Abstract
Osteoporosis is a degenerative bone disease commonly related to aging. With an increase in life expectancies worldwide, the prevalence of the disease is expected to rise. Current clinical therapeutic treatments are not able to offer long-term solutions to counter the bone mass loss and the increased risk of fractures, which are the primary characteristics of the disease. However, the combination of bioactive nanomaterials within a biomaterial scaffold shows promise for the development of a localized, long-term treatment for those affected by osteoporosis. This review summarizes the unique characteristics of engineered nanoparticles that render them applicable for bone regeneration and recaps the current body of knowledge on nanomaterials with potential for osteoporosis treatment and bone regeneration. Specifically, we highlight new developments that are shaping this emerging field and evaluate applications of recently developed nanomaterials for osteoporosis treatment. Finally, we will identify promising new research directions in nanotechnology for bone regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the world’s population is aging, the prevalence of osteoporosis is steadily growing [1–3]. Defined as a systemic skeletal disease characterized by low bone strength and an increased risk of fracture in everyday life [4], osteoporosis can significantly lower a patient’s quality of life. Healthy bone is composed of hard rigid tissue and is responsible for enabling many functions of the body. The outermost layer, cortical (or compact) bone, highly resists bending and torsion and takes the primary role of weight bearing in the body. The inner spongy section known as trabecular or cancellous bone is highly vascularized and contains bone marrow. In a healthy individual, osteoclasts break down bone while osteoblasts form it, which allows for the effective repair of the cracks and flaws resulting from normal activity. However, in an osteoporotic individual, osteoclast function overshadows that of the osteoblasts, and the trabecular region in particular loses mass. As a result of the reduced connection points, the bone becomes more brittle, which leads to an increased risk of fracture [5].
In addition to osteoblast and osteoclast activity, hormones are critical in modulating bone remodeling. Estrogen [6], parathyroid hormone [7], and testosterone [8] play important roles; estrogen is believed to be the most significantly involved hormone, as both osteoblasts and osteoclasts contain its receptors [9]. When the levels of these hormones are disturbed, particularly during menopause, bone resorption and formation rates change drastically and often lead to the onset of osteoporosis. Traditionally, this disruption in hormone levels was thought to contribute to the bone loss associated with osteoporosis [10]. Studies of hormone-based therapies demonstrate promising results; however, the increased risks associated with prolonged hormone administration limit the long-term treatment potential for these patients [4].
Alternatively, bisphosphonates, such as alendronate, inhibit osteoclast activity and thereby arrest bone degeneration [11–14]. In vitro studies using bisphosphonates to inhibit osteoclast activity yielded promising results, but the same results were not observed when translated to in vivo models [15•, 16••]. Bisphosphonates have also featured prominently in research focused on preventing the progression of osteoporosis. Unfortunately, since these treatments prevent bone breakdown, concerns relating to their long-term use [14, 17] and lack of bone reformation [3, 18] have limited their usefulness.
Another method explored in preclinical and early clinical trials to promote bone formation is the use of growth factors such as bone morphogenetic protein-2 (BMP-2) [19]. Although these growth factors promote bone formation, supraphysiological doses are required to achieve therapeutic efficiency [20, 21]. These high doses can lead to undesirable side effects such as uncontrolled bone formation, inflammation, and tumorigenesis [20, 22].
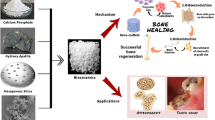
Given these complications, alternative approaches with biomaterials, specifically nanomaterials, have been investigated to combat osteoporosis and stimulate bone regeneration. Nanomaterials are defined as materials with one dimension between 1 and 100 nm. At the submicron range, properties of matter change drastically due to the quantum effects on the increased surface-to-volume ratio [23]. Modern medicine just recently began taking advantage of these unique properties in a variety of biomedical applications, from diagnostics to novel therapeutics and biopharmaceutics [24]. For example, nanoparticles have shown promise as carriers for efficient therapeutic delivery [25] and, due to their size, are more effective in targeted and intracellular delivery. The ability to shield drugs within nanoparticles increases the biostability and bioavailability of the drugs/therapeutic agents. Additionally, nanoparticles can be chemically modified to enhance therapeutic loading, or to simultaneously increase tissue specificity while decreasing dosage without sacrificing treatment efficacy [23, 26, 27].
