Abstract
Health-related quality of life (QOL) outcomes are frequently used by clinicians, patients, and researchers for assessing the effectiveness of an intervention. Small differences in QOL may be statistically significant but their clinical relevance remains undefined. The smallest changes in QOL scores of the anterior skull base surgery questionnaire (ASBS-Q) which could be considered clinically significant have not been delineated. Here we present a meta analysis and review of the literature of 273 patients undergoing skull base tumor resection. The minimal clinically important difference (MCID), defined as “the smallest change in QOL which patients perceive as beneficial”, was calculated using several statistical approaches. The MCID of the ASBS-Q was 0.4 (8%, score range 1–5). Various other instruments for QOL estimations revealed a larger range of MCID score (between 6.2%–17.5%) for the different QOL domains. The statistical analyses reveal that histology (benign vs malignant), time elapsed from surgery (< or ≥6 months), and surgical approach (open vs endoscopic) have significant clinical impact on different QOL domains. This paper brings level 1b evidence which demonstrates the importance of MCID as an adjunct for estimation of QOL in patients undergoing skull base surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Around one decade ago, investigators began to appreciate the importance of quality of life (QOL) outcomes. This is particularly relevant for patients with skull base tumors (SBTs) because the treatments of these tumors typically involve extensive surgery and adjuvant radiation therapy, which have serious morbidity and toxicity. QOL studies are particularly challenging in these patients for several reasons: 1) many of them are elderly and have multiple comorbidities, 2) the short-term survival and deteriorated general status complicates data collection, and 3) the small number of patients and histological variability of the lesions make QOL analysis more difficult than in other head and neck cancers. It is critical to bear in mind that patients’ perspectives on QOL issues cannot be assessed without direct questioning of the patients themselves [1] and that validated disease-specific instruments addressing multiple QOL domains need to be utilized for adequate assessment [2•]. Moreover, outcomes derived at by the extrapolation of numbers based on statistical significance may not reflect clinical realities.
Guyatt and colleagues [3] suggested a new index of responsiveness to assess the utility of instruments designed to measure QOL, the Minimal Clinically Important Difference (MCID) measurement. MCID is “the smallest difference in score which patients perceive as beneficial and which would, therefore, mandate a change in the patient’s management” [4]. MCID was confirmed as being a useful benchmark for assessing the effectiveness of a health care intervention as well as for determining an acceptable sample size in a clinical trial [5]. Osoba and colleagues [6] first suggested the term “subjective significance” to give meaning to QOL assessments for breast and small cell lung cancer patients receiving chemotherapy. This was followed by studies that used QOL measures to evaluate outcome and costs in women receiving chemotherapy for advanced ovarian cancer [7•]. The MCID assists clinicians in evaluating changes in QOL over time and determine appropriate sample sizes when designing clinical trials [8, 9].
There are two general approaches for establishing an MCID: one is anchor-based and the other is distribution-based [10•]. Anchor-based methods use known indicators (eg, the patient’s own assessment of change, performance status, disease stage) for changes in QOL scores. Distribution-based approaches use commonly accepted descriptive statistical measures to generate the MCID (eg, fractions of the standard deviation [SD], effect size, and standard error of measurement [SEM]) [11–13].
Valid interpretation and application of QOL data require disease-specific instruments that assess morbidity associated with a particular diagnosis or treatment. We recently reported the development and validation of the Anterior Skull Base Surgery Questionnaire (ASBS-Q) for estimating a patient’s QOL after extirpation of anterior skull base tumors [2•]. This questionnaire assesses patient function and vitality, side-effects of treatment, disease symptoms, and psychosocial issues using six scales. The ASBS-Q was validated by various authors and in different languages. However, the MCID for anterior skull base QOL instruments and for the ASBS-Q has not yet been established. Furthermore, whether the scores of QOL domains reliably reflect variations among different subgroups of patients remain unanswered.
This article attempted to determine the clinical significance of QOL scores assessed by the ASBS-Q. We also aimed to identify patients at high risk for clinically significant deterioration of their QOL scores. Towards these ends, we performed a meta-analysis of data derived from six publications and two disease-specific instruments. This is the first study to describe the MCID of QOL instruments in the field of skull base surgery.
