Abstract
Purpose of Review
The human epidermal growth factor receptor 2 (HER2) is an important prognostic and predictive biomarker in the breast cancer. The American Society of Clinical Oncology/College of American Pathology (ASCO/CAP) has published HER2 testing guidelines in breast cancer. We herein reviewed the HER2 testing guidelines in breast cancer with a focus on the application of the current guidelines.
Recent Findings
The continual investigation of HER2 testing in breast cancer has resulted in updates in the HER2 testing guidelines. The current guidelines focus on the uncommon clinical scenarios and emphasize the coordination between immunohistochemistry and in situ hybridization results, in an effort to improve clarity and accuracy.
Summary
The ASCO/CAP guidelines provide valuable recommendations to ensure the accurate evaluation of HER2 status in breast cancer patients through standardization. Additional studies, particularly those with long-term outcome data are still needed to validate the guideline recommendations, especially the uncommon cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction to HER2 in Breast Cancer
The human epidermal growth factor receptor 2 (HER2, also known as ERBB2) gene was discovered by several research groups independently in the 1980s [1,2,3,4]. The role of HER2 in the pathogenesis of human cancer was first revealed in breast cancer [5] and later in ovary, gastric, uterus, and other organs [6, 7]. When HER2 is overexpressed, it will form homodimers or heterodimers by interacting with other HER family members, especially HER3 [8,9,10,11]. Upon the dimerization, the intrinsic kinase activity of HER2 is stimulated, followed by the activation of multiple intracellular signal cascades, and ultimately involves cell proliferation, survival, differentiation, angiogenesis, invasion, and metastasis [12, 13].
HER2 overexpression/amplification is present in ~ 15–20% of breast cancers and it has been recognized as an important prognostic and predictive biomarker. In 1987, Slamon et al. identified the relationship between HER2 and the prognosis in breast cancer patients [5], and thereafter, the literature has consistently demonstrated that HER2 overexpression/amplification in breast cancers is associated with a worse prognosis [14,15,16,17]. The predictive value of HER2 was first revealed in breast cancer with the observation that HER2 status appeared to be associated with sensitivity or resistance to some endocrine and chemotherapy agents [18,19,20]. More importantly, HER2 status in breast cancer patients is now recognized as a critical test for selecting which patients will be appropriate for HER2 targeted therapy. The first HER2-targeted agent, trastuzumab is a monoclonal antibody that binds to the extracellular domain of HER2, suppressing its signaling activity and inducing antibody-dependent cell-mediated cytotoxicity. Clinical trials have demonstrated that a combination of trastuzumab and chemotherapy increase disease-free and overall survival [21,22,23,24]. Trastuzumab was first approved for use in patients with metastatic HER2-positive breast disease in 1998, for the adjuvant treatment of lymph node-positive breast cancer in 2006, and for the adjuvant treatment of lymph node-negative breast cancer in 2008 [25]. Other newly developed HER2 targeted drugs including pertuzumab, trastuzumab emtansine (T-DM1), lapatinib, and neratinib have also been evaluated, either alone or as a combined anti-HER2 approach, and have demonstrated an even more favorable outcome [26,27,28]; however, these treatments are costly with potential serious side effects [29]. Since effective HER2 targeted therapy has only been established in breast cancer patients with HER2 amplification/overexpression, accurate testing for HER2 in breast cancer is critical, and the American Society of Clinical Oncology (ASCO)/College of American Pathology (CAP) developed HER2 testing guideline recommendations.
HER2 Testing Guidelines in Breast Cancer Evolving Over the Years
In 2000, CAP published a consensus statement regarding HER2 testing in breast cancer patients [30]. ASCO subsequently recommended that HER2 needed to be tested in all patients with invasive breast cancer and published the recommendations in 2001 [31]. In 2007, in order to improve the accuracy of HER2 testing through standardization, the two professional societies published the first joint guidelines on HER2 testing in breast cancer [32•]. Based on the extensive literature review and the results of clinical trials, the ASCO/CAP HER2 testing guidelines have evolved through the years with two updates, in 2013 and again in 2018, in the attempt to reliably identify patients who will benefit from HER2 targeted therapy [33•, 34•]. Table 1 summarizes the major changes in the ASCO/CAP HER2 testing guidelines between 2007 and 2018.
Main Points in the 2007 Guideline [32•]
The 2007 guidelines focused on the following two clinical questions: (1) what is the optimal testing algorithm for the assessment of HER2 status? And (2) what strategies can help ensure optimal performance, interpretation, and reporting of established assays? The guidelines recommended that HER2 status for all invasive breast cancer should be determined and proposed an algorithmic approach for the evaluation of HER2 by immunohistochemistry (IHC) and by in situ hybridization (ISH). It also specified that important issues be considered in testing protocols such as testing requirements, tissue handling, validation and QA procedures, and others. Importantly, it defined the positive, equivocal, and negative categories for HER2 by IHC and by ISH (Table 1).
Major Updates in the 2013 Updated Guideline [33•]
The 2013 ASCO/CAP update committee revised the defining criteria for HER2 categories and recommended that HER2 test must be performed in both all newly diagnosed breast cancer and metastatic disease if tissue sample is available. The fixation time was changed from 6–48 to 6–72 h. The cut off for positivity of HER2 by IHC was decreased from > 30% (2007 guidelines) to > 10% of invasive tumor cells with complete and intense staining by IHC. The HER2/CEP17 ratio for positive ISH result was changed from > 2.2 (2007 guidelines) to ≥ 2.0, with the creation of three positive ISH “groups.” The equivocal and negative categories were also revised accordingly (Table 1). In addition, the testing methodology and quality control were also emphasized.