For the purposes of bone regeneration in osteoporosis treatments, the incorporation of nanomaterials is ideal since bone itself is a nanocomposite [28, 29]. In addition to this dimensional similarity to osseous tissue, nanoparticles have an increased surface area and roughness, aiding in the adsorption and bioactivity of neighboring proteins and cells [30]. As a result, bioactive nanoparticles hold much potential in stimulating bone growth to offset the increased turnover rate found in osteoporosis. These nanomaterial properties can be used separately or in tandem to aid in the treatment of osteoporosis, especially in the realm of drug delivery and bone tissue regeneration. For example, recent studies have demonstrated the ability to adjust the nano-topography of existing bone implants to enhance bone growth at the interface of the device and the native bone [15•, 28]. Nanomaterials can also serve as an osteoconductive agent for implantable matrices to enhance osteolineage differentiation of stem cells [31••, 32•], or as a reinforcement to increase the mechanical properties of implantable scaffolds [24, 33, 34].
Recent Advances
Current treatment options for osteoporosis generally rely on the principle of halting osteoclast activity to preserve existing bone mass but rarely aim to encourage new bone growth. However, new bone formation and decreased bone resorption have been demonstrated by bioactive coatings for implants and through the use of mineral-based therapies. Bioactive coatings have been investigated for existing titanium or ceramic bone implants to encourage both cellular infiltration and new bone growth at the implant-bone interface [15•, 16••]. Mineral-based therapeutics using calcium-based materials such as hydroxyapatite, calcium phosphate, or bisphosphonates are often implanted within a scaffolding material to direct stem cell differentiation towards osteolineage cells [31••, 32•]. These minerals have demonstrated the ability to encourage mineralization and to direct the differentiation of stem cells encapsulated within the scaffold or of host stem cells recruited to the scaffold after implantation. The design requirements for a bone tissue engineering scaffold include biocompatibility, hydrophilicity, biodegradability, and tunability of the platform for the treatment chosen. Hydrophilic materials such as chitosan, alginate, pullulan, dextran, silica, poly(lactic-co-glycolic acid) (PLGA), and poly(ethylene glycol) (PEG) have demonstrated their ability to serve as a platform for the delivery of osteoporosis treatments and bone tissue regeneration [31••, 33, 35–37]. Nanoparticles can be incorporated into these methods, independently or in synergy (Fig. 1).
Nanomaterials for Therapeutic Delivery
Traditionally, osteoporosis was believed to be caused by the loss of estrogen commonly seen in aging men and women [10]. This theory led to the investigation of several hormone-based therapies for bone regeneration, and many research groups have developed scaffold-based therapeutics with hormone-based treatments that would encourage osteoblast differentiation and inhibit osteoclast activity [35, 38]. Combining the disciplines of materials science, nanotechnology, and biochemistry, nano-based therapeutics have been developed for the localized delivery of osteoporosis treatments.
In a recent study, Hu et al. demonstrated the efficacy of loading β-estradiol into mesoporous silica nanoparticles (MSNs) as a way to enhance the osteoconductivity of the nanomaterial in vitro when implanted on a titanium substrate [35]. Increased alkaline phosphatase activity, which is an indicator of osteolineage behavior, and mineralization were observed; however, no difference in the mRNA expressed by cells seeded onto the β-estradiol MSN-incorporated scaffold compared to the control titanium substrate was observed [35]. Recent work by Kang et al. demonstrated that the combination of an anti-osteoporosis androgen, 17β-amino-11α-hydroxyandrost-1,4-diene-3-one, RGD-tetrapeptide sequences, and a succinyl spacer in a nano-globe delivery structure increased the bone weight and bone mineral density in an in vivo mouse model without increasing the risk of thrombosis or endometrial hyperplasia [38]; however, the study was conducted over a 4-week period and no long-term impacts of the hormonal therapy were investigated.
In another study, Cao et al. recently performed an in vivo rabbit study using chitosan nanoparticles with BMP-2 for long-term release in a critical-sized defect [39•]. The growth factor and chitosan nanoparticles were able to promote angiogenesis and bone formation. However, because these scaffolds were porous, the mechanical properties were not ideal for treatment in weight-bearing bones [39•]. As an alternative, a stronger material could be combined with the nanocomposite to make this treatment viable.
