Abstract
In the musculoskeletal system, muscle, tendon, and bone tissues develop in a spatially and temporally coordinated manner, and integrate into a cohesive functional unit by forming specific connections unique to each region of the musculoskeletal system. The mechanisms of these patterning and integration events are an area of great interest in musculoskeletal biology. Hox genes are a family of important developmental regulators and play critical roles in skeletal patterning throughout the axial and appendicular skeleton. Unexpectedly, Hox genes are not expressed in the differentiated cartilage or other skeletal cells, but rather are highly expressed in the tightly associated stromal connective tissues as well as regionally expressed in tendons and muscle connective tissue. Recent work has revealed a previously unappreciated role for Hox in patterning all the musculoskeletal tissues of the limb. These observations suggest that integration of the musculoskeletal system is regulated, at least in part, by Hox function in the stromal connective tissue. This review will outline our current understanding of Hox function in patterning and integrating the musculoskeletal tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How tissues are patterned and then integrated with one another during development is an important and highly complex question in developmental biology. The interaction between cells of different embryonic origins and between different tissues within the organism is essential for proper development and function. The musculoskeletal system is an example of a highly complex set of structures that integrate cell types from several distinct embryonic origins. The musculoskeletal system is composed of three basic components: muscle, tendon, and bone, which undergo substantial changes to achieve their final form. One family of highly conserved developmental regulators that have been shown to be important for the integration and patterning of the musculoskeletal tissues is the Hox genes. This review will provide an overview of the role of Hox genes in musculoskeletal development with an emphasis on how Hox regulates musculoskeletal development and patterning in the limb.
Hox Function and Skeletal Patterning
Hox genes are a family of highly conserved homeodomain-containing transcription factors that were first described in the fruit fly, Drosophila. These genes instruct what has been referred to as positional identity along the anterior to posterior (AP) body axis. The collinear arrangement of these genes along the chromosome reflects their spatial and temporal expression within the organism (Fig. 1) [1–6].
The linked Hox cluster underwent duplications during vertebrate evolution. This resulted in a total of 39 Hox genes in all mammalian species, arranged on four chromosomal clusters (Fig. 1) [2, 7]. The collinear arrangement of the Hox genes has been maintained from flies to mammals. The genes in each cluster are further subdivided into 13 paralogous groups based on sequence similarity and position within the cluster [8]. Members of each paralogous group share similar expression domains, and significant functional redundancy has been retained among paralogs. In many cases, assessing Hox gene function has required combining loss-of-function mutations in multiple members within the paralogous groups [6, 9–23].
Along the AP axis of the body, Hox genes are expressed in collinear, but overlapping domains within the somites and input from multiple Hox paralogous groups are responsible for establishing correct positional identity of the vertebra (reviewed in detail [24, 25]). This combinatorial code of Hox expression along the AP axis results in vertebrae morphology being determined by patterning information from multiple Hox genes, usually two or more paralogous groups. When loss of an entire paralogous group occurs, the region that normally receives this input is patterned by the remaining Hox genes in the region. In the majority of cases this leads to anterior homeotic transformations in which the vertebrae assume a more anterior morphology [6, 13–15].
In addition to the role of Hox genes in patterning the axial skeleton, the posterior most Hox paralogs (Hox9-13) pattern the limb skeleton along the proximodistal (PD) axis. The vertebrate limb can be divided into three segments: the proximal stylopod (humerus/femur), the medial zeugopod (radius and ulna/tibia and fibula), and the distal autopod (hand/foot bones). In contrast to the anterior homeotic transformations observed in the axial skeleton, loss of Hox paralogous groups in the limb results in a complete loss of patterning information within a specific limb segment. For example, loss of Hox10 paralogous genes results in severe stylopod mispatterning, loss of Hox11 paralogous genes results in severe zeugopod mispatterning, and loss of Hox13 paralogous genes results in a complete loss of autopod skeletal elements [6, 10–12, 26]. The difference between axial and limb patterning is because of the nonoverlapping function of Hox paralogous groups within the limb. The posterior HoxA and HoxD clusters are expressed in both the forelimb and the hindlimb, while the HoxC cluster is only expressed in the hindlimb [6, 10, 27]. With loss of function for all posterior HoxA and HoxD genes in the limb, there are severely truncated skeletal elements [28]. Additionally, posterior Shh expression is not initiated or maintained demonstrating a combined requirement for Hox function in early limb AP patterning as well as PD patterning [28].