Major Updates in the Current, 2018 Focused Update Guideline [34•]
The 2018 ASCO/CAP focused update guidelines addressed the following five clinical questions: (1) what is the most appropriate definition for IHC 2+ (IHC equivocal)? (2) Must HER2 testing be repeated on a surgical specimen if initially negative test on core biopsy? (3) Should invasive cancers with an HER2/CEP17 ratio of ≥ 2.0 but an average of HER2 copy number of < 4.0 signals per cell be considered ISH positive? (4) Should invasive cancers with an HER2/CEP17 ratio of < 2.0 but an average of HER2 copy number of ≥ 6.0 signals per cell be considered ISH positive? (5) What is the appropriate diagnostic workup for invasive cancers with an average HER2 copy number of ≥ 4.0 but < 6.0 signals per cell and an HER/CEP17 ratio of < 2.0 and initially deemed to have an equivocal HER2 ISH test result? The 2018 guidelines also re-defined the HER2 IHC equivocal cases as “weak to moderate complete membrane staining observed in > 10% of tumor cells or complete and circumferential membranous that is intense and within ≤ 10% of invasive tumor cells” and clarified the confusion in the 2013 guidelines that defined the equivocal cases as “circumferential membrane staining that is incomplete and/or weak/moderate.” The guidelines also addressed uncommon IHC staining patterns such as moderate to intense but incomplete (basolateral or lateral) staining, or circumferential, intense membrane staining in ≤ 10% of tumor cells (heterogeneous but limited in extent) and recommended that these staining patterns should be considered as equivocal as well. The 2018 guidelines revised the recommendation on repeating HER2 on excision if the prior breast biopsy was HER2 negative, by replacing the word “must” from the 2013 guidelines with “may” in the 2018 guidelines (Table 1).
One of the major updates in the 2018 focused update guidelines was the interpretation of the uncommon ISH HER2 groups 2 (monosomy), 3 (co-amplified/polysomy), and 4 (equivocal). The 2018 guidelines emphasized on coordination between IHC and ISH results and recommended that groups 2 and 3, previously positive by the 2013 recommendations, are no longer positive by default. The 2018 guidelines recommended that the definitive diagnosis for ISH HER2 groups 2 and 3 be rendered based on additional workup. An equivocal result (group 4) is no longer reported, but now rather clarified as positive or negative based on additional workup. Additionally, the 2018 guideline discontinued the option of using alternate probes for solving the group 4 equivocal status. This topic will be elaborated in more detail in the following section.
Applying the Current HER2 Testing Guideline in Breast Cancer
Specimens To Be Tested
The 2007 ASCO/CAP guidelines recommended that all primary breast cancer specimens and metastases should have at least one HER2 test performed [32•]. The majority of earlier studies demonstrated overwhelming consistency of HER2 status in the paired primary and metastatic breast cancers [35,36,37]. However, after the release of 2007 guideline, emerging data suggested that there is up to 42% discordance in HER2 results between primary and metastatic disease, likely due to testing methods, definition of metastatic disease, and the effect of HER2 targeted therapy [38,39,40]. As a result of this discordance, the 2013 and 2018 guidelines recommend that all newly diagnosed patients with breast cancer must have a HER2 test performed and patients who develop metastatic or recurrence disease must also perform HER2 testing in the metastatic site if the tissue sample is available [33•, 34•].
Tissue Handling, Fixation, and Section
Pre-analytical factors including tissue handling, fixation, and sectioning play a critical role in achieving consistent quality with accurate and reproducible result. The ASCO/CAP guidelines recommend that routinely processed formalin-fixed paraffin-embedded tissue and cell blocks should be used for all HER2 testing [32•, 33•, 34•]. Core biopsy or cytology material should be placed in 10% neutral buffered formalin as soon as possible. Resection specimens should be sliced at 5- to 10-mm intervals after appropriate gross inspection and margin designation and placed in a sufficient volume of 10% neutral buffered formalin within 1 h. The tissue fixation time in formalin for HER2 testing was changed to 6–72 h in the 2013 guidelines [32•] and remained in the 2018 guidelines [33•, 34•]. In addition, it should be noted that after 6 weeks post-tissue sectioning, these tissue sections should not be used for HER2 testing.
Strategies To Ensure Optimal Performance, Interpretation, and Reporting of Established Assays
One of the two main tasks in the 2007 guideline was to ensure that the laboratory had optimal performance, interpretation, and reporting of the established IHC and ISH assays for HER2 testing. These recommendations continued to be emphasized in the revised 2013 and 2018 guidelines. These guidelines provided detailed rejection criteria for IHC and ISH testing, optimal internal validation procedure and monitoring of test concordance between methods, optimal internal QA procedures and external proficiency assessments, as well as optimal laboratory accreditation. The guidelines strongly recommend validation of laboratory assays or modifications, strict adherence to laboratory accreditation standards, proficiency testing, and competency assessment. A recent publication reported that following the ASCO/CAP guidelines resulted in highly accurate and reproducible breast cancer biomarker results, including HER2 [41].
