Abstract
Purpose of the Review
Acute erythroleukemia (AEL) is a rare form of acute myeloid leukemia recognized by erythroblastic proliferation. Many controversies remain around diagnosis influencing prognostic and therapeutic implications relating to this unique leukemia subset.
Recent Findings
The 2016 WHO classification includes more clear and restrictive diagnostic criteria for AEL. Primary acute erythroid leukemia is associated with complex and high-risk karyotypes including chromosomes 5q and 7q abnormalities. Mutational data shows that AEL is characterized by far lower NPM1 and FLT3-ITD mutation rates and higher mutational rates in TP53 compared with other AML subtypes. Hypomethylating agents have shown therapeutic value in AEL.
Summary
In this article, we discuss the evolving diagnostic concepts of erythroleukemia, genomics, clinical outcome, and promising therapeutic targets through an appraisal of the current literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Erythroleukemia was first described as a leukemic condition by an Italian hematologist Giovanni Di Guglielmo in 1917 as a syndrome composed of immature erythroid and myeloid elements [1]. Di Guglielmo subsequently distinguished between two variants of the disease, including a pure acute (Di Guglielmo disease) and a more chronic (Di Guglielmo syndrome) form. In 1951, William Dameshek grouped Di Guglielmo syndrome under the broad umbrella of myeloproliferative diseases based on the hypothesis that a common hematopoietic cell of origin was responsible for these disorders [2]. By the 1970s, acute erythroleukemia (AEL) was no longer considered as a myeloproliferative disorder and was included in the first report of the French-American-British Cooperative Group (FAB) classification for acute leukemias [3]. The 1976 FAB classification defined AEL based upon the overall percentage of myeloblasts (Table 1). However, owing to the overwhelming erythroid component in this disease, a diagnosis of AEL, based on greater than or equal to 30% of the bone marrow elements being myeloblasts, could not be established very often. In the 1985 FAB revised its criteria by requiring at least 30% of the non-erythrocytic elements to be blasts and defined AML6 as a proliferation of > 50% erythroblasts and > 30% of myeloblasts within non-erythroid cells [4]. The 1985 FAB classification recommended that if the percentage of blasts did not reach 30% limit, the disease should be classified as a subtype of myelodysplastic syndrome (MDS) with increased erythroid component.
Over the next decade, several reports described patients presenting with a proliferation of immature erythroid cells that could be better recognized with availability of antibodies for erythroid antigens and led to description of pure erythroid leukemia that was first proposed by Kowal-Vern et al. in 1992 [5]. Patients with erythroid hyperplasia (> 50%) were classified according to blast count among non-erythroid cells (> 30% for M6a and < 30% for M6b) and proerythroblasts among erythroid cells (< 30% for M6a and > 30% for M6b). In 1998, a mixed subtype M6c was added to the classification and included patients who had 30% or more myeloid blasts among nonerythroid cells and 30% or more proerythroblasts among erythroid cells [6]. The 2001 World Health Organization (WHO) classification lowered required blast count for all types of AML from 30 to 20%, which lowered the blast count defining AEL to 20% of the nonerythroid cells (Table 1). The 2001 WHO classification categorized acute erythroid leukemia into two subtypes: erythroid/myeloid leukemia (similar to FAB M6a) and primary erythroid leukemia (similar to FAB M6b) [7]. Under this classification, erythroid/myeloid leukemia was defined by the following two criteria: [1] erythroid cells comprising ≥ 50% of total nucleated marrow cells and [2] myeloblasts comprising ≥ 20% of non-erythroid cells. Pure erythroid leukemia (PEL) was defined by maturation-arrested primitive erythroblasts making up at least 80% of nucleated marrow cells.
