Abstract
An increasing number of studies suggest an association between depression and osteoporosis. In a mouse model, depression induces bone loss, mediated by brain-to-bone sympathetic signaling. Depression and bone antianabolic sympathetic tone are alleviated by increasing central serotonin (5-hydroxytryptamine, 5-HT) levels. However, selective serotonin reuptake inhibitors (SSRIs), the first-line antidepressants, increase extracellular 5-HT levels but have deleterious skeletal effects. The skeletal serotonergic system consists of 5-HT receptors and the 5-HT transporter (5-HTT) in osteoblasts and osteocytes. 5-HTT is a transmembrane protein targeted by SSRIs. 5-HT restrains osteoblastic activity, thus leading to bone loss. Apparently, the negative skeletal effects of the peripheral SSRI-induced increase in 5-HT outweighs the skeletal benefits resulting from the enhanced central 5-HT antidepressant and antisympathetic activity. Overall, major depression appears as an important risk factor for osteoporosis. However, antidepressants, mainly SSRIs, should be evaluated in view of the causal relationship between depression and bone loss, and vis-à-vis their skeletal adverse effects. Patients with depressive disorders should undergo a routine skeletal evaluation and receive timely antiosteoporotic therapy, especially when SSRI treatment is prescribed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past three decades, the association between depression and osteoporosis has been the subject of a growing body of research, implicating major depressive disorder (MDD) as a risk factor for bone loss and osteoporosis [1••]. Like osteoporosis, MDD is a prevalent disease, considered the second leading global cause of years of life lived with disability [2]. Both depression and osteoporosis are approximately threefold more common in women than in men [3, 4].
In spite of the high prevalence of both diseases, most official publications emanating from authorities, such as the US National Institute of Health, the National Osteoporosis Foundation, the National Osteoporosis Society (United Kingdom), and Osteoporosis Canada, do not fully acknowledge depression as a risk factor for low bone loss and osteoporosis. However, in view of recent meta-analyses demonstrating a link between these maladies [1••, 5•, 6•], and experimental data suggesting a causal relationship between the two diseases [7••], it is likely for depression to be officially recognized as a risk factor for osteoporosis.
Another element affecting the depression-osteoporosis equation is the bone antianabolic activity of antidepressant drugs, in particular selective serotonin reuptake inhibitors (SSRIs). These drugs further exacerbate the skeletal status, apparently by increasing extracellular serotonin levels that inhibit osteoblastic activity thus leading to a negative bone remodeling balance and bone loss.
Association Between Depression and Osteoporosis
That the association between depression and osteoporosis has not been officially acknowledged could be because the literature on the relationship between these conditions is insufficiently conclusive. Some studies report that patients with MDD suffer up to 15% bone loss, whereas others, particularly large-scale population-based studies, show a weak or no relationship between the two conditions [1••].
Recently, three meta-analyses conducted to assess the possible impact of depression on skeletal status concluded that depression should be considered as an important risk factor for osteoporosis [1••, 5•, 6•]. We have recently carried out the largest of these analyses in terms of the number of studies and patients enrolled. It is based on a total of 23 studies that could be identified in databases and in relevant articles. These studies address the relationship between depression and skeletal status, comparing a total of 2327 depressed with 21,141 nondepressed individuals [1••]. The results of this meta-analysis demonstrated that depressed individuals display lower bone mineral density (BMD) and higher bone resorption markers than nondepressed subjects. The association of depression with lower bone mass is significant in the spine (vertebrae), hip (proximal femur), and distal radius, suggesting that the depression-associated low bone mass involves multiplicity of trabecular bone sites throughout the skeleton. This conclusion has been recently supported by findings in the spine, femoral neck, and total femur [5•]. According to standard criteria, in our study, the overall effect size of depression on BMD for the entire sample, which consisted of adult male and pre- and postmenopausal females, was rather small, with a moderate effect on resorption markers.
Like osteoporosis, depression is approximately threefold more common in women than in men [3, 4]. As in the study by Wu et al. [6•], our meta-analysis portrays women as significantly more vulnerable to depression-associated low BMD. This gender difference may be related to the greater sensitivity of females to stress in general, and to the greater responsiveness of depressed women to various stressors in particular [1••]. It should be emphasized, however, that although the effect of depression in men is smaller than in women, it is more robust, namely, it is not influenced by various moderating variables. In contrast, the association between depression and BMD in women shows substantial heterogeneity, as it is significantly moderated by at least two variables, menopausal status and the depression assessment procedure [1••].