Methods for Evaluation of MCID
Study and Patient Selection Criteria for the Meta-Analysis
All clinical studies evaluating QOL using the ASBS-Q were considered for inclusion. Suitable study populations were those comprised of patients who underwent either endoscopic or open extirpation of anterior skull base malignant or benign tumors and whose preoperative and/or postoperative QOL scores were available for assessment.
Search Strategy
During June 2011, we conducted a systematic electronic literature database search of PubMed, CINAHL, Cochrane Central Register of Clinical Trials, Cochrane Database of Systematic Reviews, and Google from 1975 to 2010. The searches were conducted using the Medical Subject Heading (MeSH) terms (skull base) AND (quality of life OR QOL OR HRQOL) and limited to “Human.” Reference lists of retrieved manuscripts were hand-searched for additional publications. Two publications in a language other than English that could not be translated because of resource constraints were excluded. Two reviewers (M.A. and Z.G.) independently screened all available article and abstract titles generated by the electronic search strategies. Articles were rejected at the initial screening if their titles or abstracts indicated that they were irrelevant. The full text of potentially relevant articles was reviewed to assess their suitability for inclusion in this meta-analysis.
The ASBS-Q
The development of the questionnaire, including its reliability and validity, is described elsewhere [2•, 14]. The questions are divided into six domains that were found relevant by factor analysis: role of performance (6 items), physical function (7 items), vitality (7 items), pain (3 items), specific symptoms (7 items: appetite, taste, smell, appearance, epiphora, nasal secretions, and visual disturbances), and impact on emotions (5 items). The internal reliability score of the questionnaire is 0.8 and the test–retest reliability score is 0.902. The answers are given on an ordinal scale with 5 levels (35 specific questions) that indicate a change in QOL within the past month. All questions have an identical level of importance. A higher score represents better QOL for all these scales.
Description of Data Collection
Three ASBS-Q cohorts with a total of 118 patients were jointly analyzed. Table 1 summarizes patients’ demographic and clinical data. The first study was based on a retrospective cohort of 35 patients operated between 1994 and 2002 for extirpation of anterior skull base tumors via the subcranial approach to the anterior skull base [14]. All patients were at least 3 months after surgery when asked to fill in the ASBS-Q. The second was a prospective study that included 39 patients who underwent extirpation of anterior skull base tumors between 2002 and 2007 by means of the subcranial approach to the anterior skull base [15•]. These 39 patients were assessed with the ASBS-Q and an additional general 3-level anchor question for assessing postoperative overall QOL as being better, same, or worse. The questionnaires were completed by the patient at two time points: 1 week before the operation, 12 months after surgery. The third study was a retrospective review of 44 patients operated between 2008 and 2011 for extirpation of anterior skull base tumors via the expanded endoscopic approach (EEA) to the anterior skull base [16]. All these patients were at least 6 months after surgery when asked to fill in the ASBS-Q. Descriptions of these studies and the approach to MCID calculation are summarized in Table 1. All 118 patients were at least 18 years old, were able to read and write, and none had any severe psychopathological or cognitive impairment. All patients had given their informed consent for their data to be used in future surveys, and the three studies were approved by the institutional review board.
Selecting the Anchors
The anchor-based approach to establish MCID requires an independent interpretable standard that correlates with the instrument being explored. We used the patient-reported outcome, which is a widely used and accepted method for assessment of clinically important differences based on patient perception [4, 17]. Patients were requested to globally rate the change in their QOL compared with their preoperative QOL. The anchor question read as follows: “Please indicate whether there has been any overall change in your quality of life since the surgery.” We used a scale of 0 = no change, 1 = better, and 2 = worse, which is equivalent to Juniper and Jaeschke scale of change on the global score [4, 17].
Statistical Analysis and Calculation of the Distribution-Based and Anchor-Based MCID
Distribution-based and anchor-based methods were used to estimate the minimal clinically important difference [18]. First, we used half SD as the statistically derived MCID for overall QOL. Then, we did a subgroup analysis to establish population-based MCIDs [19]. Patients were categorized by three parameters of time elapsed from surgery, surgical approach, and histology. One-half of the SD, the SEM, and the effect size were calculated for each subgroup in every domain. The standard error of measurement was calculated using the following formula: SD times the square root of [2 × (1 − r)] where r is the test-retest reliability coefficient previously mentioned. Effect size was defined as the average difference divided by the baseline SD and a small, medium, or large effect size was considered at 0.2, 0.5, or 0.8, respectively [11]. A medium change was considered as a minimal clinically important difference.