In the 2008 WHO classification of AML, the category of AML with myelodysplasia-related changes (AML-MRC) was introduced [8, 9]. This group includes all cases with blasts comprising 20% or more of all bone marrow cells and the presence of either morphologic evidence of significant multilineage dysplasia, specific myelodysplastic syndrome (MDS)-related cytogenetic abnormalities, or a history of MDS or a myelodysplastic/myeloproliferative neoplasm (MDS/MPN), irrespective of the presence of erythroid hyperplasia [9]. According to this classification scheme, the vast majority of AEL cases with blasts comprising 20% or more of all bone marrow cells also fulfills these criteria and are classified as AML-MRC, whereas cases with blasts comprising less than 20% of all cells but 20% or more of the non-erythroid cells are classified as AEL. Moreover, if bone marrow blasts comprise less than 20% of nonerythroid cells, the case is then classified as MDS, not AEL. In effect, the distinction of AEL from MDS or AML-MRC with erythroid hyperplasia and is based solely on the number of blasts, calculated as the proportion of nonerythroid cells in AEL, but as the proportion of total bone marrow cells in MDS and AML-MRC. Diagnostic confusion had also arisen in regard to the diagnosis of pure erythroid leukemia versus WHO categories of therapy-related myeloid neoplasm and AML with myelodysplasia-related changes, with some authors incorrectly suggesting that the presence of an MDS-associated cytogenetic abnormality or history of MDS would move a case otherwise meeting criteria for AEL into the category of AML with MRC.
In the recent update in 2016, the WHO classification has eliminated the category of erythroid/myeloid type of acute erythroleukemia and only retains PEL as a subtype of AML, NOS [10]. Other cases with erythroid hyperplasia and increased blasts under 20% should be placed into the appropriate MDS category based on the percentage of blasts among all cells counted. The non-erythroid blast cell count has been completely eliminated. For the 2016 WHO definition of PEL, the only type of acute leukemia with bona fide erythroid differentiation, at least 80% marrow erythroid precursors are required and at least 30% should be proerythroblasts. In cases with a history of prior therapy, a diagnosis of therapy-related myeloid neoplasm should be made, although a modifier of pure erythroid leukemia is recommended. AML MRC is differentiated from pure erythroid leukemia by the presence of 20% or more myeloblasts.
Clinical Presentation
AEL is a rare disease and most clinical reports of it include both PEL and the historic types of AEL that are no longer included in the WHO classification. In these reports, AEL accounts for 3–5% of all AML cases and appears to be more common in males [11]. Most patients are elderly and some studies suggest a bimodal age distribution, with a smaller peak below 40 years of age and a more definitive and broader peak in the seventh decade of life [6]. Acute erythroleukemia is also very rare in children. In one report from the Children’s Oncology Group, patients with FAB-classified AEL comprised 2.3% of all patients with AML. AEL was associated with lower white blood cell count, the presence of chromosome 7 abnormalities, and worse response to treatment and survival compared with other FAB subtypes of AML [12]. Congenital erythroleukemia is exceedingly rare, with only six cases reported in the literature (11.)
Most commonly, patients with AEL present with symptoms due to pancytopenia such as fatigue, mucocutaneous bleeding, and infections. Anemia is present in most patients and is often severe (mean, 7.5 g/dl) [13]. Thrombocytopenia and neutropenia are also common and most patients have leukopenia [13]. Organomegaly is common and varies between 20 and 40% of the series [14]. Extramedullary disease and CNS involvement are rare [15]. Peripheral blood finding may also include a high level of schistocytes and nucleated red cells and pseudo Pelger–Huet neutrophils. The most frequently observed abnormalities include spiculated red cells (echinocytes, acanthocytes), schistocytes, dacriocytes, and basophilic stippling in more than 40% of cases [16]. Additional findings in peripheral blood include dysplastic neutrophils, as well as giant and hypogranular platelets and circulating micromegakaryocytes [16]. Almost half of the patients have no significant blasts in peripheral blood.
Morphologic and Immunophenotypic Findings of PEL
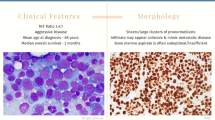
As now defined, the bone marrow in patients with erythroleukemia (specifically PEL) are typically hypercellular and are composed of sheets of leukemic cells that replace sheets of marrow (Figs. 1a and b). Blasts are large with round nuclei and often one or multiple prominent nucleoli and deeply basophilic cytoplasm (Fig. 1a). The cytoplasm often contains vacuolization and lack granules. Erythroid cells often show dysplasia. Megakaryocytes, when present, were often dysplastic and characterized by small monolobated forms, micromegakaryocytes, and/or hyperchromatic forms [17•]. Erythrophagocytosis by pathological erythroblasts has been described [18]. Interestingly, Park et al. described 7 cases of PEL with extensive necrosis [19]. Potential diagnostic pitfalls for PEL include megaloblastic leukemia, hemolytic leukemia, acute undifferentiated leukemia, other types of AML myeloid neoplasms with abundant erythroid precursors, and non-hematopoietic malignancies such as carcinoma and sarcoma.