Premenopausal women display a greater depression-associated decrease in BMD compared with postmenopausal subjects [1••]. A decreased rate of bone mass accrual has been reported in teenager females with MDD [8•], portraying reduced peak bone mass as one determinant leading to decreased BMD in depressed premenopausal women. However, most of the studies included in the cited meta-analyses do not report the age of onset of the depressive episodes. Thus, depression-induced bone loss in premenopausal patients after completion of the peak bone mass accrual cannot be ruled out at the present time.
The greater depression-associated decrease in BMD in pre- than postmenopausal subjects does not necessarily mean that depression is not associated with low bone mass after menopause. However, in postmenopausal women this association may be masked by the multiplicity of factors contributing to the development of low bone mass, such as estrogen depletion, reduced physical activity, nutritional disturbances, and drug treatments [1••]. Thus, to further assess the impact of menopausal status on depression-associated low BMD, direct comparisons for the effects of depression on BMD should be undertaken between pre- and postmenopausal women, inasmuch as studies carried out so far focused mainly on either pre- or postmenopausal subjects.
Our meta-analysis also indicates that assessment of an association between depression and BMD critically depends on the procedure used for diagnosing the depressive condition. When depression was individually diagnosed by a psychiatrist, defining major depression on the basis of the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, the magnitude of the effect was substantial. In contrast, in studies based on self-rating checklists, an overall significant relationship could not be identified between depression and BMD [1••]. Self-rating checklists are usually used in studies with large samples of community-dwelling individuals who do not seek psychiatric help. Compared with DSM-based psychiatric evaluations, these studies are rather heterogeneous and measure a restricted array of depressive symptoms. For example, some of these scales, such as the Geriatric Depression Scale (GDS), focus mainly on depressed mood and feelings of helplessness, hopelessness, and worthlessness, with less emphasis on anhedonia and vegetative symptoms such as weight loss and psychomotor retardation. In addition, some scales, such as the Center for Epidemiologic Studies Depression Scale (CES-D), assess mood and behavior over a relatively short period (eg, the past week), whereas studies using DSM-based psychiatric evaluation address depressive episodes that lasted for weeks or months [1••]. Therefore, it appears that depression-associated low bone mass afflicts mainly psychiatric patients diagnosed with major depression, who experience relatively severe symptoms over prolonged time periods. Depressed community-dwelling individuals, whose depressive symptoms are not severe enough to prompt them to seek psychiatric help, do not display significantly reduced BMD.
The contribution of potential moderator variables to depression-associated low BMD is reported in several individual studies, showing that body weight, height, number of previous depressive episodes, total duration of disease, history of estrogen treatment, smoking, and race do not modulate the association between depression and bone density. Low levels of physical activity, characteristic of depressed patients, were also suggested to be associated with low BMD [1••]. However, studies included in our meta-analysis that assessed the levels of exercise, adequate matching, or statistical adjustment for this variable did not modulate the association between depression and BMD [1••]. In addition, in several studies demonstrating depression-associated low BMD, the levels of endocrine factors believed to affect BMD do not differ between depressed and nondepressed subjects. These factors include serum 25-hydroxyvitamin D, parathyroid hormone, free T3, insulin-like growth factor-1, and thyroid-stimulating hormone [1••]. It is therefore unlikely that these variables are involved in the depression-associated low BMD.
Antidepressant therapy, especially by SSRIs, could be also a confounding variable affecting the recent meta-analyses (discussed below). However, studies using antidepressant therapy as a covariate found no evidence to its effect on the association between depression and BMD. Furthermore, several studies reporting a significant association between depression and low BMD specifically indicate that none, or almost none (<5%), of the participants were ever treated with antidepressants [1••]. Thus, the association between depression and bone density appears independent of the deleterious effects of antidepressants on the skeleton.
