Abstract
Purposeof Review
Diabetes mellitus (DM) causes systemic vascular complications. Chronic hyperglycemia is a hallmark of DM and appears to be at least partially responsible for the vascular complications. In addition, hyperglycemia during acute tissue injury has been postulated to augment the injury. This review addresses the potential therapeutic benefits related to ischemic stroke from lowering hyperglycemia in two settings, in chronic hyperglycemia and during acute ischemic stroke.
Recent Findings
A recent efficacy trial to lower hyperglycemia during acute ischemic stroke showed no significant benefit overall as well as in patient subgroups. This finding helps to establish good clinical practice protocols for patients with acute ischemic stroke and hyperglycemia.
Summary
Hyperglycemia appears to be a key mediator of the systemic vascular complications of DM. Despite current lack of evidence that lowering hyperglycemia during acute ischemic stroke improves functional outcome, unanswered questions remain in specific acute ischemic stroke settings that warrant additional research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is manifested by disturbed glucose metabolism resulting in chronic hyperglycemia, leading to systemic vascular complications. Multiple mechanisms have been postulated to explain the vascular complications in DM. [1] DM has been estimated to increase the risk of ischemic stroke by a factor of 2.27, hemorrhagic stroke by 1.56, and unspecified stroke by 1.84. [2] The severity of chronic hyperglycemia, as measured by glycated hemoglobin A1c (HbA1c) levels, is directly associated with risk of stroke. [3] Thus, until a cure for DM becomes available, lowering the accompanying chronic hyperglycemia seems a plausible therapeutic target.
In addition to multiple systemic vascular complications related to chronic hyperglycemia, during acute ischemic stroke, hyperglycemia has been suspected of exacerbating the brain injury. [4] Many preclinical studies supported this hypothesis, but they were limited by clinically unrealistic designs and provided inconsistent results. Human observational studies frequently showed an association between hyperglycemia during acute stroke and worse outcomes. [5] However, the cause and effect relationship could not be established in the observational studies and equipoise developed about the optimal blood glucose target during acute stroke. Meta-analyses of small randomized preliminary studies and one randomized efficacy trial were inconclusive. [6].
This review summarizes ischemic stroke outcomes in patients with chronic hyperglycemia and in patients with hyperglycemia during acute ischemic stroke. Despite a partially shared name, hyperglycemia, and vascular complications, type 1 and type 2 DM are considerably different metabolic conditions. DM1 also leads to systemic vascular complications. However, DM1 comprises approximately 10% of all DM and there are minimal data on its relationship to stroke as compared to DM2. This review summarizes the relationship between DM2 and stroke.
Pre-diabetes and Stroke Prevention
Analogous to pre-hypertension, it could be postulated that detection and treatment of pre-diabetes might reduce the incidence of DM and consequently of stroke. A meta-analysis among general populations with prediabetes showed a small and significantly increased stroke risk. [7•] Thus, treating prediabetes may decrease the incidence of stroke and possibly other vascular complications.
A post hoc analysis of a recurrent stroke prevention trial compared pioglitazone to placebo in 2885 patients with prediabetes. [8] After 4.8 years of follow-up, pioglitazone reduced the development of DM from 9.9 to 4.7% (p < 0.001) and stroke from 9.4 to 6.9% (p = 0.01). However, bone fractures were increased with pioglitazone from 3.2 to 4.9% (p = 0.02).
In addition to stroke prevention, prediabetes has been associated with worse outcomes from acute ischemic stroke. [9, 10, 11•] This suggests that DM impairs vascular integrity or function by an additional mechanism that is independent of prolonged hyperglycemia and creates opportunities for new therapeutic targets against DM.
Diabetes Control and Stroke Prevention
Since DM carries an increased risk of stroke and is manifested by hyperglycemia, it is reasonable to postulate that reducing chronic hyperglycemia might reduce the incidence of stroke. In addition, other vascular events may also be reduced by reducing chronic hyperglycemia.
In a meta-analysis of five randomized hyperglycemia lowering treatment trials totaling 32,629 patients with DM, the glycated hemoglobin (HbA1c) was lowered an average of 0.9% in the intensive treatment groups. This analysis showed a significant reduction of cardiovascular events, but not stroke in the intensively treated patients. [12] In addition, cardiovascular mortality was associated with severe hypoglycemia in the intensively treated patients.