Testing in Central vs Local Laboratory
When evaluating HER2 expression, the issue of local versus central laboratory testing deserves discussion. The initial overall performance in HER2 testing performed by local laboratories was disappointing, with only 74% concordance in HER2 positivity between local and central laboratories [42]. Concordance between central and reference laboratories was found to be higher, suggesting that specifics related to local laboratories may contribute to the discordance between local and central HER2 testing [43].
When To Repeat HER2 Testing
Given the evidence of a high concordance rate (97–99%) for HER2 status between the core biopsy and the excision specimen [44•], the 2018 guidelines recommended that a HER2 negative result diagnosed on core biopsy may be repeated on the resection specimen under the following situations: (1) if the tumor is high grade, (2) if the sample is scarce on core biopsy, (3) if the resection specimen shows new areas of high-grade carcinoma not encountered on the original biopsy or, (4) if the core biopsy result is equivocal after both IHC and FISH testing. A HER2 test should be not ordered on the excision specimen if HER2 was negative on the biopsy specimen when both specimens show grade 1 ductal or lobular carcinoma with ER and PR positivity, tubular, mucinous, cribriform, or adenoid cystic carcinomas. Similarly, if the excision and biopsy specimens both have the same histopathologic findings and a positive initial HER2 result in grade 1 ductal or lobular carcinoma with ER and PR positivity, tubular, mucinous, cribriform, or adenoid cystic carcinoma, a new HER2 test should be ordered. Of note, rare low-grade carcinomas such as approximately 5% of classical lobular carcinoma can be HER2 positive [45]. Our recent findings also support continued HER2 evaluation for all patients with invasive carcinoma, regardless of ductal or lobular morphology [46].
Issues Regarding Groups 2, 3, and 4
In the 2013 guidelines [33•], the HER2 ISH categories were modified, creating five different breast cancer ISH groupings, using both the HER2/CEP17 (the centromere enumeration probe for chromosome 17) ratio and the average HER2 copy number per nucleus. Group 2 (monosomy) is defined as a HER2/CEP17 ratio of ≥ 2.0, with an average HER2 copy number of < 4.0 signals/cells. Group 2 result is due to amplification of the HER2 gene and an associated increased HER2 copy number, with a loss of chromosome 17 copy number, resulting in HER2-amplified monosomy of chromosome 17 [47]. In group 2 cases, the CEP17 copy number is so low that even with slight and potentially insignificant elevations of the HER2 copy number, the HER2/CEP17 ratio is ≥ 2.0. The group 2 cases account for approximately 0.4–3.7% of breast cancers [34•, 48•, 49, 50]. Group 3 (co-amplified/polysomy) is defined as a HER2/CEP17 ratio of < 2.0, with an average HER2 copy number ≥ 6.0 signals/cell. Group 3 findings are due to either polysomy of the HER2 gene or more commonly co-amplification of both the HER2 and CEP17 genes [49, 51,52,53]. In these cases, the HER2 copy number is significantly elevated but the CEP17 copy number is also elevated to such a degree that the HER2/CEP17 ratio is < 2.0. The incidence of polysomy/co-amplification is approximately 0.4–3.0% [34•, 48•, 49]. Both groups 2 and 3 were considered as positive in the 2013 guidelines. Group 4 is defined as a HER2/CEP17 ratio of < 2.0, with an average HER2 copy number of ≥ 4.0 and < 6 signals/cell. Group 4 was considered as equivocal (neither amplified nor not-amplified) in the 2013 guidelines. The incidence of group 4 is variable, ranging from 1.9 to 14.2% [34•, 48•, 54, 55, 56•]. Due to the rare incidence of group 2, 3, and 4 cases, and limited data regarding the benefit of HER2 targeted therapy in these three groups, they were very challenging cases for both pathologists and oncologists. In addition, in the 2013 guidelines, since the incidence of true polysomy 17 in breast cancer is rare and the increased CEP17 copy number often results from amplification or copy number gains in the centromeric or pericentromeric regions, the group 4 cases were recommended to be resolved as negative or positive by assessments with alternative, non-centromeric chromosome 17 control probes such as D17S122, Smith–Magenis syndrome (SMS), lissencephaly gene 1 (LIS1), topoisomerase-II-alpha gene (TOP2A), retinoic acid receptor α (RARA), and tumor protein p53 (TP53).
After the release of the 2013 guidelines, new evidence regarding the clinical outcomes of these three groups and whether patients benefited from HER2 targeted therapy has been published. Press et al. [48•] retrospectively analyzed 10,468 breast cancer patients from the Breast Cancer International Research Group (BCIRG)-005, -006, and -007 clinical trials and found that the majority of group 2 patients did not benefit from HER2 targeted therapy. An earlier study also reported that HER2 gene-amplified breast cancers with monosomy of chromosome 17 were poorly responsive to trastuzumab-based treatment [57]. Press et al. [48•] also reported that there was not enough data to determine the benefit of HER2 targeted therapy in group 3 patients and that the outcome of group 4 (equivocal) patients was similar to the outcome of group 5 (classic negative) patients. Furthermore, the 2013 guideline recommendation regarding using alternative chromosome 17 control probes to solve the ISH equivocal cases was also challenged. For example, a recent study showed that the indiscriminate use of alternative control probes to calculate HER2 FISH ratios in HER2-equivocal breast cancers may lead to false-positive interpretations of HER2 status resulting from unrecognized heterozygous deletions of these alternative control genomic sites and incorrect HER2 ratio determinations [58]. Sneige et al. also showed no survival disadvantage between ISH group 4 compared to HER2-negative patients and suggested that alternative chromosome 17 probes may erroneously upgrade the HER2 status, again due to unrecognized reduction in signal numbers resulting from heterozygous deletions [59]. The 2018 guidelines did not recommend using an alternative control probe to resolve ISH equivocal cases.