At the tissue level, bone mass is determined by continuous bone remodeling that consists of bone formation by osteoblasts and bone resorption by osteoclasts. Consistent with the association between depression and low bone density, bone resorption markers are significantly elevated in depressed patients, suggestive of a negative remodeling balance in the depressed patients [1••]. Whereas bone resorption markers are significantly elevated in these patients, osteocalcin, the so-called bone formation marker, does not exhibit any significant trend. Although it is produced by osteoblasts and discharged into the blood circulation, a significant amount of osteocalcin is incorporated into the mineralized bone matrix and further released to the circulation during osteoclastic degradation. Thus, in view of the apparent increase in bone resorption, the lack of association between serum osteocalcin levels and depression may reflect a decrease in bone formation, accompanied by a new balance between osteocalcin production, its direct passage into the circulation, incorporation into the bone matrix, and release by osteoclasts. Such an association between depression and bone formation is supported by the results of our recent study in mice, in which we used direct bone formation measurements and showed a depression-induced inhibition of bone formation [7••].
Taken together, the published literature, in particular the three recent meta-analyses [1••, 5•, 6••], demonstrates a significant association between depression and low skeletal mass. More specifically, all individuals psychiatrically diagnosed with major depression are at risk for developing osteoporosis, with depressed women, particularly those who are premenopausal, showing a higher risk than men. These patients should be periodically evaluated for progression of bone loss and imbalances in bone remodeling.
Causal Relationship Between Depression and Bone Loss
The causal relationship between MDD and osteoporosis has not been fully elucidated. In the early 1980s, osteoporosis researchers suggested that depression is one of the major negative consequences of bone loss and osteoporotic fractures. These researchers believed that osteoporosis occurred first, leading to a reactive depression. A similar, but distinct psychiatry literature reported that low BMD appears to be an undesirable consequence of MDD [9]. The perspective that osteoporosis causes depression argues that MDD results from the pain and discomfort associated with osteoporotic fractures. The other approach, that depression is the causal process, claims that most studies demonstrate an association of depression with low BMD rather than with increased fracture rate. That depression is the causal attribute has been further proposed based on the well-established depression-induced increases in glucocorticoids and norepinephrine [10], agents also known to suppress bone formation and bone mass [11, 12].
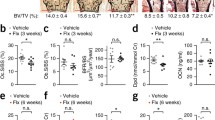
In support of the latter concept, we have recently demonstrated loss of bone mass and architecture in mice with chronic mild stress (CMS), an established rodent model for depression (Fig. 1). The bone loss in this model results mainly from decreased bone formation. Both the reduced bone formation and the trabecular bone loss, measured by microcomputed tomography in the distal femoral metaphysis and lumber vertebral bodies, as well as the depressive symptoms (reduced sucrose preference and social exploration) could be prevented by the antidepressant drug imipramine [7••]. Expectedly, we were able to demonstrate that the depressive-like state was associated with increased norepinephrine levels in bone and elevated serum corticosterone. Furthermore, the CMS-induced bone loss, but not the depressive-like state, could be prevented using the β-adrenergic antagonist, propranolol, portraying bone sympathetic innervation as a brain-to-bone pathway communicating depressive signals to the skeleton. Although serum corticosterone is also elevated in mice subjected to CMS, the role of the hypothalamic-pituitary-adrenal (HPA) axis in depression-induced bone loss is still unraveled, since it is unclear whether the elevation of serum corticosterone induced by CMS is sufficient to cause a negative bone remodeling balance and bone loss. Interestingly, although leptin has been implicated in both depression and the regulation of bone remodeling [13, 14], we were unable to establish any relationship between leptin serum levels and the CMS-induced bone loss.
Depression-induced structural impairment of the skeleton in mice exposed to chronic mild stress (CMS) for 4 weeks or left untreated (UT). Microcomputed tomography analysis. BV/TV bone volume density. (From the Yirmiya et al. [7••].)
The adrenergic system and HPA axis are the most studied pathways mediating depressive signals from the central nervous system (CNS) to the periphery. However, several other systems, implicated in both depression and osteoporosis could be involved in this process, such as the endocannabinoid system [15, 16] and inflammatory cytokines like interleukin (IL)-1 [17, 18], IL-6 [19, 20], and tumor necrosis factor-α [21, 22]. In addition, dietary and behavioral patterns commonly observed in psychiatric patients may also contribute to the pathogenesis of osteoporosis.