Overall, using nanomaterials for the delivery of protein-based therapeutics has shown promise for the treatment of osteoporosis. However, the risk of thrombosis and certain cancers rises with increased dosage and prolonged use of hormone-based therapies, limiting their implementation as a long-term anti-osteoporosis treatment [38]. Recent findings have also revealed that the pathophysiology of osteoporosis is much more complex than simply a decrease in hormones with old age [9], and a treatment aimed at enhancing bone growth would be more applicable to patients affected by the disease.
Nanomaterials for Regulating Bone Remodeling
Encouraging new bone growth is a key focus in designing the next generation of osteoporosis therapies. A number of mineral-based nanomaterials are promising, such as calcium phosphate, hydroxyapatite, bisphosphonate, and silica. Calcium phosphate has been shown to enhance new bone formation via the promotion of activity of osteoblasts [31••, 32•], and bisphosphonates, such as alendronate, have been used experimentally and clinically to actively inhibit the activity of osteoclasts [15•, 16••]. By targeting osteoblasts and osteoclasts, osteoporosis treatments could potentially reverse the osteoclast-dominant activity seen in the disease to an osteoblast-dominant system.
In order to harness the osteoblast-promoting ability of calcium-based nanomaterials, Ignjatović et al. substituted cobalt for calcium ions in the crystal lattice of hydroxyapatite, transforming the calcium phosphate-based material from a diamagnetic to a paramagnetic material [40]. The effect of this substitution on new bone formation in an in vivo model revealed increased levels of osteogenesis and replacement of osteoporotic bone and confirmed accelerated bone regeneration as a result of the hydroxyapatite paramagnetic nanoparticles [40]. Another study by Tran et al. found that osteoblasts cultured with iron oxide (Fe3O4) nanoparticles coated with hydroxyapatite possessed both increased levels of mineralization and higher levels of osteoblast differentiation into new bone, while maintaining magnetic functionality for potential direction in vivo to osteoporotic bone [28].
With the intent of combining the synergistic effect of osteoblast-promoting calcium-based materials and osteoclast-inhibiting bisphosphonates into one therapeutic, Bosco et al. demonstrated that the co-administration of hydroxyapatite nanocrystals and the bisphosphonate drug, alendronate, is capable of reducing the activity of osteoclasts by inducing apoptosis of the osteoclasts in vitro when utilized as the coating on titanium bone implants [16••].
A study by Weitzmann et al. confirmed enhanced osteogenesis as a result of silica nanoparticles in an in vivo microenvironment [41••]. In vitro, these bioactive nanoparticles were able to simultaneously promote osteoblast activity and inhibit osteoclast activity. However, the results of the in vivo study were less indicative of inhibited osteoclast differentiation; the increased bone density as compared to the no treatment control is attributed to increased osteoblast activity [41••]. An in vitro anti-resorption study conducted by Kim et al. demonstrated a similar ability of siRNAs to incorporate into bioglass nanospheres and downregulate the genes known to be essential in osteoclast genesis and a marker enzyme expressed in osteoclast differentiation. However, no in vivo studies were performed to confirm the efficacy of inhibiting osteoclast function in osteoporotic bone as a means of promoting new bone growth [42•].
In a study conducted by Alghamdi et al., a coating for bone implants was designed utilizing both calcium phosphate and bisphosphonate to simultaneously promote new bone formation at the interface and decrease osteoclast activity [15•]. In vitro, new bone formation and integration at the interface of the coated implant increased; however, the group was unable to confirm the ability of bisphosphonate to actively inhibit osteoclast activity in the osteoporotic bone in vivo. The results showed no downregulation of osteoclast gene expression at the interface of bisphosphonate-coated implants compared to non-coated implants [15•].
These collective findings indicate that future research for the next generation of osteoporosis treatments should focus on bioactive molecules known to increase osteolineage differentiation of stem cells and on factors that encourage new bone formation in vivo to counter the bone loss seen in osteoporotic patients.
Nanoengineered Biomaterials for Accelerating Bone Regeneration
Bioactive factors known to promote osteoblast activity can be incorporated within an engineered scaffold and implanted in osteoporotic bone. The platform material or matrix in which the bioactive factors are implanted must be biocompatible, hydrophilic, biodegradable, and porous to encourage cellular infiltration and proliferation [31••]. Polymeric materials such as PLGA, PEG, chitosan, dextran, alginate, pullulan, and collagen have all been demonstrated as potential platform materials in bone tissue engineering [31••, 33, 35–37].