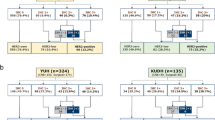
In the 2018 focused update guideline, the rare “non-classical” HER2 ISH group 2 and 3 cases are no longer considered positive by default, and the ISH group 4 category has been eliminated. Group 2, 3, or 4 cases should be reflexed back to IHC, using the same tissue samples used for ISH. If the reflex IHC is 0 or 1+, the case should be considered negative, and if the reflex IHC is 3+, then the case should be considered positive. If the reflex IHC is 2+, then an observer blinded to the previous results should recount at least 20 invasive tumor cells. If the recount shows a different ISH result, then the new result should be reviewed with reference to the 2018 guidelines and the result should be adjudicated per internal procedures to determine the final category. If the recount shows the same ISH group again, the interpretations among groups 2–4 are slightly different. For group 2 cases, the result should be reported as negative. For group 3 cases, the result should be reported as positive, with an explanatory comment. For group 4 cases, the result should be reported as negative, with an explanatory comment (Fig. 1 and Table 2).
The current 2018 focused update guidelines predominately impact the patients in ISH groups 2, 3, and 4. Gordian-Arroyo et al. found that the 2018 guidelines reclassified the HER2 status of 6% of patients, mostly from HER2 equivocal status to HER2 negative status, and that the HER2 positive rate decreased by 0.4% [60]. Two large Chinese studies also found that using the 2018 guidelines, the HER2 negative rate was increased by 8.1–13.3%, either from an originally equivocal or positive case and that 0.1% of cases were changed from equivocal to positive [61, 62]. Our unpublished data suggests that the 2018 guidelines resulted in a 2.2% decrease in ISH positive patients and an 11.2% increase in ISH-negative patients (unpublished observation).
Issues Not Included in the Current HER2 Testing Guideline
HER2 Testing in Bone Specimen
Bone is the most common site of breast cancer involvement and the bone specimen always requires decalcification. The current ASCO/CAP guidelines do not recommend a specific decalcification process for HER2 testing, although it states that samples with decalcification artifacts should be rejected and that samples that were decalcified in a strong acid solution may be rejected [34•]. Studies have shown that acidification of bone specimen may affect antigenicity of the tumor cells and DNA quality, which can alter the biomarker status of metastatic breast cancer. For example, Maclary et al. demonstrated that most HER2 equivocal cases became negative after 24 h of hydrochloric acid decalcification [63]. Darvishian et al. showed that after 1 h of decalcification in a rapid decalcifying reagent, HER2 IHC showed less intense and more sparse membranous staining with a mean drop of 1 score. Interestingly, FISH analysis on decalcified cases with scores of 3 and 1 by IHC failed to detect any signals [64]. A recent study compared three decalcification solutions including acetic acid, hydrochloric/formic acid, and EDTA and found that bone biopsy specimens can be reliably used for biomarker studies in breast cancer and that EDTA decalcification is the optional method since it minimally affects receptor expression results [65]. At the University of Rochester Medical Center, when a bone biopsy specimen from a patient with history of breast cancer is received, an in-house solution of EDTA with formalin is the preferred method for decalcification and fixation. We recommend that the fresh bone material should be in formalin for at least 1 h before placing in EDTA solution. The decalcification/fixation time depends on the size, thickness, and consistency of the sample, but should be between 6 and 72 h.
Whether to Re-test HER2 Status in the Neoadjuvant Setting
Neoadjuvant chemotherapy (NAC) has been increasingly used for breast cancer in recent years and published literature comparing pre- and post-NAC HER2 results have yielded conflicting information, ranging from no alteration to up to a 43% change. For example, several studies found NAC caused no significant modulation of HER2 status [66,67,68]. In contrast, most studies have demonstrated discordant rates of 4.7–43.0% for HER2 between before-NAC core biopsy and post-NAC resection [69,70,71,72,73,74,75,76,77,78,79]. Studies also found that loss of HER2 amplification in the residual disease was associated with a shorter recurrence-free survival or a higher risk of relapse [72, 74]. Although whether to perform HER2 re-testing on the residual disease after NAC has not been addressed in the ASCO/CAP guidelines, more studies suggest performing HER2 re-testing after NAC, since any significant changes in HER2 status may impact therapeutic management. Interestingly, a few studies have suggested that FISH analysis is preferred to IHC when re-testing, because FISH analysis is more stable than IHC [68, 73].
Challenges Remain for HER2 Testing
Despite the remarkable success realized from targeting HER2-overexpression in a subset of breast cancers over the past two decades, a number of challenges remain, and many of which are directly related to testing.
