Abstract
Purpose of Review
Urgent reperfusion treatment with intravenous thrombolysis or mechanical thrombectomy reduces disability after ischaemic stroke. Imaging plays an important role in identifying patients who benefit, particularly in extended time windows. However, the role of post-treatment neuroimaging is less well established. We review recent advances in neuroimaging after reperfusion treatment and provide a practical guide to the options and management implications.
Recent Findings
Post-treatment imaging is critical to identify patients with reperfusion-related haemorrhage and oedema requiring intervention. It also can guide the timing and intensity of antithrombotic medication. The degree of reperfusion on post-thrombectomy angiography and infarct volume and topography using CT or MRI carry important prognostic significance. Perfusion-weighted MRI and permeability analysis may help detect persistent perfusion abnormalities post-treatment and predict haemorrhagic complications.
Summary
Post-treatment neuroimaging provides clinically relevant information to identify complications, assess prognosis and perform quality assurance after acute ischaemic stroke. Recent advances in neuroimaging represent a potential avenue to explore post-reperfusion pathophysiology and uncover therapeutic targets for secondary ischaemic and haemorrhagic injury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urgent reperfusion to restore cerebral blood flow to salvageable ischaemic tissue and reduce patient disability is the primary aim of hyperacute treatment in ischaemic stroke. The efficacy and safety of intravenous thrombolysis (IVT) is well established for ischaemic stroke patients presenting within 4.5 h of onset [1]. In patients presenting with acute intracranial arterial large vessel occlusion (LVO), endovascular thrombectomy (EVT) with or without IVT has been unequivocally shown to substantially reduce disability [2].
A cornerstone of acute reperfusion treatment is the use of neuroimaging to select the appropriate patients for intervention. A pre-requisite for IVT is the exclusion of intracerebral haemorrhage based on non-contrast CT (NCCT) while accurate identification and localisation of LVO on CT or MR angiogram is required when considering EVT. The use of multimodal imaging including perfusion further enhances clinical decision-making by predicting treatment response [3]. For patients presenting with unclear time of onset or beyond the conventional therapeutic time windows, CT perfusion imaging or multimodal MRI is critical for selecting appropriate candidates for EVT and IVT respectively [4,5,6,7,8].
The role of post-treatment neuroimaging is less well established. The utility, timing and modality of follow-up imaging are often variable and a matter of debate [9,10,11]. In this article, we review recent research in neuroimaging after reperfusion treatment and aim to provide a practical guide to the rationale and management implications of imaging performed in the immediate, early post-treatment (< 24 h) and post-acute (24–48 h) period after IVT or EVT (Table 1).
Immediate Post-thrombectomy and Peri-procedural Imaging
In patients undergoing EVT, a dedicated series of digital subtraction angiography (DSA) is routinely acquired immediately following recanalisation or just before concluding the procedure to assess cerebral blood flow angiographically. This “completion angiogram” provides early prognostication, complication detection and quality assurance at a time when additional endovascular intervention can still be conveniently performed if required [12].
Recanalisation, Vessel Patency and Reperfusion
The degree of recanalisation of the primary occlusion can be assessed by the arterial occlusion lesion (AOL) score which was introduced in the first Interventional Management of Stroke (IMS) study in 2005 [13]. The AOL score assesses the occlusive lesion site using four grades where grade 0 is defined as complete occlusion and grade 3 defined as complete recanalisation with evidence of anterograde flow. Incomplete recanalisation is scored as grades 1 or 2 and carries an inherent risk of re-occlusion that may require antithrombolytic prophylaxis [14]. A limitation of the AOL score is the lack of assessment in vasculature beyond the initial occlusion as it is now well recognised that complete recanalisation of the initially occluded arterial segment may not lead to full reperfusion. The AOL score hence provides only a basic measure of procedural success.
In practice, the success of neurointervention is evaluated by the degree of tissue-level reperfusion which is visually evident as capillary blushing on DSA [15]. The most widely established scoring system is the modified treatment in cerebral ischaemia (mTICI) score which has been universally used among contemporary EVT randomised clinical trials (RCT). The mTICI stratifies the extent and speed of anterograde perfusion of the ischaemic territory into 5 grades: grade 0 with no perfusion; grade 1 as impaired anterograde flow with limited distal branch filling and little or slow distal reperfusion; grade 2a as anterograde reperfusion of less than half of the initial ischaemic territory; grade 2b as more than half but incomplete anterograde reperfusion; and grade 3 with complete anterograde perfusion.