In the study with the prospective cohort [14], we analyzed changes in the QOL scores at two time points for each patient. We used one-half of the SD as the statistically driven MCID and calculated the effect size. Then the anchor-based MCID was established using the patient response anchor. Each patient’s response was matched to that individual’s calculated change in the relevant QOL score. In an attempt to reduce bias derived from patients on each of the extremes of changes in health, the patients were categorized into two groups: “responders” were patients reporting improvement, and “nonresponders” were patients who reported “no change” or “worsening” of their QOL.
Results
Characteristics of the Study Patients
A total of 118 eligible patients participating in three studies were included. The rate of malignancy and adjuvant radiotherapy treatment was similar in the prospective and retrospective groups and in the open and endoscopic groups (P > 0.1). Preoperatively, 91% of patients had WHO performance scores of 1 or 2 reflecting independency.
Anchor-Based MCID Threshold
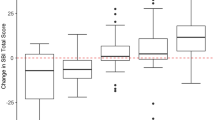
We used the anchor-based method to determine the MCID in our prospective cohort. Of 39 patients, 31 completed the questionnaire including anchor question. The difference between the overall preoperative and postoperative QOL scores was calculated for all patients who were divided according to whether they were responders (improved scores), or nonresponders (no change/deteriorated scores). There were 15/31 (48%) responders (mean change 2.01 ± 0.33, range 1.59–2.67) and 16/31 (52%) nonresponders (mean change 1.17 ± 0.76, range 0.1–2.34). The difference between the two groups was statistically significant (P < 0.03; 95% CI 0.8–1.54). Figure 1 shows the change in scores of patients reporting improvement, no change, or deterioration of their QOL. The figure demonstrates that nonresponders (either those reporting no change or deterioration in QOL) had symmetrical distribution of QOL scores (range 0–2.37), while those reporting improvement in QOL had positively skewed distribution (range 1.59–1.9, P < 0.05).
Change in overall QOL score in patients reporting improvement (top), no change (middle), or no change (bottom). Median value of change was 1.58; intervals represent half SD. The figure demonstrates that patients reporting no change or deterioration in their QOL had evenly distributed QOL scores, while those reporting improvement in QOL had positively skewed distribution (towards the right)
Distribution-Based MCID Threshold
Next we calculated the distribution-based MCID by analyzing the SEM and half SDs for the different QOL domains. Based on half SD, the MCID threshold for the ASBS-Q was 0.4 (8%) and ranged from 0.2–0.52, reflecting a 5–10% change in QOL score. Similar results were found when the SEM was used as the MCID threshold (mean 0.27, range 0.1–0.48). Data analysis of the Pittsburgh QOL study, which used the ASBS-Q for patients undergoing endoscopic skull base surgery, showed MCID threshold of 0.39 (7.8%), a similar threshold as found in the other cohorts [20].
Longitudinal Assessment of MCID Threshold
We performed a longitudinal-based statistical analysis using a prospective cohort in order to obtain an independent estimate of the MCID scores. The magnitude of change of every QOL domain score between two time points (before surgery and 12 months after the operation) was calculated for each patient. The statistically derived baseline (preoperative) MCID threshold range was 0.43–0.64, reflecting an 8.6–12.8% change for the different domains. A significant clinical improvement was demonstrated in the domains of physical function and specific symptoms (a difference of 0.64 and 0.55, respectively) with an effect size of 0.7 and 0.62, respectively. All other domains (performance, vitality, pain, and role on emotions) did not change significantly over time (difference range of 0.04–0.26, effect size range of 0.07–0.34, P > 0.1).
Subgroup Analysis
The study population was divided into subgroups based on pathology, surgical approach, and the time that elapsed from surgery. A cross-sectional analysis of the patients in these subgroups was performed in order to identify clinical and demographic predictors of QOL. First, we investigated whether the time that elapsed from surgery significantly affected the QOL scores of the patients using the MCID as threshold. Differences between early (< 6 months) and late (≥ 6 months) postoperative scores reached the MCID threshold in the performance domain (difference = 0.33), and correlated well with effect size (≥ 0.5) and P values (< 0.05). Differences in all other domains did not reach either statistical or clinical significance (Table 2).