Immunophenotypic studies are necessary but can be challenging in the evaluation of PEL. Blasts are typically negative for CD34 and HLA-DR, while CD117 is partially or weakly positive and some may show CD33 expression [19]. Further evaluation with erythroid markers is often necessary. Anti-glycophorin A is the most widely used antibody for diagnosing PEL by flow cytometry. In one series, glycophorin A was positive in 78% of PEL cases and in only 3% of non-PEL cases, but it can be negative as well [20] (Fig. 1c). CD71 (transferrin receptor) is also found in AEL, but it is nonspecific and can be positive in other subtypes of AMLs [21] (Fig. 1d). Erythroblasts may also express CD36, a thrombospondin receptor, but this marker is also expressed in megakaryocytes and monocytes [22]. E-cadherin is another marker that is frequently helpful in clinical diagnosis of PEL.
GATA1, a critical transcription factor for erythroid and megakaryocytic development, is a sensitive and specific nuclear marker for erythroid and megakaryocytic precursors. Lee et al. found that using a rabbit monoclonal antibody against GATA1, GATA1 consistently marked the blast populations of pure erythroid leukemia [23•] (Fig. 1e). Of note, GATA1 staining alone cannot distinguish between the blast forms of erythroid and megakaryocytic lineages and needs to be combined with morphologic assessment as well as additional studies that are already routinely used. Both acute pure erythroid leukemia and acute megakaryoblastic leukemia exhibit strong nuclear GATA1 reactivity and will require additional markers, such as CD61 and CD71, for subclassification [23•]. Some cases of pure erythroid markers may show expression of P53 [24] (Fig. 1f).
Molecular and Cytogenetic Findings
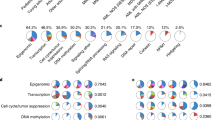
There are no recurrent cytogenetic or other molecular aberrations that appear to be specific for AEL. Karyotypically, de novo pure erythroid leukemia appears to uniformly demonstrate an abnormal karyotype. The karyotype is generally complex (≥ 3 abnormalities), with most cases showing highly complex genetic alterations (> 10 abnormalities). Many, though not all, pure erythroid leukemia cases contain recurring myeloid-associated cytogenetic abnormalities, such as del(5q), −7/del(7q), and −17/17p deletions [25•, 26]. Table 2 lists frequencies of complex karyotype in AEL and PEL.
Earlier investigational studies had associated AEL with a high frequency of mutations with mutational profiles significantly different from other AML subtypes. AEL is characterized by far lower NPM1 and FLT3-ITD mutation rates and higher mutational rates in TP53 when compared with other AML subtypes [27, 28] (Table 2). Iacobucci et al. have identified recurrent mutations in multiple genes involved in cell cycle/tumor suppression, cohesin complex formation, RNA splicing, transcription, signaling, DNA methylation, and chromatin modification [29]. Rose et al. reported on molecular mutation data in a cohort of 166 AEL patients and showed that TP53 was the only gene occurring at a higher frequency within AEL as compared with the remaining overall AML cohort [30•]. Recent data reveal an especially high prevalence of at least two TP53 abnormalities (both mutations and aberrant or deleted chromosome 17p) in > 90% of PEL [31••]. Allelic frequencies of TP53 mutations suggested that a founder TP53 mutation was always present, suggesting a crucial role of TP53 in leukemic transformation [31••].
In their study of 159 childhood and adult AEL, Iacobucci et al. found that the mutational spectrum of adult AEL was intermediate between those of MDS and AML [32••]. Of note, 85% of cases included in their study were described as M6a and reclassified under 2016 WHO criteria as MDS, AML NOS (non-erythroid subtype) and AML-MRC and only 5% of cases were PEL. Specifically, they found a lower frequency of canonical genes mutated in AML, such as FLT3 and NPM1 in AEL, compared with non-erythroid AML, but they were more common than in MDS. Conversely, MDS-associated mutations such as in SF3B1 and ASXL1 were less frequent in AEL compared with MDS but more common than in non-erythroid AML. Compared with childhood MDS, childhood AEL was characterized by a higher frequency of mutations in FLT3 and WT1 and a lower frequency of mutations in GATA2 and ASXL1. Mutation profile patterns varied in pediatric and adult AEL with NUP98-fusions, PTPN11, GATA1, and UBTF mutations more frequent in pediatric AEL and TP53 and KMT2A mutations predominant in adult AEL. Additionally, 33% of cases harbored signaling pathway gene mutations, 3 classes of which were found to be targetable by in vivo and in vitro studies; ALK mutations to crizotinib, tyrosine kinase domain mutations of NTRK1 to entrectinib, and JAK-STAT, mTOR, and PI3K pathway targeting to JAK2 inhibitor ruxolitinib [32••]. Similarly, isolated case reports of fusion genes such as NFIA-CBFA2T3 have been described [27, 33].