Roles for two other paralogous groups, Hox5 and Hox9, have recently been described in patterning the AP axis of the forelimb. With complete loss of Hox9 genes (Hoxa9 -/-; Hoxb9 -/-, Hoxc9 -/-, Hoxd9 -/-), Shh expression is not initiated, disrupting AP patterning within the developing limb bud [29]. Hox9 promotes posterior Hand2 expression resulting in the inhibition of the hedgehog pathway inhibitor Gli3, allowing for induction of Shh expression in the posterior limb bud. Gli3 expression is not repressed when Hand2 is lost in the posterior limb bud. The overexpression of Gli3 results in a complete loss of Shh expression and no AP axis is established. This results in only one skeletal element in each limb segment, identical to the phenotype resulting from loss of Shh function [29]. Loss-of-function mutations in the Hox5 paralogous group (Hoxa5 -/-, Hoxb5 -/-, Hoxc5 -/-) results in loss of repression of anterior Shh expression in the limb bud leading to anterior patterning defects in the limb [30]. In this case, Hox5 has been reported to interact with Plzf and functions to restrict Shh to the posterior limb bud [30].
Vertebrate Limb Musculoskeletal Development
Musculoskeletal development is a complex process whereby bone, tendon, and muscle tissues must be appropriately pattered and precisely connected for physiologically relevant movement. An excellent model for studying musculoskeletal system development is the vertebrate limb. During limb development, bone, tendon, and muscle precursors differentiate and are coordinately patterned and integrated into a functional unit. There is significant knowledge on the developmental regulation of bone, tendon, and muscle tissue individually (reviewed in detail [31–33]) however, our understanding on how these tissues integrate into a cohesive unit is less clear. Work in recent years has begun to tease apart the mechanisms behind these coordinated patterning events, and it is clear that there is substantial communication between these tissues as limb pattern is established.
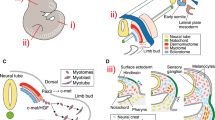
The limb musculoskeletal system is composed of mesodermal tissue that is derived from two distinct embryonic compartments (Fig. 2). The lateral plate mesoderm gives rise to the limb bud itself and the cartilage and tendon precursors arise within the lateral plate mesoderm of the limb bud [34, 35]. In contrast, muscle precursors differentiate from the dermomyotomal compartment of the axial somites adjacent to the limb field and migrate into the limb bud to form the limb musculature [36–39].
The limb musculoskeletal tissues are derived from two distinct mesodermal compartments. Tendon (green) and chondrocyte (red) precursors are derived from the lateral plate mesoderm within the limb bud, and tendon primordia arise adjacent to the centrally localized chondrocyte condensations. Muscle precursors (purple) delaminate from the ventral somite adjacent to the limb and migrate into the limb bud as dorsal and ventral muscle masses
In the mouse, the forelimb bud emerges at embryonic day 9 (E9) from the lateral plate mesoderm on each side of the embryo. The skeletal pattern of the limb arises as Sox9-positive cartilage precursors condense centrally within the limb bud mesenchyme from proximal (stylopod) to distal (autopod) beginning around E11.5 [40]. Concurrently, muscle precursor cells delaminate from the ventrolateral dermomyotome of the somites adjacent to the limb bud and migrate into the limb as dorsal and ventral masses, where they continue to proliferate, aggregate, and differentiate into muscle tissue [36]. Finally, tendon primordia arise in the dorsal and ventral limb mesenchyme directly from the lateral plate mesoderm then align between the muscle masses and the skeletal elements (Fig. 2) [41, 42]. The dorsal and ventral muscle bundles segregate into individual anatomic groups as muscle connective tissue cells align along future sites of splitting and initiate cell death [41, 43, 44]. This process generally proceeds in a proximal to distal and dorsal to ventral fashion along the limb axis. Bone, tendon, and muscle precursors undergo concurrent differentiation and patterning in a highly coordinated fashion, however, there is a significant lack of understanding on the mechanisms behind these highly coordinated patterning events.