We then determined whether the surgical approach had significant clinical impact on QOL scores. We compared the QOL domain score of patients undergoing subcranial surgery with those undergoing the EEA. This analysis involved 79 patients, 44 of whom were operated via the subcranial approach and 35 through the EEA. There were no significant clinical or demographic differences between the two groups.
Table 2 indicates that the differences in QOL scores between the open and endonasal approaches were clinically significant. There were significant differences in the domains of physical function and emotional status (0.41 and 0.37, respectively) and both had medium to large effect sizes (1.04 and 0.49, respectively). Interestingly, the difference in the performance domain (difference = 0.26) failed to reach the threshold for clinical importance, although it was statistically significant (P = 0.01).
The pathology-based difference analysis (malignant vs benign tumors) revealed a statistically and clinically significant difference in the performance domain (0.47, P = 0.01). Differences were above the MCID threshold in the domains of emotional status and specific symptoms (effect sizes of 0.47 and 0.54, respectively), but did not reach statistical significance (P = 0.09 and 0.08, respectively). The data are found in Table 2.
Evaluating the MCIDs of Other Instruments
Our search of the literature yielded five clinical studies that evaluated the QOL of patients undergoing skull base tumor extirpation. Two studies were not in English and were excluded. A total of 158 patients were studied and different tools for QOL assessment had been used [20–22]. The first published study used the general SF-36 questionnaire on skull base tumor patients and its distribution-based MCID analysis showed a significant difference that ranged between 6.2% and 17.5% in different QOL domains [21]. Analysis of the Toronto study by Palme and colleagues which used several questionnaires to assess QOL (EORTC, emotional status, and midface dysfunction) in patients undergoing skull base surgery revealed MCID threshold of 8.5% (range 8.5%–11.8%) [22].
Discussion and Review of the Literature
QOL instruments play an increasing role in evaluating the efficacy of treatment for cancer. The QOL scores between different subgroups can be statistically significant; however, the interpretation of these data remains unclear because of the lack of any explanation of what defines their clinical importance. The evaluation of QOL in patients with anterior skull base tumors is particularly challenging since many of these patients are at risk for postoperative cognitive dysfunction, prolonged hospitalization, and long periods of rehabilitation [23]. Issues that may influence the functional outcome of patients are directly related to surgery, and they include anosmia, hearing loss, meningitis, cerebrospinal fluid leak, osteoradionecrosis and fistula, impaired nasal function, cosmesis, and visual disturbances. In an early study, Janecka and colleagues [24] used the Karnofsky performance score to evaluate the effect of cranial base surgery on patients’ QOL. They showed that 83% of these patients had improved or unchanged scores after surgery. In another cohort of patients with esthesioneuroblastoma who were undergoing the subcranial approach, the Karnofsky score was not sensitive enough to record changes in patients’ clinical status following resection [25]. There was, therefore, a need for disease-specific instruments designed to evaluate health-related QOL issues of these patients [26]. Such an instrument became available in 2003 by the Tel Aviv group [14].
The current study was designed to address an important question in QOL research—the clinical significance of change in QOL scores. To achieve this goal we first quantify the smallest difference in ASBS-Q scores required for an individual patient undergoing anterior skull base tumor resection to experience a noticeable difference in his subjective level of QOL. Since there is no consensus over a single method for assessing the power of an instrument to capture an MCID, we used several commonly accepted methods. Our analysis of the ASBS-Q data revealed an MCID of 0.4, reflecting an 8% change in QOL score. In accordance with this threshold, our analysis of several studies using the ASBS-Q revealed similar MCID scores [20–22]. In one study which used several other instruments on a similar population of skull base patients, Palme and colleagues [22] reported an MCID threshold equal to 8–12%, again similar to that of the ASBS-Q. These data further support the utility of the ASBS-Q, as a single disease-specific instrument for estimating clinically relevant QOL issues in patients undergoing open or endoscopic surgeries.
The long-term QOL of patients undergoing anterior skull base tumor resection was previously assessed by a number of studies that used the ASBS-Q [2•, 14, 27, 28]. Their results showed that 38% of the patients reported a significant improvement in overall QOL, 36% reported no change, and 26% reported that the surgical procedure worsened their QOL. In the current paper, we found a significant difference of 20% in the median change in scores between patients who reported improvement (responders) and those who reported no change (non-responders) in their overall QOL, 1 year after surgery. Interestingly non-responders had a mean positive QOL score change. This exemplifies the significance of a multidimensional QOL questionnaire which gives better perception of the true impact of surgery on daily function [28]. In addition, our results exemplify previous findings showing that the direction of change in QOL scores is more important than its absolute value [29].