Quantitative Measurement of HER2
While the IHC and ISH methodologies for assessing HER2 in breast cancer have undergone extensive analytic and clinical validation over the years to demonstrate clinical utility, both of these methodologies have limitations [32•, 33•]. IHC utilizes chromogenic dyes that enable semi-quantitative measurement of HER2 receptor overexpression; however, this detection methodology has a limited dynamic range where the test is linear, restricting the capability to obtain accurate quantitative results [80]. Furthermore, the intensity of staining is dependent on the enzymatic activity of the detection system being employed, the reaction time, and temperature as well as substrate concentration, thus limiting the quantitative sensitivity of IHC [81]. A number of studies have established a correlation between the level of HER2 protein expression and breast cancer dependence on HER2 signaling, which in turn would be predictive of sensitivity to HER2-targeted therapy [82]. Given the limitation of IHC for accurate quantitative assessment of HER2 protein levels, more reliable methods to quantify HER2 protein expression have been proposed for more accurate prediction of therapeutic efficacy. The development of quantitative methodologies for the measurement of a predictive biomarker such as HER2 has in fact provided compelling evidence to suggest that this approach can improve the prediction of the response to HER2-targeted therapies in breast cancer [83, 84]. The measurement of HER2 protein by a selected reaction monitoring mass spectrometry has been shown to significantly correlate with both IHC and ISH, and these quantitative measurements were superior to IHC and ISH for predicting outcome after HER2-targeted therapy in the metastatic and adjuvant settings [85]. In addition, a number of studies have shown that compared to IHC and ISH, the quantitative measurement of HER2 protein, regardless of the methodology, is associated with a significantly higher pathologic complete response rate (a surrogate for patient outcome) following NAC that included HER2-targeted therapy [84, 86,87,88]. As with any other predictive biomarkers or companion diagnostics, these new methodologies will need to undergo rigorous analytic and clinical validation to demonstrate level-1 evidence of clinical utility before entering clinical practice [89].
Expansion of HER2 Indications and the Need for Testing in Other Solid Tumors
The remarkable clinical benefit achieved by targeting the HER2-pathway in HER2-positive breast cancer has generated intense interest in expanding the clinical indications for this treatment approach to other solid tumors [90]. With increased genomic profiling of many tumor types, there is an emerging recognition that HER2 gene amplification and activating mutations can occur in a number of different solid tumor types including lung, colon, bladder, salivary gland, ovary, and uterine serous carcinomas, as well as adenocarcinomas of the upper gastrointestinal tract [91,92,93,94]. Clinical trials, such as the MY PATHWAY basket trial have shown objective responses across a variety of solid tumors with HER2 overexpression and/or amplification in 30 of 114 treatment refractory patients (26%, 95% confidence interval 19–35%), with the highest response rates seen in patients with colorectal carcinoma [95]. The data from this and other trials support the notion that an activated HER2 pathway may be a potential therapeutic target in a number of different solid tumors and has led to a number of trials exploring the clinical efficacy of this approach. The Trastuzumab for Gastric cancer trial (ToGA) was the first phase III randomized, controlled study that showed a survival benefit for patients with metastatic HER2-positive gastric cancer, which led to the approval of trastuzumab for these patients in both the USA and Europe in 2010 [96]. Before the initiation of the ToGA trial, the investigators needed to validate which analytic method should be used for HER2 assessment in determining the most appropriate scoring criterion for HER2 analysis, so that an appropriate inclusion algorithm could be used [97]. This HER2 gastric validation study led to the development of a modified scoring system that was distinctive for gastric cancer, incorporating pathologic and biologic features unique to upper gastrointestinal tract tumors [97, 98]. Going forward, similar rigorous validation studies will need to be performed to help determine the most appropriate assessment methodology and HER2 scoring criterion for each new emerging HER2 indication.
Conclusion
Accurate assessment of HER2 status in breast cancer is critically important and clinically relevant. The ASCO/CAP current guidelines provide very valuable recommendations for the accurate evaluation of HER2 status, based on the results of clinical trials and an extensive literature review. Additional studies, especially those with long-term outcome data are needed to confirm the clinical validity of the 2018 ASCO/CAP guideline recommendations, in order to evaluate the clinical utility of the new criteria and to help with the development of new recommendations. The expansion of HER2 indications in other solid tumors also emphasizes the need for the most appropriate assessment methodology and HER2 scoring criterion for each new emerging HER2 indication.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–6.
King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB related gene in a human mammary carcinoma. Science. 1985;229:974–6.
Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–9.
Semba K, Kamata N, Toyoshima K, Yamamoto T. A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci U S A. 1985;82:6497–501.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Elizalde PV, Cordo Russo RI, Chervo MF, Schillaci R. ErbB-2 nuclear function in breast cancer growth, metastasis and resistance to therapy. Endocr Relat Cancer. 2016;23(12):T243–57.
Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198(2–3):165–84.
Tzahar E, Pinkas-Kramarski R, Moyer JD, Klapper LN, Alroy I, Levkowitz G, et al. Bivalence of EGF-like ligands drives the ErbB signaling network. EMBO J. 1997;16:4938–50.
Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, et al. Diversification of neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–67.
Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–52.
Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER-2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40.
Grant S, Qiao L, Dent P. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front Biosci. 2002;7:d376–89.
Atalay G, Cardoso F, Awada A, Piccart MJ. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann Oncol. 2003;14:1346–63.
Borg A, Tandon AK, Sigurdsson H, Clark GM, Fernö M, Fuqua SA, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50(14):4332–7.
Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, et al. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992;10(4):599–605.
Schnitt SJ. Breast cancer in the 21st century: neu opportunities and neu challenges. Mod Pathol. 2001;14(3):213–8.
Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, et al. The HER-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8(4):307–25.
Yamauchi H, Stearns V, Hayes DF. The role of c-erbB-2 as a predictive factor in breast cancer. Breast Cancer. 2001;8(3):171–83.
Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357(15):1496–506.