Early Patterning Events are Tissue Autonomous
Muscle precursors themselves have no intrinsic or predetermined patterning information. Classical chick-quail graft experiments, where somites outside the normal limb region are grafted into the axial region adjacent to the limb, demonstrated that muscles from any somite are capable of migrating into the limb and forming normal limb musculature [36, 37]. More complex single-cell lineage analysis has demonstrated that transplanted somitic muscle precursors are not biased to form specific anatomic muscles [45]. If tendon primordia are surgically removed, muscle progenitors still migrate into the limb bud mesenchyme and are able to undergo differentiation demonstrating that the differentiation of muscle tissue is autonomous and does not require signals from the tendon [41]. Markers of early tendon progenitors have been described relatively recently, but it is not clear if they mark the earliest tendon progenitors [42]. Therefore, there has been no genetic ablation of all limb tendon progenitors. The generation of a tendon-less limb mouse will be necessary to test whether early muscle patterning events occur in the complete absence of tendon progenitors.
Various muscle-less limb models have demonstrated that early patterning of the connective tissue and skeletal elements do not require the presence of muscle. Initiation of tendon and muscle connective tissue pattern occurs normally in muscle-less limb models in both mouse and chick [41, 42, 46, 47]. The Sox9+ skeletal elements are also normally patterned in the absence of muscle.
Muscle and Tendon/Muscle Connective Tissue Interactions are Required for Later Patterning Events
After the initial prepatterning events of the tendon and the muscle connective tissue occur, integration of the muscle, bone, and tendon requires interaction between tissues. While early specification and patterning of tendon and muscle connective tissue is normal in muscle-less limbs, tendon progenitors are progressively lost in a proximal to distal fashion [41]. After migrating into the limb, patterning of the dorsal and ventral muscle masses requires interactions with the tendon. If tendon primordia are surgically removed from the limb, proper muscle patterning is disrupted and aberrant muscles form [41]. The formation of aberrant muscle in the absence of tendon demonstrates a role for tendon in negatively regulating muscle formation in specific regions of the limb [41].
There is growing evidence that the connective tissue elements of the limb, tendons, and muscle connective tissue, have critical roles in patterning the muscle. The T-box transcription factors Tbx4 and Tbx5 are broadly expressed in the limb bud mesenchyme including the muscle connective tissue and tendon precursors [48]. Loss of Tbx5 gene function results in ectopic splitting of the dorsal and ventral limb muscle masses and disruption of the muscle connective tissue organization. Additionally, levels of β-Catenin and N-cadherin were reduced. These data suggest that the organization of connective tissue and catenin/cadherin mediated cell adhesion are required for connective tissue-mediated muscle patterning.
The transcription factor Tcf4, a member of the Tcf/Lef family which acts downstream of Wnt signaling, has been identified as a marker of muscle connective tissue cells [49]. Overexpression of Tcf4 results in the formation of ectopic muscle and loss of Tcf4 disrupts muscle fiber type switching, suggesting that the muscle connective tissue directs differentiation of muscle precursors [49, 50•]. There is also evidence that Fgf4 signals from muscle at sites of tendon attachment function to maintain expression of tendon specific genes [47]. Together these data demonstrate a critical requirement for muscle-tendon interactions for maintenance and patterning of both tissues.
Muscle and Tendon Interactions with Bone are Necessary for Patterning Bone Ridges
Attachment of muscle to the bone through tendon is important in patterning the skeletal elements at sites of tendon attachment. It has long been appreciated that mechanical forces generated in muscle and transmitted to the bone through tendon attachments influence the final skeletal morphology [51–53]. In Scx null mutants, tendon condensation and differentiation is disrupted and consequently the deltoid tuberosity does not form on the humerus demonstrating a requirement for proper tendon formation in the development of this bone process [54]. Recent data provide evidence that bone ridge cells originate from a pool of Scx+;Sox9+ progenitor cells [55–57]. Bone ridge patterning appears to be a biphasic process of initiation and growth stages, and signals from the tendon are critical to initiate formation of these skeletal structures [58••]. Additionally, there is also a well-described role for muscle loading in regulating proper tendon structure [57, 59, 60]. These studies highlight the critical requirement for muscle, tendon and bone interactions in regulating bone morphology. What determines the sites of tendon-bone attachment is still a largely unanswered question in the field.