The most commonly used material for bone tissue engineering is chitosan, due to its similarity in structure to glycosaminoglycans (GAGs), a component found in extracellular matrix tissue. A study by Tripathi et al. incorporated chitosan with nano-hydroxyapatite (nHA) and a nano-copper-zinc (nCu-Zn) composite [37]. The zinc metal ion plays a role in mineralization and copper possesses antimicrobial ability. Theoretically, these metal ions combined with hydroxyapatite should increase osteo-differentiation and mineralization of stem cells and actively decrease the risk of a bacterial infection following implantation. However, no cell studies were performed, leaving these postulations as mere speculation [37]. Another in vitro study by Saravanan et al. incorporated keratin nanoparticles derived from chicken feathers into a chitosan scaffold [43]. Protein adsorption to the keratin-incorporated scaffold was increased; however, no studies other than a cytotoxicity assay were performed [43].
In another in vitro work, Sowjanya et al. combined chitosan with alginate into a platform material and then incorporated silica nanoparticles to increase the osteoconductivity of the scaffolding material [36]. Preliminary protein adsorption studies and a cytotoxicity assay were performed; while increased adsorption and no cytotoxicity were demonstrated, no further studies were conducted to examine the material’s ability to aid in bone regeneration in vivo [36].
Moving away from chitosan and choosing a scaffold design requirement to match the mechanical properties of native bone, Xu et al. reinforced polyetheretherketone (PEEK) with carbon fibers and incorporated nano-hydroxyapatite crystals. This reinforced material created a scaffold for directed stem cell differentiation and new bone formation [32•]. In vitro results showed increased osteolineage differentiation and mineralization of stem cells, and in vivo results displayed increased interaction with the carbon fiber-reinforced PEEK material compared to a titanium control [32•].
In another study, Fricain et al. developed a novel delivery system of nano-hydroxyapatite through the polysaccharide composite formed by pullulan and dextran [31••]. The extensive study included both in vitro and in vivo studies in large and small animal models. The results demonstrated the material’s ability to encourage cellular differentiation and proliferation in vitro and cellular infiltration and mineralization for bone healing in vivo [31••]. This ability to stimulate new bone formation by targeting the promotion of osteoblasts shows promise for the future of osteoporosis treatments. Although the use of nanomaterials in protein-based therapies and the incorporation of nanomaterials within engineered scaffolds shows promise in promoting bone formation, future methods are emerging that utilize novel 2D and bioactive nanomaterials.
Incorporation of bioactive nanomaterials such as synthetic silicates and nano-hydroxyapatite within PEG- or gelatin-based hydrogels has already been demonstrated [24, 33, 34, 44, 45•] and future work in bone tissue engineering will most likely continue to investigate the properties of these bioactive nanomaterials and their impact on stem cell differentiation and new bone formation.
Combinatorial Nanoengineered Approaches
The future of bone tissue engineering and osteoporosis treatments will likely build on the current technologies listed above, relying on a synergistic approach of combining elements into an intricately designed, implantable scaffold that promotes osteoblast differentiation, allows for cellular infiltration and adhesion, and provides robust mechanical stability to the defective or osteoporotic bone until new bone growth is able to replace the damaged tissue. These approaches utilize therapeutic delivery for accelerated bone regeneration along with bioactive nanomaterial delivery. For example, Liu et al. fabricated a scaffold of gelatin, nano-hydroxyapatite, and fibrin and then utilized this bioactive scaffold for the controlled release of BMP-2 for repairing segmental bone defects [46]. In this design, BMP-2 was loaded in fibrin glue and was incorporated into the gelatin-based nanocomposite scaffold. The proposed combinatorial approach allowed for the controlled delivery of BMP-2 as well as provided a 3D porous structure to support cellular activity [46]. In another study, El-Fiqi et al. designed a polycaprolactone-gelatin fiber matrix with mesoporous bioactive glass nanospheres (mBGn) for sequential drug delivery [47]. The drug intended for sustained release was carried in the mBGn while the drug intended for initial release was incorporated into the fiber matrix. Although the study showed promising results for sequential delivery, only model drugs were incorporated [47]. In a similar study, mBGn were used to carry fibroblast growth factor 18 (FGF18) which enhances osteogenic activity [48••]. These loaded nanospheres were then incorporated into an electrospun core of polyethylene oxide/polycaprolactone where an additional growth factor, fibroblast growth factor 2 (FGF2), was loaded. FGF2 was released quickly to allow for cell proliferation while the release of FGF18 was sustained to allow for osteogenic stimulation. This nanocomposite scaffold allowed for dual growth factor delivery as well as innate bioactivity with the use of the mBGn [48••]. By building on a specifically designed polymeric platform, osteoblast-specific binding sites can be incorporated, along with cell degradable linkages to allow the osteoblasts to proliferate within the scaffold and replace osteoporotic bone tissue.