Another meta-analysis assessed the relation between DM control and both, macrovascular (myocardial infarction, stroke, peripheral artery disease) and microvascular (retinopathy, nephropathy, neuropathy) complications. [13] This analysis was based on eight randomized trials totaling 32,710 patients with DM. Three of these trials totaling 23,182 patients achieved HbA1c levels < 7.0% among the intensive treatment patients, but no significant difference in macrovascular or microvascular complications was observed between the two treatment groups. In addition, severe hypoglycemia was significantly more common among the intensive treatment patents.
Five of the eight randomized trials in the meta-analysis [13] totaling 9528 patients achieved HbA1c levels 7.0–7.9% among the intensively treated patients and no significant difference in macrovascular complications between the two treatment groups was seen. However, significantly lower rates of microvascular complications were observed among the intensively treated patients.
Although small reductions in A1c levels do not clearly lower the risk of stroke, they have other health benefits, if severe hypoglycemia can be avoided. By extrapolation, it seems reasonable to expect that larger reductions in HbA1c that can usually be safely achieved when the baseline HbA1c is > 10% for example will have greater therapeutic effects than those reported in the randomized clinical trials.
We should consider the possibility that hyperglycemia may primarily be a marker of DM, and that additional metabolic derangements may contribute to the observed vascular complications. For example, in addition to hyperglycemia, DM is manifested by excessive pancreatic secretions of the peptide amylin that forms cytotoxic aggregates deposited in vessel walls. [14] These vascular amylin deposits lead to microvascular complications. This may partly account for a lack of association between reduction in HbA1c and stroke prevention.
Hyperglycemia During Acute Ischemic Stroke
In addition to the well-defined vascular complications from chronic hyperglycemia, during acute ischemic stroke, hyperglycemia has been associated with worse functional outcomes compared to normoglycemia. [15, 16] The types of complications from hyperglycemia that worsen functional outcome during acute ischemic stroke can be divided into augmentation of the ischemic cell injury, impaired thrombolysis, and hemorrhagic transformation of the infarct. [17, 18].
Evidence for augmentation of cerebral ischemic cell injury by hyperglycemia is currently largely based on accumulated preclinical studies. [19] However, one intriguing post hoc clinical study found that hyperglycemia was associated with greater severity of ischemic brain injury than normoglycemia on MRI, measured by apparent diffusion coefficients. [20] In addition, this association was present regardless of middle cerebral artery recanalization status.
Evidence for impaired thrombolysis in the presence of hyperglycemia comes from studies in tPA-treated patients with MCA occlusions monitored with Doppler ultrasonography. [21, 22] These studies showed that complete MCA recanalization 2 h after tPA bolus was significantly reduced in patients with hyperglycemia by approximately 50%. Hyperglycemia was defined as > 158 mg/dL in one study and ≥ 140 mg/dL in the other.
In patients with acute ischemic stroke treated with intravenous thrombolysis, the risk of symptomatic hemorrhagic transformation (sHT) is increased with acute hyperglycemia [23, 24] as well as chronic hyperglycemia indicated by HbA1c, [24, 25] which contributes to worse functional outcome. Without thrombolysis in acute ischemic stroke, the risk of sHT is less than 1%, and the impact of hyperglycemia on sHT in that population is likely very small. In a post hoc analysis of a randomized acute stroke anticoagulation trial, using low molecular weight heparinoid, admission hyperglycemia was not associated with increased risk of sHT. [26].
Despite none or relatively small doses of thrombolytics used with mechanical thrombectomy, this intervention is also associated with an increased risk of sHT. [27•, 28•] Perhaps in this setting the hyperglycemia increases ischemic tissue reperfusion injury. [29].
Despite uncertainty about its mechanism, the accumulating evidence that hyperglycemia during acute ischemic stroke is associated with worse functional outcomes has led to the development of clinical protocols to rapidly and safely lower hyperglycemia during acute ischemic stroke. Intravenous insulin is the natural choice to achieve rapid reductions in hyperglycemia. Usually, such insulin infusion protocols are computerized, which can optimize adherence and minimizes human error.
Preliminary clinical studies showed reasonable feasibility and safety of intensive intravenous insulin treatments for hyperglycemia during acute ischemic stroke, [30, 31] and informed the design of a subsequent efficacy trial. Hypoglycemia was more common in the intensive insulin treatment groups, but not resulting in serious or permanent complications.