Di Leo A, Chan S, Paesmans M, Friedrichs K, Pinter T, Cocquyt V, et al. HER-2/neu as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Breast Cancer Res Treat. 2004;86(3):197–206.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72.
Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–44.
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14(4):320–68.
Voigtlaender M, Schneider-Merck T, Trepel M. Lapatinib. Recent results. Cancer Res. 2018;211:19–44.
Escrivá-de-Romaní S, Arumí M, Bellet M, Saura C. HER2-positive breast cancer: current and new therapeutic strategies. Breast. 2018;39:80–8.
Baselga J, Coleman RE, Cortés J, Janni W. Advances in the management of HER2-positive early breast cancer. Crit Rev Oncol Hematol. 2017;119:113–22.
Hayes DF, Picard MH. Heart of darkness: the downside of trastuzumab. J Clin Oncol. 2006;24:4056–8.
Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124(7):966–78.
Bast RC Jr, Ravdin P, Hayes DF, Bates S, Fritsche H Jr, Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–78.
• Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45. This is the first ASCO/CAP HER2 testing guideline, which provided the expert panel’s recommendation on HER2 testing to improve the accuracy of test result through standardization.
• Wolff AC, Hammond ME, Hicks DG, Dowsett M, LM MS, Allison KH, et al. American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. Based on an extensive literature review and the results of clinical trials, the 2013 HER2 updated HER2 testing guidelines defined the criteria for HER2 categories and suggested the criteria when to consider repeat testing.
• Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–22. This is the most updated ASCO/CAP HER2 testing guideline, which focused on how to interpret the uncommon HER2 ISH group 2–4 test results.
Masood S, Bui MM. Assessment of HER-2/neu overexpression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann Clin Lab Sci. 2000;30(3):259–65.
Xu R, Perle MA, Inghirami G, Chan W, Delgado Y, Feiner H. Amplification of HER-2/neu gene in HER-2/neu-overexpressing and -nonexpressing breast carcinomas and their synchronous benign, premalignant, and metastatic lesions detected by FISH in archival material. Mod Pathol. 2002;15(2):116–24.
Tapia C, Savic S, Wagner U, Schönegg R, Novotny H, Grilli B, et al. HER2 gene status in primary breast cancers and matched distant metastases. Breast Cancer Res. 2007;9(3):R31.
Lower EE, Glass E, Blau R, Harman S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat. 2009;113(2):301–6.
Chan A, Morey A, Brown B, Hastrich D, Willsher P, Ingram D. A retrospective study investigating the rate of HER2 discordance between primary breast carcinoma and locoregional or metastatic disease. BMC Cancer. 2012;12:555.
van Rooijen JM, de Munck L, de Graaf JC, Siesling S, de Vries EG, Boers JE. Limited human epidermal growth factor receptor 2 discordance in metastatic breast cancer patients treated with trastuzumab, a population based study. Eur J Cancer. 2014;50(5):885–91.
Zhang H, Han M, Varma KR, Clark BZ, Bhargava R, Dabbs DJ. High fidelity of breast biomarker metrics: a 10-year experience in a single, large academic institution. Appl Immunohistochem Mol Morphol. 2018;26(10):697–700.
Roche PC, Suman VJ, Jenkins RB, Davidson NE, Martino S, Kaufman PA, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002;94(11):855–7.
Perez EA, Suman VJ, Davidson NE, Martino S, Kaufman PA, Lingle WL, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24(19):3032–8.
• Rakha EA, Pigera M, Shaaban A, Shin SJ, D’Alfonso T, Ellis IO, et al. National guidelines and level of evidence: comments on some of the new recommendations in the American Society of Clinical Oncology and the College of American Pathologists human epidermal growth factor receptor 2 guidelines for breast cancer. J Clin Oncol. 2015;33(11):1301–2. This study showed only 2% of cases had a different HER2 status identified in the core biopsy and excision specimens, providing evidence to suggest to revise the 2013 guideline regarding when to repeat the test in cases of histopathological discordance.
Yu J, Dabbs DJ, Shuai Y, Niemeier LA, Bhargava R. Classical-type invasive lobular carcinoma with HER2 overexpression: clinical, histologic, and hormone receptor characteristics. Am J Clin Pathol. 2011;136(1):88–97.
Zhang H, Moisini I, Ajabnoor RM, Turner BM, DAguiar M, Cai X, et al. Frequency, clinicopathologic characteristics and follow-up of HER2 positive nonpleomorphic invasive lobular carcinoma of breast: a retrospective analysis of 11-year study in an academic institution. Am J Clin Pathol. 2019; [Epub ahead of print].
Page DB, Wen H, Brogi E, Dure D, Ross D, Spinelli KJ, et al. Monosomy 17 in potentially curable HER2-amplified breast cancer: prognostic and predictive impact. Breast Cancer Res Treat. 2018;167(2):547–54.
• Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, et al. HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-College of American Pathologists guidelines with FISH scores used for enrollment in Breast Cancer International Research Group clinical trials. J Clin Oncol. 2016;34(29):3518–28. This study retrospectively analyzed 10,468 breast cancer patients and provided the fundamental data regarding clinical outcomes and benefit from HER2 target therapy of patients with HER2 ISH group 2–4 results.
Ballard M, Jalikis F, Krings G, Schmidt RA, Chen YY, Rendi MH, et al. ‘Non-classical’ HER2 FISH results in breast cancer: a multi-institutional study. Mod Pathol. 2017;30(2):227–35.