Establishment of Regional Patterning
Despite significant data on the requirement for crosstalk between muscle, tendon, and bone during musculoskeletal patterning, the mechanisms responsible for these processes are still under active exploration. A critical component in patterning the musculoskeletal system is the incorporation of regional differences in patterning along the AP axis of the vertebrae and the PD axis of the limb. The same tissue components, muscle, tendon, and bone, are present throughout the limb, but substantial morphologic differences and unique connections occur within these tissues along the axial and limb axes. While there is significant information on the development of muscle, tendon, and bone, how these tissues form precise connections and become patterned and integrated into a functional unit is largely unknown. Work published recently from our laboratory suggests that this regional pattering information is provided, at least in part, by the master developmental regulators, the Hox genes.
Hox genes expression is initiated very early in limb bud development. The posterior Hox genes are expressed in a nested and highly overlapping pattern within the limb bud. As the limb develops and grows in length, the Hox paralogous groups become progressively restricted to their appropriate functional domains such that Hox9 and Hox10 are expressed in the stylopod, Hox11 is expressed in the zeugopod, and Hox13 is expressed in the autopod (Fig. 3) [6, 10–12, 26, 61•]. This restricted expression pattern is then maintained throughout embryonic development [61•].
Posterior Hox paralogous genes are progressively restricted to specific limb regions during development. In the early limb bud, Hox 9-13 paralogs are expressed in an overlapping and nested pattern. As the limb develops, Hox paralogs are progressively restricted resulting in Hox9/10 (yellow) expressed proximally (stylopod), Hox11 (green) expressed medially (zeugopod), and Hox13 (blue) expressed distally (autopod)
The regionalized expression and function of Hox genes along the limb axis makes them an attractive candidate for directing regionalized patterning and integration of the musculoskeletal tissues. A Hoxa11eGFP knock-in mouse model was utilized to examine Hox11 expression in detail within the developing limb. Characterization of Hoxa11eGFP expression in whole mount and in longitudinal limb sections shows broad expression throughout the distal limb bud mesenchyme early in development. This expression rapidly becomes restricted to the presumptive zeugopod [61•]. Interestingly, Hoxa11eGFP+ cells are largely excluded from the Sox9+ chondrogenic precursor population at all stages of development [61•]. Careful examination of Hoxa11eGFP expression shows Hoxa11eGFP+ cells in the outer perichondrial layer surrounding the presumptive zeugopod condensations (Fig. 4A and B). Analysis with markers for muscle and tendon revealed Hoxa11eGFP expression within the muscle connective tissue and all cells of the tendon (Fig. 4A and C). Throughout limb development, Hoxa11eGFP+ cells are always excluded from muscle and bone and remain expressed in the stromal connective tissues (Fig. 4) [61•]. Hoxa13GFP expression has also been reported to be expressed in the perichondrium and excluded from condensing cartilaginous elements, however, detailed co-expression analysis with muscle and tendon markers has not yet been examined [62].
Hox11 is expressed in the connective tissue (stromal) compartment of the forelimb. A, Hoxa11eGFP expression is restricted to the zeugopod and excluded from bone and muscle cells. Hox11 is expressed in the outer perichondrium surrounding the bones, in the muscle connective tissue, and in the tendons. B, Longitudinal section of E14.5 forelimb showing expression of Hox11eGFP (green) surrounding the Sox9+ (red) skeletal elements. C, Transverse view of an individual forelimb muscle bundle at E14.5 showing expression of Hox11eGFP (green) in the muscle connective tissue surrounding individual muscle (red) cells. My32 myosin 32
The cartilage elements destined to become the limb skeletal elements still condense in Hox loss-of-function mutants but they are not elaborated to form the appropriate anatomic bones [26]. The observation that Hox is not expressed in the skeletal elements but in the perichondrial stromal layer surrounding the elements significantly changes how the skeletal phenotypes observed in these mutants are interpreted. These data strongly suggest that signals from the perichondrial stromal cells have an important role in determining the morphology and patterning of the skeletal elements.