Overall, different approaches currently used for osteoporosis include (a) nanomaterials for therapeutic delivery, (b) nanomaterials for regulating bone remodeling, (c) nanoengineered biomaterials for accelerated bone regeneration, and (d) combinatorial nanoengineered approaches. Table 1 summarizes each approach and highlights their advantages and disadvantages. For example, approaches incorporating therapeutic delivery of growth factors have aided in new bone formation at the defect site; however, in some cases these factors have led to uncontrolled or unwanted tissue formation in healthy tissue. The use of mineral-based nanomaterials for regulating bone remodeling in vitro has provided promising results especially with the incorporation of bioactive nanomaterials which can direct cells to an osteogenic lineage. Many of these strategies for regulating bone remodeling rely on inhibiting osteoclast activity though, and in vivo nanomaterials such as bisphononates have not inhibited this activity. Alternatively, some studies have focused on bone regeneration using nanoengineered biomaterials which have allowed for increased mechanical properties of the scaffold as well as increased osteoblast activity. Although promising, the cytotoxicity of some of the nanomaterials is unknown and further studies must be completed for these approaches to be applicable for osteoporosis treatment. Finally, combinatorial nanoengineered approaches have been explored and while these incorporate osteoinductive cues, cell binding sites, and enhance the mechanical properties, little research has been done to explore the efficacy of multi-component systems.
Future Directions
Recently, a new class of bioactive nanomaterials has emerged for bone regeneration and osteoporosis treatments. These osteoconductive materials include 2D nanostructures, which allow for simple incorporation into the matrix of interest, and in some cases, they possess completely different properties than the 3D arrangement of the same material [33]. Graphene, silicate nanoparticles (also known as layered clay), and layered double hydroxides (LDHs) are emerging as strong contenders for a bioactive nanomaterial approach to new bone formation for the next generation of bone tissue engineering and osteoporosis treatments [24, 33, 44].
Graphene’s electrically conductive properties have been documented extensively in industry and in myocardial and neural tissue engineering, but the carbon-based material has also demonstrated osteo-differentiation capability in stem cells [33, 49•]. In a study by Crowder et al., graphene was utilized to create 3D porous foams. The increased mechanical stiffness of the graphene-based matrix induced the differentiation of stem cells into osteoblasts in the absence of osteoinductive factors, suggesting that an osteolineage stem cell fate can be controlled by the mechanical stiffness of the environment in which the stem cells are implanted [49•].
Other inorganic nanomaterials, such as silica nanoparticles and nano-hydroxyapatite, have been shown to induce osteogenic differentiation of stem cells and promoted new bone formation. Nano-hydroxyapatite is well documented in its ability to stimulate osteolineage differentiation of stem cells and promote mineralization for new bone tissue formation [24, 44], and has been used to coat orthopedic implants to increase osseointegration and help lengthen the lifetime of these medical devices [15•, 16••]. Silicate nanoparticles, however, are emerging as a novel osteoconductive material. These 2D nanostructures are capable of being a versatile carrier for targeted drug delivery and have demonstrated the ability to induce osteo-differentiation of stem cells. The center of the particles is negatively charged, which forces positive charges to accumulate on the perimeter of the material. This surface charge distribution permits increased stability in an aqueous solution and allows for shear-thinning, thereby showing promise for implementation as an injectable or 3D-printed osteoconductive system [24, 33, 44]. The positively charged surface turns synthetic clays into a versatile carrier for drug loading and subsequent encapsulation into a matrix for localized and sustained therapeutic release [33].