The mTICI score is an important prognostic marker as the likelihood of good functional outcome correlates strongly with increasing degree of reperfusion. Grade 2b has been used as the yardstick of procedural success in the positive thrombectomy trials but only represents restoration of flow to > 50% of the involved territory [16, 17]. Recent data have confirmed that each incremental increase in reperfusion from 50–66% to 67–90% to 90–99% and 100% is associated with further improvements in functional outcome and cost effectiveness [18••]. This granular “expanded” TICI (eTICI) has yet to be widely adopted and is likely to predominantly be a research tool. Nonetheless aiming for eTICI 2c/3 (> 90%) reperfusion should probably be the aim in clinical practice.
Procedural-Related Complication Monitoring
Immediate post-intervention DSA is also important in detecting procedural-related complications that include clot embolisation, vessel perforation with subarachnoid haemorrhage, vasospasm and dissection [19•].
Peri-procedural embolisation may occur in the same territory of the target vessel when clot fragments dissipate distally or may occur in a new vascular territory. The incidence has been reported to be up to 8.6% in the multicentre randomised clinical trial of endovascular treatment for acute ischaemic stroke in the Netherlands (MR CLEAN) among the contemporary EVT RCTs [20]. The risk of embolisation is higher in more proximal occlusions and may be reduced by achieving proximal flow arrest, e.g. with a balloon guide catheter [21]. Recognition of distal embolisation on the completion angiogram allows the interventionist potential to address the new occlusion by additional clot retrieval or intraarterial thrombolysis [22].
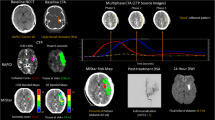
Iatrogenic vessel perforation is a rare but feared complication of neurointervention that is associated with significant morbidity and mortality. In the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration of contemporary EVT RCTs, vessel perforation occurred in 12 of 868 patients (1.4%). A recent case series of 1599 patients reported 16 (1%) incidence of vessel perforations and 9 patients (56%) died in hospital [23]. Vessel perforations most commonly occurred at distal occlusion sites, during technically challenging occlusion navigation or when removing stent retriever devices (Fig. 1a, b).
Intraprocedural haemorrhage due to vessel perforation is identified on digital subtraction angiography (a, b) and assessed on volumetric flat-panel angiographic CT (c). In a separate patient with recanalised middle cerebral artery occlusion (d), post-thrombectomy MRI performed within 24 h showed striatocapsular infarct (e) and presence of concurrent post-ischaemic hyperperfusion and persistent hypoperfusion on MR perfusion Tmax map (f) where dark blue indicates hyperperfusion, and light-blue/yellow-red indicates hypoperfusion
Occasionally, active haemorrhagic transformation involving deep perforator arteries may also develop during neurointervention without obvious vessel perforation [24]. Intraprocedural rotational flat-panel CT performed using the angiography equipment may help assess the extent of intracranial haemorrhage by providing cross-sectional images rapidly without the need to transport the patient out of the angio-suite to a conventional CT scanner (Fig. 1c) [25]. Immediate endovascular rescue techniques such as intracranial balloon occlusion for arterial tamponade or permanent occlusion with vessel sacrifice may be required in patients with active haemorrhage from vessel perforation.
Arterial dissection and vasospasm have been reported to occur in up to 3.9% and 23% respectively among contemporary EVT RCTs. These are thought to arise as a result of vessel wall manipulation with subsequent intimal injury, vascular reactivity and inflammation [26, 27]. Although usually asymptomatic [28], haemodynamically significant flow limitation has been reported [22]. Management options for flow-limiting dissections include balloon angioplasty without or without stenting while judicious blood pressure manipulation and intraarterial calcium channel antagonist such as verapamil may be required for vasospasm.
Finally, post-intervention DSA may also help identify patients at risk of symptomatic haemorrhagic transformation after thrombectomy. Early venous filling and prominent cerebral vascularity with capillary blush detected on post-intervention DSA are presumed to be due to altered cerebral haemodynamics and loss of autoregulation due to ischaemia. These findings have been shown to be predictive of symptomatic parenchymal haematoma development [29,30,31]. Although there is no proven prophylaxis for the development of reperfusion haemorrhage, these findings may help identify an at-risk population who may benefit from closer blood pressure monitoring and a longer delay before initiating antiplatelet or anticoagulation therapy.
