Maxillofac Surg 2011.
Attal N, Bouhassira D, Gautron M, et al. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain. 2009;144:245–52.
Ducreux D, Attal N, Parker F, et al. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129:963–76.
Eisenberg E, Backonja MM, Fillingim RB, et al. Quantitative sensory testing for spinal cord stimulation in patients with chronic neuropathic pain. Pain Pract. 2006;6:161–5.
Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation (CPM) predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012. doi:10.1016/j.pain.2012.02.021.
Cruccu G, Anand P, Attal N, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–62.
Stiasny-Kolster K, Magerl W, Oertel WH, et al. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127:773–82.
Finnerup NB, Biering-Sorensen F, Johannesen IL, et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–30.
Gormsen L, Finnerup NB, Almqvist PM, et al. The efficacy of the AMPA receptor antagonist NS1209 and lidocaine in nerve injury pain: a randomized, double-blind, placebo-controlled, three-way crossover study. Anesth Analg. 2009;108:1311–9.
Yucel A, Ozyalcin S, Koknel Talu G, et al. The effect of venlafaxine on ongoing and experimentally induced pain in neuropathic pain patients: a double blind, placebo controlled study. Eur J Pain. 2005;9:407–16.
Walk D. Role of skin biopsy in the diagnosis of peripheral neuropathic pain. Curr Pain Headache Rep. 2009;13:191–6.
Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am. 2003;14:261–86.
Arezzo J, Bolton C, Boulton A, et al. Quantitative sensory testing: a consensus report from the Peripheral Neuropathy Association. Neurology. 1993;43:1050–2.
• Dyck PJ, Kennedy WR, Kesserwani H, et al. Limitations of quantitative sensory testing when patients are biased toward a bad outcome. Neurology. 1998;50:1213. This review reports on limitations of QST.
Leffler AS, Hansson P. Painful traumatic peripheral partial nerve injury-sensory dysfunction profiles comparing outcomes of bedside examination and quantitative sensory testing. Eur J Pain. 2008;12:397–402.
Woolf CJ, Bennett GJ, Doherty M, et al. Towards a mechanism-based classification of pain? Pain. 1998;77:227–9.
Geber C, Birklein F. Dissecting post-herniotomy pain–scratching the surface? Pain. 2010;150:215–6.
Treede RD: Funktionsprüfung der nozizeptiven Bahnen durch SEP nach schmerzhaften Laser-Hitzereizen, in Evozierte Potenziale, Hess CW, et al., Editors. 2005, Springer: Heidelberg.599-621.
Disclosures
The authors work in a quantitative sensory testing training center of the German Research Network on Neuropathic Pain, receiving payment for training sessions.
Dr. Doreen B. Pfau has received honoraria and travel expense compensation from Pfizer. C. Geber: none. Dr. Frank Birklein has served on the boards of Eli Lilly & Co., Pfizer, and Astellas Pharma; has received grants from Eli Lilly & Co.; has received honoraria from Eli Lilly & Co., Pfizer, Shire Pharmaceuticals, and Grünenthal GmbH; and has received travel expense compensation from Eli Lilly & Co. and Pfizer. Dr. Rolf-Detlef Treede has served as a consultant for Astellas Pharma, Boehringer Ingelheim, Galderma, Grünenthal GmbH, Eli Lilly & Co., Dr. Kade Merz, Mundipharma, Nycomed, and Pfizer; has been issued patents on behalf of Merz; and has received travel expense compensation from Boehringer Ingelheim, Grünenthal GmbH, Mundipharma, and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfau, D.B., Geber, C., Birklein, F. et al. Quantitative Sensory Testing of Neuropathic Pain Patients: Potential Mechanistic and Therapeutic Implications. Curr Pain Headache Rep 16, 199–206 (2012). https://doi.org/10.1007/s11916-012-0261-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-012-0261-3