Therapy and Outcome
Due to the rarity of the disease (2–5% of all leukemias), there are no prospective clinical trials available in patients with AEL and most do not discriminate between PEL and older types of AEL. Patients with AEL are usually treated similarly to patients with other types of AML [11]. When treated with intensive chemotherapy, the median overall survival (OS) of AEL patients range between 7.6 and 9 months [34]. Santos et al. reported on clinical outcomes of AML M6 in 91 patients and found no significant difference in survival between M6 and other AML types [35]. Interestingly, subtype of AML M6 (6a vs 6b) was not an independent prognostic factor. The authors concluded that AML-M6 by itself did not carry additional prognostic import. Whether prognosis in AEL is linked solely to its association with unfavorable karyotype or is as result of additional disease-specific characteristics is not well understood. Studies that have looked at PEL specifically note a median overall survival ranging from 1.4 to 6.6 months [17•].
Recent emphasis has been on evaluating efficacy of hypomethylating agents (HMAs) in treating TP53 mutated leukemia. In a study of 36 AEL patients (81% considered as MDS using 2016 WHO classification), decitabine showed comparable overall survival when compared with cytarabine-based agents [36]. Montalban-Bravo found in their cohort that survival of both AEL and PEL patients is unfavorable and HMAs did not improve outcome, in contrast to the recent data reporting high response rates to decitabine in patients with TP53-mutant AML [37]. Almeida et al. found that in their largest series to date of 217 AEL patients treated with hypomethylating agents (HMA) showed an overall response rate of 46% in the front-line setting, with a complete response (CR) rate of 30% [38••]. Standard induction led to a higher OS rate when compared with first-line HMA but similar progression-free survival. Initial responses were seen after a median of 79 days, but the best responses were documented after a median of 120 days, confirming that responses improve with continued treatment and reinforcing the importance of not interrupting treatment too early due to a lack of response.
Although HMAs may represent a good treatment option for patients not eligible for transplant, allogeneic transplant improves outcome of patients with AEL and should be utilized when possible. Novel therapies based on a more detailed molecular pathway in AEL may improve outcome.
Conclusions
Erythroleukemia is a distinct form of AML characterized by unfavorable risk karyotype and disease features, especially as defined in the updated WHO 2016 diagnostic criteria. Controversies remain around diagnosis, and some authors have questioned the 30% proerythroblast requirement, while others advocate for a diagnosis of AEL with lower erythroid cell count [24]. Many cases of AEL evolve from prior MDS or occur in the setting of prior therapy; however, therapy-related cases should be diagnosed as therapy-related myeloid neoplasms. Patients with prior MDS may have an increase in marrow erythroid precursors secondary to therapy [39]. Studies have provided valuable insights into the genetic landscape of AEL and may pave the way for the transformation from a morphologic/phenotypically based classification to an enhanced molecular classification of prognostic and therapeutic relevance. Further refinements in future definitional criteria are expected, incorporating emerging mutation and chromosomal data garnered from studies inspired by a growing interest and recognition of PEL.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schwartz SO, Critchlow J. Erythremic myelosis (DI Guglielmo’s disease); critical review with report of four cases, and comments on erythroleukemia. Blood. 1952;7(8):765–93.
Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372–5.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG, Gralnick HR, et al. Proposals for the classification of the acute leukemias. Br J Haematol. 1976;33:451–8.
Villeval JL, Cramer P, Lemoine F, Henri A, Bettaieb A, Bernaudin F, et al. Phenotype of early erythroblastic leukemias. Blood. 1986;68(5):1167–74.
Kowal-Vern A, Cotelingam J, Schumacher HR. The prognostic significance of proerythroblasts in acute erythroleukemia. The prognostic significance of proerythroblasts in acute erythroleukemia. Am J Clin Pathol. 1992;98(1):34–40.