Early Post-treatment Imaging (< 24 H)
In many centres, follow-up CT or MRI is routinely performed 24 h following reperfusion treatment. Earlier neuroimaging is indicated in the event of unexpected clinical deterioration.
Haemorrhagic Transformation
The major concern in patients with abrupt neurological deterioration after reperfusion treatment is symptomatic intracerebral haemorrhage (sICH). The rates of sICH were reported to be 7.6% and 4.4% among pooled thrombolysis and thrombectomy trials, respectively [32•]. The fundamental underlying pathophysiology is disruption to the blood-brain barrier (BBB) secondary to ischaemic endothelial injury, extracellular matrix breakdown, inflammation and abnormal cerebrovascular haemodynamics leading to increased tissue permeability [33•, 34].
Haemorrhagic transformation (HT) in ischaemic stroke is commonly classified into 4 radiological categories using the European Cooperative Acute Stroke Study (ECASS) system. In this system, haemorrhagic infarction type 1 (HI1) is defined as isolated petechiae within the infarcted area, haemorrhagic infarction type 2 (HI2) as confluent petechiae without mass effect, parenchymal haematoma type 1 (PH1) as haematoma occupying less than 30% of the infarcted area and parenchymal haematoma type 2 (PH2) as haematoma occupying more than 30% of the infarcted area with significant mass effect [35]. Of the four categories, PH2 has consistently correlated with early neurological deterioration and poor clinical outcome whereas the other subtypes generally have not [36]. Early definitions of symptomatic haemorrhage included any degree of clinical deterioration and any degree of haemorrhagic transformation which was overly conservative and led to many patients with large infarcts as the cause of their deterioration being classified as sICH. The SITS definition requires PH2 and ≥ 4 point increase in National Institute of Health Stroke Scale (NIHSS) as well as limiting to 36 h after thrombolysis to be deemed related. Recently, the Heidelberg classification system was proposed in recognition of the increasing role of EVT in acute stroke care and incorporates thrombectomy-related intraventricular, subarachnoid and subdural haemorrhages into the classification [37••].
Clinically significant sICH usually develops early following reperfusion treatment with 80% of fatal haemorrhages occurring within 12 h of thrombolysis and the remainder occurring within 24 h [38]. Notably, iodine-based contrast administered during EVT often remains in the brain parenchyma during the first 24 to 48 h and appears hyperdense on NCCT [39•]. While the absence of mass effect and excessive hyperattenuation beyond that expected for acute blood product (Hounsfield Unit exceeding 120) suggest artefactual contrast-staining, differentiation between acute blood product and residual contrast on conventional NCCT can be challenging [19•]. In theory, iodine-based contrast at high concentration may also impact on T1 and T2-relaxation time on early MRI performed within 48 h of EVT [40].
This problem can be overcome by two strategies. Dual-energy CT (DECT) uses two different ionizing energy levels (kV) and translates the differences in the photoelectric and Compton scattering properties between iodine and blood into different pixel attenuation. DECT therefore can generate an iodine-only image which can then be subtracted to generate a virtual non-contrast image. If that still shows hyperdensity then there is true haemorrhage. The sensitivity and specificity of this technique is reported to be 100% and 92.8%, respectively, although regions of blood-brain barrier permeability to iodine have an increased risk of haemorrhagic transformation in the ensuing days. CT scanners with DECT capabilities are not widely available, are expensive to acquire and maintain, and require specialised post-processing that often renders DECT impractical beyond major academic institutions. However, as contrast-staining mainly follows EVT, DECT is primarily relevant at comprehensive stroke centres rather than primary stroke centres.
An alternative strategy to distinguish between contrast and haemorrhage is the use of MRI gradient-echo T2*-weighted (GRE) and susceptibility-weighted imaging (SWI). Both sequences are highly sensitive for haemorrhage and relatively insensitive to iodine contrast [41, 42]. SWI can also detect hypointense thrombus fragments as a marker of distal embolisation. MRI is superior in spatial resolution for characterizing cerebral pathology and infarct volume compared to NCCT in the early post-treatment period when infarcted brain may still appear isodense. Despite being much more widely available than DECT, urgent MRI access is often limited and the longer scan times and greater susceptibility to motion artefact can make MRI more challenging in unstable acute stroke patients.