Cigarette smoking is more common within psychiatric populations [23]. It increases the risk for the onset of MDD [24, 25] and has repeatedly been shown to negatively influence bone mass in cross-sectional studies of both men and women [26, 27]. Likewise, depression and excessive alcohol consumption are common comorbidities, and alcohol abuse is a recognized risk factor for osteoporosis [28, 29]. Finally, although with a less well-defined cause-effect relationship, changes in food consumption are typically associated with depression and certain nutrients reported to be deficient in patients with MDD are required in the maintenance of good bone health [30].
The Skeletal Serotonergic System
Biogenic amine transporters are important regulators, controlling the synaptic and extracellular concentrations of these amines in the CNS by their high-affinity reuptake from the extra- to the intracellular milieu. They are also major targets for many antidepressant drugs that inhibit their activity, thereby potentiating the effect of the biogenic amines. The skeletal role of these drugs, in particular that of the SSRIs, which are targeted to the serotonin transporter, has recently attracted substantial interest because of its potential impact on osteoporosis and resultant fractures.
Serotonin (5-hydroxytryptamine, 5-HT) is a monoamine neurotransmitter known best for its role in the CNS, gastrointestinal (GI) tract, and cardiovascular system. In the CNS, it is produced by presynaptic neurons, released into the synaptic gap and activates pre- and postsynaptic 5-HT receptors, thus influencing a handful of behavioral, physiologic and cognitive functions [31, 32]. In the GI tract, 5-HT is synthesized and secreted by enterochromaffin cells and diffuses to enteric nerve endings to stimulate peristalsis [33]. In the CNS and GI tract, the duration and intensity of serotonergic activity are regulated by the sodium chloride-dependent 5-HT transporter (5-HTT) [34]. In the cardiovascular system, 5-HT is primarily taken up by platelets via 5-HTT and stored in dense granules [35]. It is released by activated platelets and induces blood vessel constriction or dilation [36], and smooth muscle cell hypertrophy and hyperplasia [37].
Osteoblasts, osteocytes, and osteoclasts express functional 5-HT receptors and 5-HTT [38]. In osteoblasts, 5-HT receptor agonists influence cell proliferation, potentiate parathyroid hormone-induced increase in activator protein-1 activity, and modulate the cellular response to mechanical stimulation. In osteocytes, 5-HT increases whole-cell cyclic adenosine monophosphate and prostaglandin E2 levels, which are also involved in the transduction of mechanical stimuli [39]. In osteoclasts, 5-HT and 5-HTT have been shown to affect differentiation, but not activity [38].
What is the source of 5-HT in bone tissue? The CNS does not appear to be a likely source of 5-HT available to bone cells, as the blood-brain barrier is impermeable to 5-HT and serotonergic neurons have not yet been demonstrated in the skeleton. As in the case of other neurotransmitters, such as endocannabinoids [15], 5-HT could be synthesized and released by bone cells and act in an autocrine/paracrine mode. mRNA transcripts for tryptophan hydroxylase-1 (Tph1), the rate-limiting enzyme in 5-HT synthesis, have been detected in osteoblast and osteocyte cell lines [40]. Most of the organism’s 5-HT is produced in the GI tract and stored in dense granules in platelets. Because 5-HT from this source is released only upon platelet activation [35], it is an unlikely activator of the bone cell 5-HT receptors. However, a small fraction of the GI-derived 5-HT remains in the serum [41]. Because the osteoblastic Tph1 mRNA level is more than 1000-fold lower than the duodenal, the serum “free” 5-HT could still be the major source for the skeleton. It has been suggested that the serum 5-HT is a negative regulator of osteoblast proliferation, bone formation, and bone mass [42••]. Alleviating the 5-HT tonic inhibition of bone formation using a peripherally selective Tph1 inhibitor has a bone anabolic effect in normal and osteopenic mice [43]. Still, in view of the expression of Tph1 in osteoblasts, the local production of 5-HT should be determined directly in osteoblast cultures and bone tissue following pharmacologic and genetic ablation of Tph1 and possibly other genes involved in 5-HT metabolism.