Zare SY, Lin L, Alghamdi AG, Daehne S, Roma AA, Hasteh F, et al. Breast cancers with a HER2/CEP17 ratio of 2.0 or greater and an average HER2 copy number of less than 4.0 per cell: frequency, immunohistochemical correlation, and clinicopathological features. Hum Pathol. 2019;83:7–13.
Ballinger TJ, Sanders ME, Abramson VG. Current HER2 testing recommendations and clinical relevance as a predictor of response to targeted therapy. Clin Breast Ca. 2015;15:171–80.
Vranic S, Teruya B, Repertinger S, Ulmer P, Hagenkord J, Gatalica Z. Assessment of HER2 gene status in breast carcinomas with polysome of chromosome 17. Cancer. 2011;117:48–53.
Marchiò C, Lambros MB, Gugliotta P, Di Cantogno LV, Botta C, Pasini B, et al. Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol. 2009;219:16–24.
Shah MV, Wiktor AE, Meyer RG, Tenner KS, Ballman KV, Green SJ, et al. Change in pattern of HER2 fluorescent in situ hybridization (FISH) results in breast cancers submitted for FISH testing: experience of a reference laboratory using US Food and Drug Administration criteria and American Society of Clinical Oncology and College of American Pathologists guidelines. J Clin Oncol. 2016;34:3502–10.
Stoss OC, Scheel A, Nagelmeier I, Schildhaus HU, Henkel T, Viale G, et al. Impact of updated HER2 testing guidelines in breast cancer-re-evaluation of HERA trial fluorescence in situ hybridization data. Mod Pathol. 2015;28:1528–34.
• Press MF, Villalobos I, Santiago A, Guzman R, Cervantes M, Gasparyan A, et al. Assessing the new American Society of Clinical Oncology/College of American Pathologists guidelines for HER2 testing by fluorescence in situ hybridization: experience of an academic consultation practice. Arch Pathol Lab Med. 2016;140:1250–8. This is one of the largest study (7526 cases) on reporting the distribution of HER2 testing results by ISH and IHC.
Risio M, Casorzo L, Redana S, Montemurro F. HER2 gene-amplified breast cancers with monosomy of chromosome 17 are poorly responsive to trastuzumab-based treatment. Oncol Rep. 2005;13(2):305–9.
Press MF, Seoane JA, Curtis C, Quinaux E, Guzman R, Sauter G, et al. Assessment of ERBB2/HER2 status in HER2-equivocal breast cancers by FISH and 2013/2014 ASCO-CAP guidelines. JAMA Oncol. 2019;5(3):366–75.
Sneige N, Hess KR, Multani AS, Gong Y, Ibrahim NK. Prognostic significance of equivocal human epidermal growth factor receptor 2 results and clinical utility of alternative chromosome 17 genes in patients with invasive breast cancer: a cohort study. Cancer. 2017;123(7):1115–23.
Gordian-Arroyo AM, Zynger DL, Tozbikian GH. Impact of the 2018 ASCO/CAP HER2 guideline focused update. Am J Clin Pathol. 2019;152(1):17–26.
Liu ZH, Wang K, Lin DY, Xu J, Chen J, Long XY, et al. Impact of the updated 2018 ASCO/CAP guidelines on HER2 FISH testing in invasive breast cancer: a retrospective study of HER2 fish results of 2233 cases. Breast Cancer Res Treat. 2019;175(1):51–7.
Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L. Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract. 2019;215(2):251–5.
Maclary SC, Mohanty SK, Bose S, Chung F, Balzer BL. Effect of hydrochloric acid decalcification on expression pattern of prognostic markers in invasive breast carcinomas. Appl Immunohistochem Mol Morphol. 2017;25(2):144–9.
Darvishian F, Singh B, Krauter S, Chiriboga L, Gangi MD, Melamed J. Impact of decalcification on receptor status in breast cancer. Breast J. 2011;17(6):689–91.
van Es SC, van der Vegt B, Bensch F, Gerritse S, van Helden EJ, Boon E, et al. Decalcification of breast cancer bone metastases with EDTA does not affect ER, PR, and HER2 results. Am J Surg Pathol. 2019;43(10):1355–60.
Taucher S, Rudas M, Mader RM, Gnant M, Sporn E, Dubsky P, et al. Influence of neoadjuvant therapy with epirubicin and docetaxel on the expression of HER2/neu in patients with breast cancer. Breast Cancer Res Treat. 2003;82(3):207–13.
Bottini A, Berruti A, Bersiga A, Brunelli A, Brizzi MP, Marco BD, et al. Effect of neoadjuvant chemotherapy on Ki67 labelling index, c-erbB-2 expression and steroid hormone receptor status in human breast tumours. Anticancer Res. 1996;16(5B):3105–10.
Kasami M, Uematsu T, Honda M, Yabuzaki T, Sanuki J, Uchida Y, et al. Comparison of estrogen receptor, progesterone receptor and HER-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast. 2008;17(5):523–7.
Burcombe RJ, Makris A, Richman PI, Daley FM, Noble S, Pittam M, et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer. 2005;92(1):147–55.
Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24(12):1831–8.
Adams AL, Eltoum I, Krontiras H, Wang W, Chhieng DC. The effect of neoadjuvant chemotherapy on histologic grade, hormone receptor status, and HER2/neu status in breast carcinoma. Breast J. 2008;14(2):141–6.
Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15(23):7381–8.