Studies on patients with malignant intracranial tumors have demonstrated severely deteriorating QOL measures, with marked decline in cognitive, physical, emotional, and social functioning after surgery [30]. Similarly, one QOL analysis of patients with carcinomas of the paranasal sinuses showed that the worst affected domains were mood, anxiety, and activity [31]. Our study further demonstrates that differences in the QOL between patients with benign and malignant histology are clinically significant. This could be expected not only because of general cancer-related influences on different aspects of QOL but also due to the impact of early and long-term morbidity related to the adjuvant radiotherapy [14, 22, 32].
The type of surgical approach also showed clinically significant difference in QOL scores. Our analysis showed that the EEA to the skull base was associated with better QOL scores than open surgery, especially in the domains of physical function and impact on emotions. The differences in QOL scores were larger than the MCIDs, suggesting that they were clinically important. These findings support a potential advantage for minimally invasive surgery on the psychosocial and emotional status of patients. Interestingly, the scores for sinonasal morbidity (specific symptoms domain) were similar in both groups, suggesting that the influence of the surgical approach on QOL may be related to other factors, such as skin incisions, craniotomy, visual function, osteonecrosis or encephalomalacia, as well as on psychological issues, which are associated with open surgery [33, 34]. Estimation of the influence of surgical procedures on QOL can serve as a means by which the most appropriate surgical approach can be selected for a given patient [35]. For example, information on QOL could assist in deciding whether a specific treatment is associated with a better impact on patients’ well being than other procedures. A main question in skull base surgery is whether endoscopic approaches, with their potential for causing permanent nasal morbidity, are advantageous over frontal craniotomy, which is associated with a different kind of morbidity. Our QOL findings support a clinical benefit for minimally invasive techniques over traditional approaches. Further studies of QOL scores using MCID thresholds among skull base patients are needed to establish superiority of one surgical procedure over the other.
Conclusions
This study demonstrates the role of MCID analysis in estimating the clinical significance of QOL scores. The MCID of the ASBS-Q is 0.4, reflecting an 8% difference in threshold. Any change above this score can be considered as being clinically significant. Histology, length of the follow-up period, and surgical approach have significant clinical impact on different QOL domains. The endoscopic approach is preferred in terms of physical function and emotional status. Malignancy and < 6 months since surgery are associated with worse scores.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Gil Z, Cohen JT, Spektor S, Shlomi B, Fliss DM. Anterior skull base surgery without prophylactic airway diversion procedures. Otolaryngol Head Neck Surg. 2003;128(5):681–5.
• Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss DM. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg 2004;100(5):813–9. First to assess and validate a skull base surgery—specific questionnaire.
Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis. 1987;40(2):171–8.
Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15.
Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002;11(3):207–21.
Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17(6):1654–63.
• Doyle C, Crump M, Pintilie M, Oza AM. Does palliative chemotherapy palliate? Evaluation of expectations, outcomes, and costs in women receiving chemotherapy for advanced ovarian cancer. J Clin Oncol 2001;19(5):1266–74. Group showed that patient expectations from palliative chemotherapy are often unrealistic. Objective responses were low; still it was associated with substantive improvement in patients’ emotional function and global QOL.
Purcell A, Fleming J, Bennett S, Burmeister B, Haines T. Determining the minimal clinically important difference criteria for the Multidimensional Fatigue Inventory in a radiotherapy population. Support Care Cancer 18(3):307–15.
Maringwa J, Quinten C, King M, Ringash J, Osoba D, Coens C, et al. Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann Oncol.
• Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution- and anchor-based approaches in interpretation of changes in health-related quality of life. Med Care 2001;39(10):1039–47. This study showed that effect size and anchor-based approaches provide equivalent information in chronic disease.
J. C. Statistical Power Analysis for the Behavioural Sciences. London: Academic Press; 1969.
Testa MA. Interpreting quality-of-life clinical trial data for use in the clinical practice of antihypertensive therapy. J Hypertens Suppl. 1987;5(1):S9–13.
Wyrwich KW, Tierney WM, Wolinsky FD. Using the standard error of measurement to identify important changes on the Asthma Quality of Life Questionnaire. Qual Life Res. 2002;11(1):1–7.