What is the physiologic role of the skeletal serotonergic system? The diversity of actions of 5-HT results from the occurrence of multiple 5-HTRs, which are divided into seven classes based on their signaling pathways [44•]. Of these, only 5-HT1AR, 5-HT2AR, 5-HT1BR, and 5-HT2BR are expressed in osteoblasts and only the expression of 5-HT2BR is increased during osteoblast differentiation. Mice deficient of 5-HT2BR have accelerated age-related low turnover bone loss, secondary to impaired osteoblast recruitment and proliferation [45]. In line with these findings, rats treated with 5-HT have increased BMD [46]. By contrast, mice deficient of osteoblastic 5-HT1BR have a high bone mass phenotype, secondary to increases in osteoblast number and bone formation [42••]. Disruption of the 5-HTT gene or pharmacologic inhibition of 5-HTT by SSRIs leads to a low bone mass phenotype in growing mice [38]. These apparently paradoxical findings may suggest that 5-HT has different skeletal effects. It has been recently suggested that the peripheral and central 5-HT signaling have opposite effects on bone. Peripherally, 5-HT directly activates osteoblastic 5-HTR(s) to inhibit bone formation, whereas centrally it inhibits the sympathetic nervous system, thus alleviating the negative adrenergic tone on osteoblasts [47••].
SSRIs and Bone Health
SSRIs are 5-HTT inhibitors. Use of the term “reuptake” was initially coined to describe the phenomenon in the CNS, whereby the serotonergic system is downregulated through the reuptake of 5-HT by its producer, the presynaptic neuron. The principal component of this process is 5-HTT (also referred to as SERT), which is also expressed by peripheral 5-HT target cells, including osteoblasts and osteocytes [38, 44•].
In humans, SSRIs have emerged as the first-line agents in the treatment of depressive disorders. Most clinical studies report that antidepressants in general, but mainly SSRIs, are associated with low BMD and a dose-dependent increase in the risk of fractures and low bone mass in children [48]. The reason for these deleterious effects is not known but may be linked to direct and indirect 5-HT effects on bone cells and the risk of falls, which is increased in SSRI users especially after prolonged administration [48]. Importantly, it appears that the negative skeletal effects of peripheral SSRI-induced increase in 5-HT outweighs the skeletal benefits resulting from the enhanced central 5-HT antidepressant and antisympathetic activity. Thus, physicians treating growing children and elderly depressive patients should be aware of the unfavorable respective short- and long-term consequences of SSRIs on BMD and fracture risk.
Conclusions
An increasing number of studies, including recent meta-analyses, suggest that osteoporosis is disproportionately prevalent among patients with depressive disorders. In a recent study using a mouse model for depression, we demonstrated a causal relationship between depressive-like behavior and bone loss, which could be prevented by tricyclic antidepressant treatment. The depression-induced bone loss was associated with increases in skeletal norepinephrine and serum corticosterone levels. Bone loss, but not the depressive behavior, could be blocked by a β blocker, suggesting an important role for adrenergic signaling in communicating depressive brain-to-bone signals. Both depression and bone antianabolic sympathetic tone are alleviated by increasing central 5-HT levels.
However, although SSRIs constitute the first-line of antidepressant agents, presumably by increasing extracellular 5-HT levels in the brain, they have deleterious skeletal effects. Several studies demonstrate a skeletal serotonergic system consisting of 5-HTRs in osteoblasts and osteocytes as well as the 5-HTT, the main transmembrane protein targeted by SSRIs. Increased 5-HTR activation restrains osteoblastic activity, thus leading to a negative bone remodeling balance. It appears that the negative skeletal effects of the peripheral SSRI-induced increase in 5-HT outweigh the skeletal benefits resulting from the enhanced central 5-HT antidepressant and antisympathetic activity.
Taken together, these studies portray major depression as an important risk factor for osteoporosis. However, antidepressant therapy, in particular by SSRIs, has to be evaluated not only in view of the causal relationship between depression and bone loss, but also vis-à-vis its skeletal adverse effects. Practically, it is recommended that patients with depressive disorders undergo a careful skeletal evaluation and receive timely antiosteoporotic therapy, especially when SSRI treatment is prescribed.