Synthetic silicate nanoparticles also show promise for use in bone tissue regeneration because the degradation products—sodium, magnesium (Mg2+), orthosilicic acid (Si(OH)4), and lithium—are all biocompatible [44]. The Mg2+ produced during degradation of the silicates has been shown to promote cell adhesion to scaffolds, and Si(OH)4 encourages the synthesis of type I collagen. Although the silicate nanoparticles exhibited cytotoxicity in high doses in the studies performed, the concentrations needed to promote new bone formation without osteoinductive factors such as BMP-2 or dexamethasone did not reach these toxic levels, which further distinguished this novel approach in regulating stem cell fate in bone tissue regeneration [24]. In another study by Xavier et al., these silicate nanoparticles were incorporated within a gelatin-based scaffold [45•]. Not only did the addition of these particles provide an osteoinductive feature but also increased the mechanical properties of the scaffold due to their strong interactions with synthetic and natural polymers [50–52].
Other recently investigated 2D nanomaterials are layered double hydroxides (LDHs), composed of cationic metal atoms layered between hydroxide sheets. The incorporation of these nanosheets into matrices results in increased mechanical properties, and they show promise as use as a vessel for the delivery of both therapeutics and genetic material given their layered and ionic structure [33]. The use of Mg2+ as one of the cationic metals sandwiched between hydroxide layers has been investigated [53], but little research has been performed to demonstrate the material’s ability to potentially control stem cell fate through the release of ions such as Mg2+ or calcium upon dissolution, or through the synergistic effect of controlled ion or drug release and increased mechanical stiffness of the matrix. Given their potential as a blank slate for the incorporation and controlled release of ions and therapeutics, LDHs show promise for the development of new osteoporosis treatments in the future.
Conclusions
Ongoing nanomaterials research in bone tissue engineering shows promise for the next generation of potential osteoporosis therapies. Traditional therapies for the degenerative disease have centered on hormone-based therapies and inhibition of osteoclast activity. However, in recent years, increased risk of thrombosis and certain types of cancer as a result of prolonged hormone therapy have fueled the need to attack the bone loss seen in osteoporosis from a different angle [4]. Many therapies currently available on the market utilize the approach of inhibiting osteoclast activity, specifically through the use of bisphosphonates such as alendronate [15•, 16••]. However, recent research has revealed more promising methods in treating osteoporosis by utilizing a multi-component treatment approach instead of focusing solely on osteoclast inhibition [41••, 42•]. By taking advantage of recent advances in nanotechnology, a novel approach of utilizing nanomaterials for the promotion of new bone growth shows promise for the future of osteoporosis therapy [54]. Minerals such as hydroxyapatite have been shown to encourage osteolineage differentiation of stem cells and increase levels of mineralization when implanted within a scaffold [31••, 32•]. Bioactive coatings have been developed from calcium phosphate-based nanomaterials and combined with silica to encourage bone regeneration and halt the progress of osteoporosis. Implantation of nanomaterials known to encourage new bone growth (nano-hydroxyapatite, synthetic nano-silicates, and LDHs) within an implantable matrix not only aid in directing an osteolineage stem cell fate but also serve as a biocompatible substrate to encourage cellular infiltration upon implantation [24, 33, 44]. Choosing a synergistic approach of incorporating osteoconductive nanomaterials built on a biomaterial scaffold that is hydrophilic, biocompatible, and specifically incorporated with biochemical cues for directed bone regeneration shows promise for the next generation of therapies to reverse the bone loss seen in osteoporosis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Felsenberg D, Silman A, Lunt M, Armbrecht G, Ismail A, Finn J, et al. Incidence of vertebral fracture in Europe: results from the European prospective osteoporosis study (EPOS). J Bone Miner Res. 2002;17:716–24.
Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33.
Mackey PA, Whitaker MD. Osteoporosis: a therapeutic update. J Nurs Pract. 2015;11:1011–7.
Ehrlich P, Lanyon L. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700.
Parfitt AM. Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med. 1987;82:68–72.
Riggs BL, Khosla S, Melton LJ. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–73.
Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–5.
Francis R. The effects of testosterone on osteoporosis in men. Clin Endocrinol. 1999;50:411–4.
Zallone A. Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann N Y Acad Sci. 2006;1068:173–9.
Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300.
Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122:S14–21.
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437–44.
Lozano-Calderon SA, Colman MW, Raskin KA, Hornicek FJ, Gebhardt M. Use of bisphosphonates in orthopedic surgery: pearls and pitfalls. Orthop Clin N Am. 2014;45:403–16.
Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67:994–1001.
Alghamdi HS, Bosco R, Both SK, Iafisco M, Leeuwenburgh SC, Jansen JA, et al. Synergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic rats. Biomaterials. 2014;35:5482–90. Nanoparticles are utilized on the surface of an implant to concurrently promote osteoblast activity while also decreasing osteoclast activity.
Bosco R, Iafisco M, Tampieri A, Jansen JA, Leeuwenburgh SC, van den Beucken JJ. Hydroxyapatite nanocrystals functionalized with alendronate as bioactive components for bone implant coatings to decrease osteoclastic activity. Appl Surf Sci. 2015;328:516–24. This study highlights the use of hydroxyapatite nanoparticles as not only an osteoconductive material but also as a delivery vehicle for a drug to decrease osteoclast activity.
Diab DL, Watts NB. Bisphosphonates in the treatment of osteoporosis. Endocrinol Metab Clin N Am. 2012;41:487–506.
Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14.
Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35:1794–800.
Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91.
Lad SP, Nathan JK, Boakye M. Trends in the use of bone morphogenetic protein as a substitute to autologous iliac crest bone grafting for spinal fusion procedures in the United States. Spine. 2011;36:E274–81.
Ehnert S, Jian Z, Pscherer S, Freude T, Dooley S, Kolk A, et al. Transforming growth factor b1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Med. 2012;10:101–11.
Karunaratne DN. Nanotechnology in medicine. Journal of the National Science Foundation of Sri Lanka. 2010;35:149–52.
Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111:441–53.
Rawat M, Singh D, Saraf S, Saraf S. Nanocarriers: promising vehicle for bioactive drugs. Biol Pharm Bull. 2006;29:1790–8.
Carrow JK, Gaharwar AK. Bioinspired polymeric nanocomposites for regenerative medicine. Macromol Chem Phys. 2015;216:248–64.
Kerativitayanan P, Carrow JK, Gaharwar AK. Nanomaterials for engineering stem cell responses. Adv Healthc Mater. 2015;4:1600–27.
Tran N, Webster TJ. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater. 2011;7:1298–306.
Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4:66–80.
Webste T. Nanophase ceramics: the future orthopedic and dental implant material. In: Ying J, editor. Advances in chemical engineering, vol. 27. New York: Academic Press; 2001. p. 125–66.
Fricain JC, Schlaubitz S, Le Visage C, Arnault I, Derkaoui SM, Siadous R, et al. A nano-hydroxyapatite–pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials. 2013;34:2947–59. When tested in several in vivo models, the composite scaffold induces high mineralization as well as maintains incorporated growth factors.
Xu A, Liu X, Gao X, Deng F, Deng Y, Wei S. Enhancement of osteogenesis on micro/nano-topographical carbon fiber-reinforced polyetheretherketone–nanohydroxyapatite biocomposite. Mater Sci Eng C. 2015;48:592–8. By incorporating nanohydroxyapatite into the composite, stem cell osteo-differentiation, mineralization, and interaction with the composite are increased.
Chimene D, Alge DL, Gaharwar AK. Two‐dimensional nanomaterials for biomedical applications: emerging trends and future prospects. Adv Mater. 2015;27:7261–84.
Gaharwar AK, Dammu SA, Canter JM, Wu C-J, Schmidt G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly (ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules. 2011;12:1641–50.
Hu Y, Cai K, Luo Z, Jandt KD. Layer‐by‐layer assembly of β‐estradiol loaded mesoporous silica nanoparticles on titanium substrates and its implication for bone homeostasis. Adv Mater. 2010;22:4146–50.
Sowjanya J, Singh J, Mohita T, Sarvanan S, Moorthi A, Srinivasan N, et al. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf B: Biointerfaces. 2013;109:294–300.
Tripathi A, Saravanan S, Pattnaik S, Moorthi A, Partridge NC, Selvamurugan N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper–zinc for bone tissue engineering. Int J Biol Macromol. 2012;50:294–9.
Kang G, Wang Y, Liu J, Wu J, Zhao M, Li G, et al. Development of three-component conjugates: to get nano-globes with porous surfaces, high in vivo anti-osteoporosis activity and minimal side effects. J Mater Chem. 2012;22:21740–8.