Gil Z, Abergel A, Spektor S, Cohen JT, Khafif A, Shabtai E, et al. Quality of life following surgery for anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2003;129(12):1303–9.
• Abergel A, Fliss DM, Margalit N, Gil Z. A prospective evaluation of short-term health-related quality of life in patients undergoing anterior skull base surgery. Skull Base;20(1):27–33. A prospective study that showed that the overall deteriorated QOL of patients after anterior skull base tumor resection returns to baseline by 1 year after surgery. Histology and radiotherapy are significant predictors of health-related QOL in this population.
Gil Z. Unpublished data. 2011.
Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47(1):81–7.
Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–9.
Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262(7):907–13.
Pant H, Bhatki AM, Snyderman CH, Vescan AD, Carrau RL, Gardner P, et al. Quality of life following endonasal skull base surgery. Skull Base 20(1):35–40.
Kelleher MO, Fernandes MF, Sim DW, O’Sullivan MG. Health-related quality of life in patients with skull base tumours. Br J Neurosurg. 2002;16(1):16–20.
Palme CE, Irish JC, Gullane PJ, Katz MR, Devins GM, Bachar G. Quality of life analysis in patients with anterior skull base neoplasms. Head Neck. 2009;31(10):1326–34.
Levine NB, Demonte F. Functional outcome in the neurosurgical patient and its impact on quality of life. Skull Base 20(1):19–22.
Janecka IP, Sen C, Sekhar LN, Ramasastry S, Curtin HD, Barnes EL, et al. Cranial base surgery: results in 183 patients. Otolaryngol Head Neck Surg. 1994;110(6):539–46.
Ward PD, Heth JA, Thompson BG, Marentette LJ. Esthesioneuroblastoma: Results and Outcomes of a Single Institution’s Experience. Skull Base. 2009;19(2):133–40.
Witgert ME, Veramonti T, Hanna E. Instruments for estimation of health-related quality of life in patients with skull base neoplasms. Skull Base 20(1):5–10.
Cooper LA, Ford DE, Ghods BK, Roter DL, Primm AB, Larson SM, et al. A cluster randomized trial of standard quality improvement versus patient-centered interventions to enhance depression care for African Americans in the primary care setting: study protocol NCT00243425. Implement Sci 5:18.
Gil Z, Abergel A, Spektor S, Khafif A, Fliss DM. Patient, caregiver, and surgeon perceptions of quality of life following anterior skull base surgery. Arch Otolaryngol Head Neck Surg. 2004;130(11):1276–81.
Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000;18(5):419–23.
Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55(4):992–9.
Martinez-Devesa P, Barnes ML, Alcock CJ, Kerr RS, Milford CA. Evaluation of quality of life and psychiatric morbidity in patients with malignant tumours of the skull base. J Laryngol Otol. 2006;120(12):1049–54.
Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23(5):389–98.
Reimer J, Gilg K, Karow A, Esser J, Franke GH. Health-related quality of life in blepharospasm or hemifacial spasm. Acta Neurol Scand. 2005;111(1):64–70.
Gil Z, Abergel A, Leider-Trejo L, Khafif A, Margalit N, Amir A, et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17(1):25–37.
French EA, Gilkey MB, Earp JA. Patient advocacy: putting the vocabulary of patient-centered care into action. N C Med J. 2009;70(2):114–9.
Acknowledgments
This research was supported by the Legacy Heritage Biomedical Science Partnership Program of the Israel Science Foundation (No. 1680/08), the Israel Cancer Association (grant donated by Ellen and Emanuel Kronitz in memory of Dr. Leon Kronitz No. 20090068), the Israeli Ministry of Health (No. 3-7355), the Weizmann Institute—Sourasky Medical Center Joint Grant, the Tel Aviv Sourasky Intramural Grant, the ICRF Barbara S. Goodman Endowed Research Career Development Award (2011-601-BGPC), and a grant from the US–Israel Binational Science Foundation (No. 2007312) to Z.G. Esther Eshkol is thanked for her editorial assistance.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amit, M., Abergel, A., Fliss, D.M. et al. The Clinical Importance of Quality-of-Life Scores in Patients with Skull Base Tumors: A Meta-Analysis and Review of the Literature. Curr Oncol Rep 14, 175–181 (2012). https://doi.org/10.1007/s11912-012-0222-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-012-0222-3