Li P, Liu T, Wang Y, Shao S, Zhang W, Lv Y, et al. Influence of neoadjuvant chemotherapy on HER2/neu status in invasive breast cancer. Clin Breast Cancer. 2013;13(1):53–60.
Guarneri V, Dieci MV, Barbieri E, Piacentini F, Omarini C, Ficarra G, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24(12):2990–4.
Yang YF, Liao YY, Li LQ, Xie SR, Xie YF, Peng NF. Changes in ER, PR and HER2 receptors status after neoadjuvant chemotherapy in breast cancer. Pathol Res Pract. 2013;209(12):797–802.
Cockburn A, Yan J, Rahardja D, Euhus D, Peng Y, Fang Y, et al. Modulatory effect of neoadjuvant chemotherapy on biomarkers expression; assessment by digital image analysis and relationship to residual cancer burden in patients with invasive breast cancer. Hum Pathol. 2014;45(2):249–58.
Zhou X, Zhang J, Yun H, Shi R, Wang Y, Wang W, et al. Alterations of biomarker profiles after neoadjuvant chemotherapy in breast cancer: tumor heterogeneity should be taken into consideration. Oncotarget. 2015;6(34):36894–902.
Gahlaut R, Bennett A, Fatayer H, Dall BJ, Sharma N, Velikova G, et al. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression—implications for the practising oncologist. Eur J Cancer. 2016;60:40–8.
Xian Z, Quinones AK, Tozbikian G, Zynger DL. Breast cancer biomarkers before and after neoadjuvant chemotherapy: does repeat testingimpact therapeutic management? Hum Pathol. 2017;62:215–21.
Goldstein NS, Hewitt SM, Taylor CR, Yaziji H, Hicks DG. Members of ad-hoc committee on immunohistochemistry standardization. Recommendations for improved standardization of immunohistochemistry. Appl Immunohistochem Mol Morphol. 2007;15(2):124–33.
Downs-Kelly E, Yoder BJ, Stoler M, Tubbs RR, Skacel M, Grogan T, et al. The influence of polysomy 17 on HER2 gene and protein expression in adenocarcinoma of the breast: a fluorescent in situ hybridization, immunohistochemical, and isotopic mRNA in situ hybridization study. Am J Surg Pathol. 2005;29(9):1221–7.
Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol. 2019;139:53–66.
Yardley DA, Kaufman PA, Huang W, Krekow L, Savin M, Lawler WE, et al. Quantitative measurement of HER2 expression in breast cancers: comparison with ‘real-world’ routine HER2 testing in a multicenter collaborative biomarker study and correlation with overall survival. Breast Cancer Res. 2015;17:41.
Hicks DG, Buscaglia B, Goda H, McMahon L, Natori T, Turner B, et al. A novel detection methodology for HER2 protein quantitation in formalin-fixed, paraffin embedded clinical samples using fluorescent nanoparticles: an analytical and clinical validation study. BMC Cancer. 2018;18(1):1266.
Nuciforo P, Thyparambil S, Aura C, Garrido-Castro A, Vilaro M, Peg V, et al. High HER2 protein levels correlate with increased survival in breast cancer patients treated with anti-HER2 therapy. Mol Oncol. 2016;10(1):138–47.
Cheng H, Bai Y, Sikov W, Sinclair N, Bossuyt V, Abu-Khalaf MM, et al. Quantitative measurements of HER2 and phospho-HER2 expression: correlation with pathologic response to neoadjuvant chemotherapy and trastuzumab. BMC Cancer. 2014;14:326.
Scaltriti M, Nuciforo P, Bradbury I, Sperinde J, Agbor-Tarh D, Campbell C, et al. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin Cancer Res. 2015;21(3):569–76.
Bianchini G, Kiermaier A, Bianchi GV, Im YH, Pienkowski T, Liu MC, et al. Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. 2017;19(1):16.
Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101(21):1446–52.
Hicks DG, Whitney-Miller C. HER2 testing in gastric and gastroesophageal junction cancers: a new therapeutic target and diagnostic challenge. Appl Immunohistochem Mol Morphol. 2011;19(6):506–8.
Ogoshi Y, Shien K, Yoshioka T, Torigoe H, Sato H, Sakaguchi M, et al. Anti-tumor effect of neratinib against lung cancer cells harboring HER2 oncogene alterations. Oncol Lett. 2019;17(3):2729–36.
Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res. 2019;25(7):2033–41.
Chmielecki J, Ross JS, Wang K, Frampton GM, Palmer GA, Ali SM, et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist. 2015;20(1):7–12.
Hicks DG, Whitney-Miller CL. The evolving role of HER2 evaluation for diagnosis and clinical decision making for breast and gastric adenocarcinoma. Biotech Histochem. 2013;88(3–4):121–31.
Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36:536–42.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805.
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35(4):446–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Huina Zhang declares that she has no conflict of interest.
Ioana Moisini declares that she has no conflict of interest.
Rana M. Ajabnoor declares that she has no conflict of interest.
Bradley M. Turner declares that he has no conflict of interest.
David G. Hicks has received compensation from Genentech for participation on speakers’ bureaus.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Zhang, H., Moisini, I., Ajabnoor, R.M. et al. Applying the New Guidelines of HER2 Testing in Breast Cancer. Curr Oncol Rep 22, 51 (2020). https://doi.org/10.1007/s11912-020-0901-4
Published:
DOI: https://doi.org/10.1007/s11912-020-0901-4