References
Papers of particular interest, published recently, have been highlighted as:• Of importance •• Of major importance
•• Yirmiya R, Bab I: Major depression is a risk factor for low bone mineral density: a meta-analysis. Biol Psychiatry 2009, 66:423–432. This article reports the most comprehensive meta-analysis demonstrating a significant correlation between MDD and low bone mass, which is stronger in women than in men and in pre- than postmenopausal women. The article further provides a review of the studies dealing with depression and bone and portrays depression as a risk factor for osteoporosis.
Murray CJ, Lopez AD: Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997, 349:1498–1504.
Riggs BL, Khosla S, Melton LJ 3rd: Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002, 23:279–302.
Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Arlington, VA: American Psychiatric Association; 1994.
• Cizza G, Primma S, Coyle M, et al.: Depression and osteoporosis: a research synthesis with meta-analysis. Horm Metab Res 2010, 42:467–482. This study shows that the low BMD is associated with MDD and involves the anterior-posterior spine, femoral neck, and total femur, suggesting that depression-associated low bone mass is site independent.
• Wu Q, Magnus JH, Liu J, et al.: Depression and low bone mineral density: a meta analysis of epidemiologic studies. Osteoporos Int 2009, 20:1309–1320. This meta-analysis found that depression is associated with a significantly decreased BMD, with a substantially lower BMD in depressed women and in cases of clinical depression.
•• Yirmiya R, Goshen I, Bajayo A, et al.: Depression induces bone loss through stimulation of the sympathetic nervous system. Proc Natl Acad Sci U S A 2006, 103:16876–16881. Using CMS, a mouse model for depression, this article suggests a causal relationship whereby depression induces bone loss, mainly by inhibiting bone formation. The article further portrays the sympathetic nervous system as the main pathway communicating depressive signals to the skeleton.
• Dorn LD, Susman EJ, Pabst S, et al.: Association of depressive symptoms and anxiety with bone mass and density in ever-smoking and never-smoking adolescent girls. Arch Pediatr Adolesc Med 2008, 162:1181–1188. Consistent with the increased association between depression and low bone mass in premenopausal women, this article demonstrates an association between depressive symptoms and low bone density in adolescent girls, implicating depression in the inhibition of bone mass accrual.
Gold DT, Solimeo S: Osteoporosis and depression: a historical perspective. Curr Osteoporos Rep 2006, 4:134–139.
Ilias I, Alesci S, Gold PW, Chrousos GP: Depression and osteoporosis in men: association or casual link? Hormones (Athens) 2006, 5:9–16.
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC: Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 1998, 102:274–282.
Elefteriou F: Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys 2008, 473:231–236.
Lu XY: The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 2007, 7:648–652.
Karsenty G: Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 2006, 4:341–348.
Tam J, Trembovler V, Di Marzo V, Petrosino S, et al.: The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J 2008, 22:285–294.
Hill MN, Carrier EJ, McLaughlin RJ, et al.: Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem 2008, 106:2322–2336.
Bajayo A, Goshen I, Feldman S, et al.: Central IL-1 receptor signaling regulates bone growth and mass. Proc Natl Acad Sci U S A 2005, 102:12956–12961.
Goshen I, Kreisel T, Ben-Menachem-Zidon O, et al.: Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 2008, 13:717–728.
Adler UC, Marques AH, Calil HM: Inflammatory aspects of depression. Inflamm Allergy Drug Targets 2008, 7:19–23.
Mundy GR: Osteoporosis and inflammation. Nutr Rev 2007, 65:S147–S151.
Yu B, Becnel J, Zerfaoui M, et al.: Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther 2008, 327:316–323.
Roggia C, Gao Y, Cenci S, et al.: Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A 2001, 98:13960–13965.
Poirier MF, Canceil O, Baylé F, et al.: Prevalence of smoking in psychiatric patients. Prog Neuropsychopharmacol Biol Psychiatry 2002, 26:529–537.
Klungsøyr O, Nygård JF, Sørensen T, Sandanger I: Cigarette smoking and incidence of first depressive episode: an 11-year, population-based follow-up study. Am J Epidemiol 2006, 163:421–432.
Steuber TL, Danner F: Adolescent smoking and depression: which comes first? Addict Behav 2006, 31:133–136.