Cao L, Wang J, Hou J, Xing W, Liu C. Vascularization and bone regeneration in a critical sized defect using 2-N, 6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials. 2014;35:684–98. Demonstrates ability of nanoparticle system to deliver BMP-2 for short and long-term treatment to improve bone regeneration as well as promote vascularization.
Ignjatović N, Ajduković Z, Savić V, Najman S, Mihailović D, Vasiljević P, et al. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J Mater Sci Mater Med. 2013;24:343–54.
Weitzmann MN, Ha S-W, Vikulina T, Roser-Page S, Lee J-K, Beck GR. Bioactive silica nanoparticles reverse age-associated bone loss in mice. Nanomed Nanotechnol Biol Med. 2015;11:959–67. Successful in vivo mice studies show potential for administration of silica nanoparticles to counteract age-related bone loss.
Kim T-H, Singh RK, Kang MS, Kim J-H, Kim H-W. Inhibition of osteoclastogenesis through siRNA delivery with tunable mesoporous bioactive nanocarriers. Acta Biomater. 2015. siRNA is incorporated into bioglass nanospheres, which are biocompatible and biodegradable, and successfully delivered to inhibit osteoclast activity.
Saravanan S, Sameera D, Moorthi A, Selvamurugan N. Chitosan scaffolds containing chicken feather keratin nanoparticles for bone tissue engineering. Int J Biol Macromol. 2013;62:481–6.
Gaharwar AK, Mihaila SM, Swami A, Patel A, Sant S, Reis RL, et al. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv Mater. 2013;25:3329–36.
Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, et al. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano. 2015;9:3109–18. Incorporation of two-dimensional silicate nanoparticles not only increases the structural properties of the scaffold but provides a growth-factor free approach to stimulating osteogenic differentiation.
Liu Y, Lu Y, Tian X, Cui G, Zhao Y, Yang Q, et al. Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials. 2009;30:6276–85.
El-Fiqi A, Kim H-W. Mesoporous bioactive nanocarriers in electrospun biopolymer fibrous scaffolds designed for sequential drug delivery. RSC Adv. 2014;4:4444–52.
Kang MS, Kim J-H, Singh RK, Jang J-H, Kim H-W. Therapeutic-designed electrospun bone scaffolds: Mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015;16:103–16. A nanocomposite system is designed to deliver two different growth factors at two different rates; the nanocarriers allowed for sustained release of the later acting growth factor.
Crowder SW, Prasai D, Rath R, Balikov DA, Bae H, Bolotin KI, et al. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2013;5:4171–6. Graphene is novel two-dimensional material and its incorporation into a three-dimensional matrix not only improves the scaffolds mechanical properties but also enhances stem cell osteogenic differentiation.
Thakur T, Xavier JR, Cross L, Jaiswal MK, Mondragon E, Kaunas R, et al. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J Biomed Mater Res A. 2016;104(4):879–888.
Kerativitayanan P, Gaharwar AK. Elastomeric and mechanically stiff nanocomposites from poly (glycerol sebacate) and bioactive nanosilicates. Acta Biomater. 2015;26:34–44.
Gaharwar AK, Mukundan S, Karaca E, Dolatshahi-Pirouz A, Patel A, Rangarajan K, et al. Nanoclay-enriched poly (ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2014;20(15–16):2088–2101.
Saifullah B, Arulselvan P, El Zowalaty ME, Fakurazi S, Webster TJ, Geilich BM, et al. Development of a biocompatible nanodelivery system for tuberculosis drugs based on isoniazid-Mg/Al layered double hydroxide. Int J Nanomedicine. 2014;9:4749.
Tran PA, Sarin L, Hurt RH, Webster TJ. Opportunities for nanotechnology-enabled bioactive bone implants. J Mater Chem. 2009;19:2653–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mikayla Barry, Hannah Pearce, Lauren Cross, Marco Tatullo, and Akhilesh K. Gaharwar declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Regenerative Biology and Medicine in Osteoporosis
Mikayla Barry and Hannah Pearce contributed equally to this work.
Rights and permissions
About this article
Cite this article
Barry, M., Pearce, H., Cross, L. et al. Advances in Nanotechnology for the Treatment of Osteoporosis. Curr Osteoporos Rep 14, 87–94 (2016). https://doi.org/10.1007/s11914-016-0306-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-016-0306-3