Hoxa11eGFP expression in the connective tissues of the limb was a surprising finding and prompted analysis of the muscle and tendon phenotypes in Hox11 mutants. A potential role for Hox in mediating muscle or tendon development had not been previously investigated in detail. In addition to the well-described regional skeletal phenotypes observed in Hox paralogous loss-of-function mutants, severe mispatterning of the zeugopod muscles and tendons is observed in Hox11 loss-of-function mutants [61•]. Early in development, tendon progenitors are specified in Hox11 mutants and appear similar to controls, suggesting that Hox functions in patterning and integration but does not function in specification of connective tissue. In Hox11 mutants, the migration of muscle precursors into the limb occurs normally and dorsal and ventral muscle masses are present. However, the subsequent splitting of the masses into individual muscle bundles is abnormal and loss or fusion of various muscle groups is observed [61•]. Muscle differentiation, however, still occurs as mature muscle cells are observed in these mutants. The muscle-specific phenotypes in Hox11 mutants suggest a critical role for Hox in connective tissue-mediated patterning of the muscle but not in directing muscle migration or differentiation. These data provide strong evidence that Hox function in the connective tissue stromal cells is critical for regionally patterning all components of the musculoskeletal system.
Development and patterning of the skeletal elements remains normal in Hox11 compound mutants where one of the four functional alleles of forelimb Hox11 (either Hoxa11 or Hoxd11) is still expressed [6, 10, 26, 61•]. Hox11 compound mutants allow us to address the question of whether the muscle and tendon phenotypes observed in Hox11 double mutant animals represent independent roles for Hox11 in patterning these tissues, or whether the phenotypes are a consequence of the severely disrupted skeletal patterning. In Hox11 compound mutants, skeletal patterning is preserved but regional mispatterning of the muscles and tendons is still observed, demonstrating that the muscle and tendon phenotype is independent of skeletal pattern [61•]. These analyses also demonstrate that skeletal patterning and muscle/tendon patterning can be uncoupled. Analysis of limb muscle and tendon phenotypes in other posterior Hox gene paralogous mutants has not been investigated in detail, but we hypothesize that similar muscle and tendon phenotypes will be observed.
Perspectives and Future Directions
These observations raise very important questions of how Hox genes function in the musculoskeletal system and how they contribute to integrating the patterning of muscles, tendons, and bones. Many studies highlight the importance of the connective tissues in patterning the limb, and the expression of Hoxa11eGFP in the stromal connective tissues of the limb is consistent with an important role for Hox in these stromal cells in directing the pattern of musculoskeletal tissues [41, 48–50•, 58••, 61•]. The continuum of Hoxa11eGFP expression from the muscle connective tissue, into the tendon, and then the perichondrium surrounding the bone suggests of a role for Hox11 in integrating patterning information between these tissues. The connective tissue extracellular matrix is made up of largely the same components throughout the limb and identification of region and tissue specific markers is important for understanding how these tissues direct regional patterning. Expression of Hoxa11eGFP provides a unique tool to specifically identify these connective tissue elements for analysis within the zeugopod. Generation of fluorescent alleles and conditional loss of function experiments for other Hox genes will be beneficial in addressing these questions. Our recent work strongly suggests that connective tissue cells possess regional identity information and play a critical role in establishing proper region-specific pattern of the musculoskeletal tissues [61•].
Musculoskeletal injury and disease can have a devastating impact on a patient’s quality of life and there is a great need for novel and improved treatments and therapies. As master developmental regulators, understanding and exploiting the Hox signaling program could be invaluable for the development of usable stem cell therapies designed to treat musculoskeletal injury and disease. Our observations suggest that different Hox programs may need to be activated to direct appropriate stem cell activity for use in repair at specific axial or proximal/distal levels.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Duboule D. Patterning in the vertebrate limb. Curr Opin Genet Dev. 1991;1(2):211–6. doi:10.1016/S0959-437X(05)80072-3.
Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78(2):191–201.
Lewis EB. Genes and Developmental Pathways. Am Zool. 1963;3(1):33–56. doi:10.2307/3881152.
Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–70.
Lewis EB. Regulation of the genes of the bithorax complex in Drosophila. Cold Spring Harb Symp Quant Biol. 1985;50:155–64.
Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301(5631):363–7. doi:10.1126/science.1085672.
Scott MP. Vertebrate homeobox gene nomenclature. Cell. 1992;71(4):551–3.
Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nature Rev Genet. 2005;6(12):893–904. doi:10.1038/nrg1726.
Condie BG, Capecchi MR. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature. 1994;370(6487):304–7. doi:10.1038/370304a0.
Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375(6534):791–5. doi:10.1038/375791a0.
Fromental-Ramain C, Warot X, Lakkaraju S, Favier B, Haack H, Birling C, et al. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development. 1996;122(2):461–72.
Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dolle P, Chambon P. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122(10):2997–3011.
Horan GS, Ramirez-Solis R, Featherstone MS, Wolgemuth DJ, Bradley A, Behringer RR. Compound mutants for the paralogous hoxa-4, hoxb-4, and hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 1995;9(13):1667–77.
McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, et al. Hox patterning of the vertebrate rib cage. Development. 2007;134(16):2981–9. doi:10.1242/dev.007567.
van den Akker E, Fromental-Ramain C, de Graaff W, Le Mouellic H, Brulet P, Chambon P, et al. Axial skeletal patterning in mice lacking all paralogous group 8 Hox genes. Development. 2001;128(10):1911–21.
Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 2002;16(11):1423–32. doi:10.1101/gad.993302.
Chen F, Capecchi MR. Targeted mutations in hoxa-9 and hoxb-9 reveal synergistic interactions. Dev Biol. 1997;181(2):186–96. doi:10.1006/dbio.1996.8440.
Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci U S A. 1999;96(2):541–6.
Chen F, Greer J, Capecchi MR. Analysis of Hoxa7/Hoxb7 mutants suggests periodicity in the generation of the different sets of vertebrae. Mech Dev. 1998;77(1):49–57.
Gavalas A, Trainor P, Ariza-McNaughton L, Krumlauf R. Synergy between Hoxa1 and Hoxb1: the relationship between arch patterning and the generation of cranial neural crest. Development. 2001;128(15):3017–27.
Manley NR, Capecchi MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol. 1998;195(1):1–15. doi:10.1006/dbio.1997.8827.
Studer M, Gavalas A, Marshall H, Ariza-McNaughton L, Rijli FM, Chambon P, et al. Genetic interactions between Hoxa1 and Hoxb1 reveal new roles in regulation of early hindbrain patterning. Development. 1998;125(6):1025–36.
Wahba GM, Hostikka SL, Carpenter EM. The paralogous Hox genes Hoxa10 and Hoxd10 interact to pattern the mouse hindlimb peripheral nervous system and skeleton. Dev Biol. 2001;231(1):87–102. doi:10.1006/dbio.2000.0130.
Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev Dynam. 2007;236(9):2454–63. doi:10.1002/dvdy.21286.
Wellik DM. Hox genes and vertebrate axial pattern. Curr Top Dev Biol. 2009;88:257–78. doi:10.1016/s0070-2153(09)88009-5.
Boulet AM, Capecchi MR. Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development. 2004;131(2):299–309. doi:10.1242/dev.00936.
Hostikka SL, Capecchi MR. The mouse Hoxc11 gene: genomic structure and expression pattern. Mech Dev. 1998;70(1–2):133–45.
Kmita M, Tarchini B, Zakany J, Logan M, Tabin CJ, Duboule D. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435(7045):1113–6. doi:10.1038/nature03648.
Xu B, Wellik DM. Axial Hox9 activity establishes the posterior field in the developing forelimb. Proc Natl Acad Sci U S A. 2011;108(12):4888–91. doi:10.1073/pnas.1018161108.
Xu B, Hrycaj SM, McIntyre DC, Baker NC, Takeuchi JK, Jeannotte L, et al. Hox5 interacts with Plzf to restrict Shh expression in the developing forelimb. Proc Natl Acad Sci U S A. 2013;110(48):19438–43. doi:10.1073/pnas.1315075110.
Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Ann Rev Cell Dev Biol. 2009;25:629–48. doi:10.1146/annurev.cellbio.042308.113308.
Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development. 2010;137(17):2807–17. doi:10.1242/dev.047498.
Murphy M, Kardon G. Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Curr Top Dev Biol. 2011;96:1–32. doi:10.1016/B978-0-12-385940-2.00001-2.
Kieny M, Chevallier A. Autonomy of tendon development in the embryonic chick wing. J Embryol Exp Morphol. 1979;49:153–65.
Shellswell G, Wolpert L. The pattern of muscle and tendon development in the chick wing. Vert Limb Somite Morphogen. 1977:71–86.