Randomized Efficacy Trials in Acute Ischemic Stroke
The first efficacy trial to lower hyperglycemia during acute ischemic stroke, GIST-UK (Glucose Insulin Stroke Trial – United Kingdom), was stopped earlier than planned and did not demonstrate efficacy from the intensive intervention. [32] However, after the GIST-UK trial, many questions about treating hyperglycemia during acute stroke remained unanswered. Because 80% of the patients in the GIST-UK trial did not have DM, which may be the target population for this intervention, it remained unclear if patients with DM might benefit from intensive hyperglycemia treatment during acute ischemic stroke. Consequent to the high proportion of patients without DM enrolled in the GIST-UK trial, the mean blood glucose level was only 10 mg/dL lower in the intervention group than in the control group during the intervention, which does not seem to be clinically significant. In addition, the 14 h after stroke onset therapeutic intervention delay may have been too long.
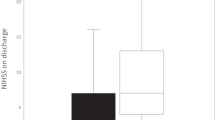
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial [33••] aimed to address the residual questions after the GIST-UK trial. The intensive intervention with intravenous insulin versus usual care subcutaneous insulin began approximately 8 h after stroke onset (1 h after enrollment). The mean blood glucose level during the 72-h study treatment was 61 mg/dL lower in the intensive than in the control group. However, the functional outcomes were similar in the two treatment groups. In addition, there was no indication that a specific patient subgroup might have benefited from the intensive intervention (Fig. 1). Severe hypoglycemia (< 40 mg/dL) occurred only in the intensively treated patients and was rapidly detected and corrected, and no related permanent injuries were observed.
Acute Ischemic Stroke Subgroup Analyses
Based on other studies, post hoc analysis of the SHINE trial data focused on patients with specific metabolic parameters that might benefit from the intensive treatment of hyperglycemia. The metabolic parameters included undiagnosed DM, glycemic gap (admission blood glucose minus the average daily blood glucose based on the HbA1c), stress hyperglycemia ratio (admission blood glucose divided by the average daily blood glucose based on the HbA1c), and blood glucose variability (defined by the standard deviation of all blood glucose measurements during protocol treatment). [34•] In this analysis, none of the specified subgroups appeared to benefit from the intensive SHINE treatment. This result should be kept in the perspective of a post hoc exploratory analysis, rather than a definitive trial. Further trials in specific subpopulation of acute stroke patients at risk for complications caused by acute hyperglycemia may be warranted.
Hyperglycemia and Reperfusion in Acute Ischemic Stroke
In the SHINE trial, only 12% of patients were treated with mechanical thrombectomy and the reperfusion status was not studied, which limits the opportunity to further analyze such data. However, subsequent increased use of mechanical thrombectomy to treat acute ischemic stroke due to large vessel occlusion provided valuable data to help answer additional important questions about hyperglycemia during acute stroke.
One important question about hyperglycemia during mechanical thrombectomy for acute ischemic stroke is whether the hyperglycemia decreases the recanalization rate. It could be expected that a metabolic derangement (hyperglycemia) will not significantly affect a mechanical intervention. Recent analyses of mechanical thrombectomy data support this expectation. [28•] Individual studies and meta-analysis consistently show no significant association between hyperglycemia or HbA1c and arterial recanalization rates with mechanical thrombectomy.
Another important consideration raised by some preclinical studies [35, 36] is whether tissue reperfusion modifies the relation between hyperglycemia and acute ischemic brain injury and consequently functional outcome. Two relatively small studies (143–341 patients) suggest that in acute stroke with incomplete recanalization by mechanical thrombectomy, hyperglycemia worsens functional outcome more than in with complete recanalization. [37, 38] However, in another analysis of 2908 patients treated with mechanical thrombectomy for acute stroke, recanalization did not modify the detrimental effect of hyperglycemia on functional outcome. [39].
A majority of the SHINE trial patients (64%) were treated with standard intravenous thrombolysis, which has been shown to nearly double the arterial recanalization rate, from 24 (spontaneous recanalization) to 46%. [40] However, there was no indication that the subgroup of patient treated with intravenous thrombolysis in the SHINE trial benefited from the intensive treatment (Fig. 1).
Lacunar type strokes have been traditionally considered permanent occlusion small vessel strokes with no significant collateral blood supply to the ischemic region. Thus, acute lacunar strokes could be considered having no recanalization or reperfusion. In the SHINE trial, 23% of patients had a lacunar stroke, and there was no indication that this stroke subtype modified the effect of the intensive intervention (Fig. 1).
In addition, results from large mechanical thrombectomy in acute stroke studies show an association between hyperglycemia and symptomatic intracerebral hemorrhage risk. [28•] Whether this association is modified by recanalization and whether intensive lowering of blood glucose will significantly decrease this complication requires further research.