Lorentzon M, Mellström D, Haug E, Ohlsson C: Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab 2007, 92:497–503.
Gerdhem P, Obrant KJ: Effects of cigarette-smoking on bone mass as assessed by dual-energy X-ray absorptiometry and ultrasound. Osteoporos Int 2002, 13:932–936.
Chakkalakal DA: Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res 2005, 29:2077–2090.
Turner RT, Sibonga JD: Effects of alcohol use and estrogen on bone. Alcohol Res Health 2001, 25:276–281.
Zalloua PA, Hsu YH, Terwedow H, et al.: Impact of seafood and fruit consumption on bone mineral density. Maturitas 2007, 56:1–11.
Kroeze WK, Kristiansen K, Roth BL: Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem 2002, 2:507–528.
Raymond JR, Mukhin YV, Gelasco A, et al.: Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther 2001, 92:179–212.
Talley NJ: Serotoninergic neuroenteric modulators. Lancet 2001, 358:2061–2068.
Wade PR, Chen J, Jaffe B, et al.: Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 1996, 16:2352–2364.
McNicol A, Israels SJ: Platelet dense granules: structure, function and implications for haemostasis. Thromb Res 1999, 95:1–18.
Egermayer P, Town GI, Peacock AJ: Role of serotonin in the pathogenesis of acute and chronic pulmonary hypertension. Thorax 1999, 54:161–168.
Lee SL, Wang WW, Lanzillo JJ, Fanburg BL: Serotonin produces both hyperplasia and hypertrophy of bovine pulmonary artery smooth muscle cells in culture. Am J Physiol 1994, 266:L46–L52.
Warden SJ, Bliziotes MM, Wiren KM, et al.: Neural regulation of bone and the skeletal effects of serotonin (5-hydroxytryptamine). Mol Cell Endocrinol 2005, 242:1–9.
Cherian PP, Cheng B, Gu S, et al.: Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem 2003, 278:43146–43156.
Bliziotes M, Eshleman A, Burt-Pichat B, et al.: Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone 2006, 39:1313–1321.
Rand M, Reid G: Source of ‘serotonin’ in serum. Nature 1951, 168:385.
•• Yadav VK, Ryu J-H, Suda N, et al.: Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 2008, 135:825–837. This article shows that gut-derived 5-HT reaches bone via the blood circulation and inhibits osteoblast proliferation, bone formation, and bone density by activating osteoblastic Htr1b receptor and CREB. It is the first report implicating gut- rather than bone cell-derived 5-HT in the regulation of skeletal remodeling.
Yadav VK, Balaji S, Suresh PS, et al.: Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med 2010, 16:308–312.
• Collet C, Schiltz C, Geoffroy V, et al.: The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J 2008, 22:418–427. This article reports a low bone mass phenotype in female Htr2b receptor null mice, due to restrained osteoblast recruitment. Compared with Yadav et al. [42••], these findings suggest an opposite skeletal role for 5-HT, namely, stimulation of bone formation and bone mass.
Gustafsson BI, Westbroek I, Waarsing JH, et al.: Long-term serotonin administration leads to higher bone mineral density, affects bone architecture, and leads to higher femoral bone stiffness in rats. J Cell Biochem 2006, 97:1283–1291.
Bonnet N, Bernard P, Beaupied H, et al.: Various effects of antidepressant drugs on bone microarchitectecture, mechanical properties and bone remodeling. Toxicol Appl Pharmacol 2007, 221:111–118.
•• Yadav VK, Oury F, Suda N, et al.: A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 2009, 138:976–989. This article shows that 5-HT in the brain downregulates the skeletal antianabolic sympathetic tone, thus assigning opposite skeletal effects to central and peripheral 5-HT signaling.
Ziere G, Dieleman JP, van der Cammen TJ, et al.: Selective serotonin reuptake inhibiting antidepressants are associated with an increased risk of nonvertebral fractures. J Clin Psychopharmacol 2008, 28:411–417.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bab, I., Yirmiya, R. Depression, Selective Serotonin Reuptake Inhibitors, and Osteoporosis. Curr Osteoporos Rep 8, 185–191 (2010). https://doi.org/10.1007/s11914-010-0026-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-010-0026-z