Mazzella FM, Kowal-Vern A, Shrit MA, Wibowo AL, Rector JT, Cotelingam JT. et alR. Acute erythroleukemia: evaluation of 48 cases with reference to classification, cell proliferation, cytogenetics, and prognosis. Am J Clin Pathol. 1998;110(5):590–8.
Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:626–9.
Brunning RD, Matutes E, Flandrin G, Jaffe ES, Harris NL, Stein H, Vardiman JW. et al. Acute myeloid leukaemia not otherwise categorised., World Health Organization Classification of Tumours, 2001 Lyon, FranceI ARC Press(pg. 91-105)
Arber DA, Brunning RD, Orazi A, Porwit A, Peterson L, Thiele J, et al. Acute myeloid leukemia, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classifiction of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. p. 134–6.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Santos FPS, Bueso-Ramos CE, Ravandi F. Acute erythroleukemia: diagnosis and management. Expert Rev Hematol. 2010;3(6):705–18.
Barnard DR, Alonzo TA, Gerbing RB, Lange R, Woods WG. Children’s Oncology Group Comparison of childhood myelodysplastic syndrome, AML FAB M6 or M7, CCG 2891: report from the Children's Oncology Group. Pediatr Blood Cancer. 2007;49(1):17–22.
Hasserjian RB, Zuo Z, Garcia C, Tang G, Kasyan A, Luthra R, et al. Acute erythroid leukemia: a reassessment using criteria refined in the 2008 WHO classification. Blood. 2010;115(10):1985–92.
Olopade OI, Thangavelu M, Larson RA, Mick R, Kowal-Vern A, Schumacher HR, et al. Clinical, morphologic, and cytogenetic characteristics of 26 patients with acute erythroblastic leukemia. Blood. 1992;80:2873–82.
Li H, Hasserjian RP, Kroft SH, Harrington AM, Wheaton SE, Pildain A, et al. Pure Erythroid Leukemia and Erythroblastic Sarcoma Evolving From Chronic Myeloid Neoplasms. Am J Clin Pathol. 2016;145(4):538–51.
Domingo-Claros A, Larriba I, Rozman M, Irriguible D, Vallespí T, Aventin A, et al. Acute erythroid neoplastic proliferations. A biological study based on 62 patients. Haematologica. 2002;87(2):148–53.
• Reinig EF, Greipp PT, Chiu A, Howard MT, Reichard KK. De novo pure erythroid leukemia: refining the clinicopathologic and cytogenetic characteristics of a rare entity. Mod Pathol. 2018;31:705–17 This series of pure erythroid leukemia illustrates high rate of very complex karyotype in this disease.
Sondergaard-Petersen H Erythrophagocytosis by pathological erythroblasts in the Di Guglielmo syndrome. A study of 18 cases. Scand J Haematol. 1974;13(4):260-5. (19). Histopathology 2017, 71, 316-321
Hasserjian RP. Erythroleukemia and Its Differential Diagnosis. Surg Path Clin. 2013;6:641–59.
Greaves MF, Sieff C, Edwards PA. Monoclonal antiglycophorin as a probe for erythroleukemias. Blood. 1983;61(4):645–51.
Gupta AD, Dhond SR. Phenotypic heterogeneity of erythroblasts in erythroblastic leukemia revealed by monoclonal antibodies. Am J Hematol. 1988;29(1):12–7.
Garand R, Duchayne E, Blanchard D, Robillard N, Kuhlein E, Fenneteau O, et al. Minimally differentiated erythroleukaemia (AML M6 ‘variant’): a rare subset of AML distinct from AML M6. Groupe Français d'Hématologie Cellulaire. Br J Haematol. 1995;90(4):868–75.
• Lee WY, Weinberg OW, Pinkus GS. GATA1 Is a sensitive and specific Nuclear marker for erythroid and megakaryocytic lineages. Am J Clin Pathol. 2017;147(4):420–6 This study shows that blasts in acute erythroleukemia exhibit intense nuclear GATA1 positivity.
Wong E, Ling V, Westerman D, Morgan S, Juneja S. How unique is pure erythroid leukaemia? A retrospective analysis of seven cases and review of the literature. JCP. 2015;68:301–5.