Future Potentially Fruitful Therapeutic Opportunities in Acute Ischemic Stroke
Now, additional questions may be asked for possible future investigation (Table 1). One, was the 8-h delay to start the SHINE intensive treatment too long? In other words, is there a time threshold below which lowering hyperglycemia during acute ischemic stroke improves functional outcome?
Two, was the 61-mg/dL difference in average blood glucose levels between the two SHINE treatment groups large enough to be clinically significant? Perhaps a larger reduction of hyperglycemia would have been beneficial. Naturally, testing such hypothesis would require a higher glucose level in the control group, such as 250 mg/dL or more. However, there is no support for this hypothesis in the SHINE trial data (Fig. 1), where patients with baseline blood glucose levels 251–549 mg/dL did not appear to benefit from the intensive intervention.
Three, does reperfusion of the ischemic brain modify the effect of intensive hyperglycemia treatment? Perhaps achieving lower blood glucose levels faster in patients with good reperfusion is more beneficial than without reperfusion by limiting reperfusion injury.
Conclusions
Although cautious reduction of chronic hyperglycemia in DM has clinical benefits, cautious rapid lowering of hyperglycemia during acute ischemic stroke does not seem to improve functional outcome. One simple explanation for this recent finding is that the functional outcomes after stroke are somehow determined by DM and premorbid chronic hyperglycemia. However, there may be high-risk patient subgroups, such as those with acutely recanalized large vessel occlusions, where rapid lowering of hyperglycemia may limit brain reperfusion injury during acute stroke and improve functional outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Investig. 2010;1:77–89.
Sarwar N, Gao P, KondapallySeshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22.
Mitsios JP, Ekinci EI, Mitsios GP, Churilov L, Thijs V. Relationship between glycated hemoglobin and stroke risk: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(11):007858.
Luitse MJA, Biessels GJ, Rutten GEHM, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11:261–71.
Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diabetes Investig. 2019;10(3):780–92.
Ntaios G, Papavasileiou V, Bargiota A, Makaritsis K, Michel P. Intravenous insulin treatment in acute stroke: a systematic review and meta-analysis of randomized controlled trials. Int J Stroke. 2014;9(4):489–93.
• Cai X, Zhang Y, Li M, Wu JHY, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:848–9. This report summarizes the major vascular complications associated with prediabetes.
Spence JD, Viscoli CM, Inzucchi SE, Dearborn-Tomazos J, Ford GA, Gorman M, et al. Pioglitazone therapy in patients with stroke and prediabetes: a post hoc analysis of the IRIS randomized clinical trial. JAMA Neurol. 2019;76(5):526–35.
Ago T, Matsuo R, Hata J, Wakisaka Y, Kuroda J, Kitazono T, et al. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology. 2018;90(17):E1470–7.
Zhou M, Pan Y, Jing J, Wang Y, Zhao X, Liu L, et al. Association between β-cell function estimated by HOMA-β and prognosis of non-diabetic patients with ischaemic stroke. Eur J Neurol. 2018;25(3):549–55.
• Pan Y, Chen W, Wang Y. Prediabetes and outcome of ischemic stroke or transient ischemic attack a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2019;28(3):683–92. This report summarizes the functional outcomes after cerebral ischemic events associated with prediabetes.
Mannucci E, Monami M, Lamanna C, Gori F, Marchionni N. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2009;19(9):604–12.
Ma J, Yang W, Fang N, Zhu W, Wei M. The association between intensive glycemic control and vascular complications in type 2 diabetes mellitus: a meta-analysis. Nutr Metab Cardiovasc Dis. 2009;19(9):596–603.
Despa F, Goldstein LB. Amylin dyshomeostasis hypothesis: small vessel-type ischemic stroke in the setting of type-2 diabetes. Stroke. 2021;52:E244–9.
Desilles JP, Meseguer E, Labreuche J, Lapergue B, Sirimarco G, Gonzalez-Valcarcel J, et al. Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke. 2013;44:1915–23.
Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34(9):2208–14.
Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol Nat Pub Group. 2010;6:145–55.
Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37:267–73.
Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64(6):654–63.
Rosso C, Pires C, Corvol JC, Baronnet F, Crozier S, Leger A, et al. Hyperglycaemia, insulin therapy and critical penumbral regions for prognosis in acute stroke: further insights from the INSULINFARCT trial. PLoS ONE. 2015;10(3): e0120230.
Ribo M, Molina C, Montaner J, Rubiera M, Delgado-Mederos R, Arenillas JF, et al. Acute hyperglycemia state is associated with lower tPA-induced recanalization rates in stroke patients. Stroke. 2005;36(8):1705–9.