Chevallier A, Kieny M, Mauger A. Limb-somite relationship: origin of the limb musculature. J Embryol Exp Morphol. 1977;41(1):245–58.
Christ B, Jacob HJ, Jacob M. Experimental analysis of the origin of the wing musculature in avian embryos. Anatomy Embryol. 1977;150(2):171–86.
Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114(2):339–53.
Wachtler F, Christ B, Jacob HJ. On the determination of mesodermal tissues in the avian embryonic wing bud. Anatomy Embryol. 1981;161(3):283–9.
Thorogood PV, Hinchliffe JR. An analysis of the condensation process during chondrogenesis in the embryonic chick hind limb. J Embryol Exp Morphol. 1975;33(3):581–606.
Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125(20):4019–32.
Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128(19):3855–66.
Schroeter S, Tosney KW. Spatial and temporal patterns of muscle cleavage in the chick thigh and their value as criteria for homology. Am J Anat. 1991;191(4):325–50. doi:10.1002/aja.1001910402.
Schroeter S, Tosney KW. Ultrastructural and morphometric analysis of the separation of two thigh muscles in the chick. Am J Anat. 1991;191(4):351–68. doi:10.1002/aja.1001910403.
Kardon G, Campbell JK, Tabin CJ. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev Cell. 2002;3(4):533–45.
Brent AE, Braun T, Tabin CJ. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development. 2005;132(3):515–28. doi:10.1242/dev.01605.
Edom-Vovard F, Schuler B, Bonnin M-A, Teillet M-A, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247(2):351–66. doi:10.1006/dbio.2002.0707.
Hasson P, DeLaurier A, Bennett M, Grigorieva E, Naiche LA, Papaioannou VE, et al. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell. 2010;18(1):148–56. doi:10.1016/j.devcel.2009.11.013.
Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a pre-pattern for vertebrate limb muscle patterning. Dev Cell. 2003;5(6):937–44.
Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138(2):371–84. doi:10.1242/dev.057463. The authors show that muscle connective tissue fibroblasts express Tcf4. Generation of Tcf4GFPCre mice provides the first genetic tool for manipulating these fibroblasts. This work presents definitive evidence that muscle connective tissue regulates muscle development in addition to muscle structure and function.
Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol. 1990;206(1):45–56. doi:10.1002/jmor.1052060105.
Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J Anatomy. 1991;177:169–78.
Sharir A, Stern T, Rot C, Shahar R, Zelzer E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development. 2011;138(15):3247–59. doi:10.1242/dev.063768.
Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134(14):2697–708. doi:10.1242/dev.001933.
Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005;102(41):14665–70. doi:10.1073/pnas.0504750102.
Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. 2013;140(13):2680–90. doi:10.1242/dev.093906.
Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140(11):2280–8. doi:10.1242/dev.096354.
Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17(6):861–73. doi:10.1016/j.devcel.2009.10.010. This work highlights the critical interaction between bone and tendon during the integration of these two tissues during development as well as key regulators of these processes.
Ralphs JR, Benjamin M, Waggett AD, Russell DC, Messner K, Gao J. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anatomy. 1998;193(Pt 2):215–22.
Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthopaed Res. 2003;21(3):413–9. doi:10.1016/S0736-0266(03)00057-3.
Swinehart IT, Schlientz AJ, Quintanilla CA, Mortlock DP, Wellik DM. Hox11 genes are required for regional patterning and integration of muscle, tendon and bone. Development. 2013;140(22):4574–82. doi:10.1242/dev.096693. This paper shows that loss of Hox11 results in mis-patterning of the muscles and tendons of the zeugopod in addition to the well described skeletal phenotypes. Hoxa11eGFP is expressed in connective tissue stromal cells, suggesting that Hox function in these cells is required for proper patterning of the musculoskeletal system.
Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128(21):4177–88.
Acknowledgements
The authors would like to acknowledge Holly Fischer for creating the illustrations.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
K. M. Pineault and D. M. Wellik declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by K. M. Pineault and D. M. Wellik involving animal research were performed after approval by the appropriate institutional review boards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pineault, K.M., Wellik, D.M. Hox Genes and Limb Musculoskeletal Development. Curr Osteoporos Rep 12, 420–427 (2014). https://doi.org/10.1007/s11914-014-0241-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-014-0241-0