• Wang SA, Patel KP, Pozdnyakova O, Peng J, Zuo Z, Dal Cin P, et al. Acute erythroid leukemia with <20% bone marrow blasts is clinically and biologically similar to myelodysplastic syndrome with excess blasts. Mod Pathol. 2016;29:1221–31 The results of this study supports WHO change in definition of acute erythroid leukemia.
Lessard M, Struski S, Leymarie V, Flandrin G, Lafage-Pochitaloff M, Mozziconacci MJ, et al. Cytogenetic study of 75 erythroleukemias. Cancer Genet Cytogenet. 2005;163:113–22.
Grossmann V, Bacher U, Haferlach C, Schnittger S, Pötzinger F, Weissmann S, et al. Acute erythroid leukemia (AEL) can be separated into distinct prognostic subsets based on cytogenetic and molecular genetic characteristics. Leukemia. 2013;27(9):1940–3.
Cervera N, Carbuccia N, Garnier S, Guille A, Adélaïde J, Murati A, et al. Molecular characterization of acute erythroid leukemia (M6-AML) using targeted next-generation sequencing. Leukemia. 2016;30:966–70.
Ko PS, Liu YC, Yeh CM, Gau JP, Yu YB, Hsiao LT, et al. The uniqueness of morphological features of pure erythroid leukemia in myeloid neoplasm with erythroid predominance: A reassessment using criteria revised in the 2016 World Health Organization classification. PLoS One. 2017;12(2):e0172029.
• Rose D, Haferlach T, Schnittger S, Perglerová K, Kern W, Haferlach C. Subtype-specific patterns of molecular mutations in acute myeloid leukemia. Leukemia. 2017;31(1):11–7 This study illustrates mutation profile in acute erythroleukemia.
•• Montalban-Bravo G, Benton CB, Wang SA, Ravandi F, Kadia T, Cortes J, et al. More than 1 TP53 abnormality is a dominant characteristic of pure erythroid leukemia. Blood. 2017;129(18):2584–7 This study illustrates high rate of TP53 mutations in acute erythroleukemia.
•• Iacobucci I, Wen J, Meggendorfer M, Choi JK, et al. Genomic subtyping and therapeutic targeting of acute erythroleukemia. Nat Genet. 2019;51:694–704 This large study compares genomic features of pediatric and adult acute erythroleukemia.
Panagopoulos I, Micci F, Thorsen J, Haugom L, Buechner J, Kerndrup G, et al. Fusion of ZMYND8 and RELA genes in acute erythroid leukemia. PLoS One. 2013;8(5):e63663.
Colita A, Belhabri A, Chelghoum Y, Charrin C, Fiere D, Thomas X. Prognostic factors and treatment effects on survival in acute myeloid leukemia of M6 subtype: a retrospective study of 54 cases. Ann Oncol. 2001;12(4):451–5.
Santos F, Faderl S, Garcia-Manero G, Koller C, Beran M, O'Brien S, et al. Adult Acute Erythroleukemia: An Analysis of 108 patients treated at a single institution. Leukemia. 2009;23(12):227–80.
Greco R, Petrungaro A, Recchia AG, De Stefano L, Bossio S, Palummo A, et al. Treatment Acute Myeloid Leukemia Using Cytoreductive Chemotherapy Cytarabine (Ara-C) Followed Azacitidine (AZA) Maintenance: A Real Life Single Center Experience. Blood. 2016;128(22):5204.
Welch JS, Petti AA, Miller CA, Fronick CC, O'Laughlin M, Fulton RS, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. NEJM. 2016;375(21):2023–36.
•• Almeida AM, Prebet T, Itzykson R, Ramos F, Al-Ali H, Shammo J, et al. Clinical Outcomes of 217 Patients with Acute Erythroleukemia According to Treatment Type and Line: A Retrospective Multinational Study. Int J Mol Sci. 2017;18(4):837 This study supports use of HMAs in acute erythroleukemia.
Peng J, Hasserjian RP, Tang G, Patel KP, Goswami M, Jabbour EJ, et al. Myelodysplastic syndromes following therapy with hypomethylating agents (HMAs): development of acute erythroleukemia may not influence assessment of treatment response. Leuk Lymphoma. 2016;57(4):812–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Neither of the authors has any conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Weinberg, O.K., Arber, D.A. Erythroleukemia: an Update. Curr Oncol Rep 23, 69 (2021). https://doi.org/10.1007/s11912-021-01060-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01060-8