Saqqur M, Shuaib A, Alexandrov AV, Sebastian J, Khan K, Uchino K. The correlation between admission blood glucose and intravenous rt-PA-induced arterial recanalization in acute ischemic stroke: a multi-centre TCD study. Int J Stroke. 2015;10(7):1087–92.
Ahmed N, Dávalos A, Eriksson N, Ford GA, Glahn J, Hennerici M, et al. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR). Arch Neurol. 2010;67(9):1123–30.
Masrur S, Cox M, Bhatt DL, Smith EE, Ellrodt G, Fonarow GC, et al. Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post-thrombolysis: findings from get with the guidelines-stroke. J Am Heart Assoc. 2015;4(10):e002193.
Rocco A, Heuschmann PU, Schellinger PD, Köhrmann M, Diedler J, Sykora M, et al. Glycosylated hemoglobin a1 predicts risk for symptomatic hemorrhage after thrombolysis for acute stroke. Stroke. 2013;44(8):2134–8.
Bruno A, Biller J, Adams HP Jr, Clarke WR, Woolson RF, Williams LS, et al. Acute blood glucose level and outcome from ischemic stroke. Neurology. 1999;52(2):280–4.
• Zang L, Zhang D, Yao Y, Wang Y. Symptomatic intracranial hemorrhage in patients with admission hyperglycemia and diabetes after mechanical thrombectomy: a systematic review and meta-analysis. Am J Emerg Med WB Saunders. 2021;45:23–8. This report summarizes the risk of symptomatic hemorrhagic transformation of acute brain infarcts after mechanical thrombectomy in relation to hyperglycemia.
• Perez-Vega C, Domingo RA, Tripathi S, Ramos-Fresnedo A, Kashyap S, Quinones-Hinojosa A, et al. Influence of glucose levels on clinical outcome after mechanical thrombectomy for large-vessel occlusion: a systematic review and meta-analysis. J Neurointerv Surg. 2022;14:17–21. This report sumamrizes the functional outcomes after mechanical thrombectomy for acute stroke in relation to hyperglycemia.
Wu MY, Yiang GT, Liao WT, Tsai APY, Cheng YL, Cheng PW, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46:1650–67.
Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, et al. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39(2):384–9.
Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. Glucose regulation in acute stroke patients (GRASP) trial: a randomized pilot trial. Stroke. 2009;40(12):3804–9.
Gray CS, Hildreth AJ, Sandercock PA, O’Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK glucose insulin in stroke trial (GIST-UK). Lancet Neurol. 2007;6(5):397–406.
•• Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA - J Am Med Assoc. 2019;322(4):326–35. This is the latest efficacy trial of intensive hyperglycemia lowering during acute ischemic stroke.
• Torbey MT, Pauls Q, Gentile N, Falciglia M, Meurer W, Pettigrew CL, et al. Intensive versus standard treatment of hyperglycemia in acute ischemic stroke patient: a randomized clinical trial subgroups analysis. Stroke. 2022;53(5):1510–5. This post hoc analysis of the SHINE efficacy trial focuses on patient subgroups with glycemic parameters of special interest.
Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM, et al. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab. 1997;17(5):553–9.
Liu L, Wang Z, Wang X, Song L, Chen H, Bémeur C, et al. Comparison of two rat models of cerebral ischemia under hyperglycemic conditions. Microsurg Wiley-Liss, Inc Microsurg. 2007;27(4):258–62.
Kim JT, Jahan R, Saver JL. Impact of glucose on outcomes in patients treated with mechanical thrombectomy: a post hoc analysis of the solitaire flow restoration with the intention for thrombectomy study. Stroke. 2016;47(1):120–7.
Lee SJ, Hwang YH, Hong JM, Choi JW, Yoon BS, Kang DH, et al. Impact of varying levels of hyperglycemia on clinicoradiographic outcomes after endovascular reperfusion treatment. Sci Rep. 2018;8(1):1–9.
Rinkel LA, Nguyen TTM, Guglielmi V, Groot AE, Posthuma L, Roos YBWEM, et al. High admission glucose is associated with poor outcome after endovascular treatment for ischemic stroke. Stroke. 2020;51:3215–23.
Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Human and Animal Rights and Informed Consent
This article does not involve studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stroke.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bruno, A. Pre-diabetes, Diabetes, Hyperglycemia, and Stroke: Bittersweet Therapeutic Opportunities. Curr Neurol Neurosci Rep 22, 781–787 (2022). https://doi.org/10.1007/s11910-022-01236-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01236-0