Management of Antithrombotics in Patients with Stents
In selected cases of acute tandem extracranial carotid artery occlusion, pre-existing intracranial atherosclerotic disease or iatrogenic vascular dissection, acute stenting may be performed as part of acute revascularisation. Very early clinical deterioration may be due to acute in-stent thrombosis which is reported to occur in up to 12.5% of patients in earlier case series [43]. Acute intracranial stenting is avoided where possible due to the unfavourable risk benefit ratio demonstrated in randomised trials. Extracranial carotid stenting may be required to gain access to intracranial vasculature or to maintain patency of critical stenosis after endovascular thrombectomy. Patients with stents warrant close clinical observation and may require emergency follow-up imaging to diagnose acute stent thrombosis and trigger rescue revascularisation.
The early introduction of antiplatelet medications may be considered in patients after stent insertion. This may be in violation of the usual exclusion of antithrombotics within 24 h of thrombolysis if the risk of acute stent thrombosis is felt to be high. Knowledge of the infarct volume and presence of haemorrhagic transformation is useful information when balancing the risk of stent thrombosis versus haemorrhagic transformation.
Cerebral Oedema
Cerebral oedema after ischaemic stroke can cause severe secondary injury by rapidly increasing intracranial pressure. It is associated with substantial morbidity and is a cause of death in 5% of patients with ischaemic stroke [44]. Reported risk factors for cerebral oedema after ischaemic stroke include high baseline NIHSS, hyperdense artery sign, elevated baseline serum glucose level, large ischaemic core on baseline CTP and large established infarction on baseline NCCT [45•].
The association between reperfusion and cerebral oedema is unclear. Experimental evidence suggests that ischaemic BBB disruption combined with reperfusion is a cause of cerebral oedema [46]. However, clinical data from IVT studies suggest reperfusion may attenuate oedema, presumably by successfully salvaging tissue and reducing the final infarct volume [47•, 48]. The latter is supported by a recent secondary analysis of the MR CLEAN trial which is the only study to date using contemporary thrombectomy RCT data to investigate the relationship between reperfusion and cerebral oedema [49••]. Further data from pooled thrombectomy RCTs may help clarify the interaction between cerebral oedema, reperfusion and treatment modality.
The presentation and management of cerebral oedema after ischaemic stroke differs according to the infarct location. Extensive middle cerebral artery (MCA) infarct involving the entire vascular territory is the most common supratentorial infarction causing oedema. The risk of subsequent neurological deterioration and death in this setting has been reported to be up to 80% if untreated [50]. Radiological findings of MCA oedema include ipsilateral sulcal effacement, compression of the ipsilateral ventricular system, progressive shift of midline anatomical structures away from the infarct, subfalcine and uncal herniation [51•]. The distance of midline shift (MLS) is commonly used as the semi-quantitative radiological measure of oedema in practice and research. At present, a critical threshold of MLS to predict surgical decompression has not been established.
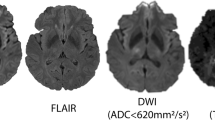
For patients with MCA-territory stroke investigated with MRI, acute diffusion-weighted imaging (DWI) lesion volume has been used to predict neurological deterioration from cerebral oedema. Acute DWI volume of > 80 ml on MRI acquired within 6 h of stroke onset and > 145 ml when imaged 14 h from stroke onset has been shown to predict rapid early neurological deterioration and a need for neurosurgery [52, 53]. However, post-reperfusion DWI often demonstrates temporary diffusion lesion reversal. The apparent diffusion coefficient recovers temporarily so the standard threshold of < 620 × 10−6 mm2/s may not segment the entire visually evident lesion and in some cases the lesion is also not visible on the B1000 image [54]. Decompressive craniectomy performed within 48 h of stroke onset for patients 60 years old or younger reduces mortality by 50% and 72% of survivors post-hemicraniectomy achieve minimal to moderate disability [55••].
In infra-tentorial infarcts, progressive oedema most commonly results from expanding cerebellar hemisphere infarct. Infra-tentorial oedema may obliterate the fourth ventricle causing acute obstructive hydrocephalus and potentially fatal tonsillar herniation with direct compression of brainstem structures. Decompressive suboccipital craniectomy with dural expansion with or without ventriculostomy are recommended in guidelines [51•]. In the absence of concurrent brain stem infarction, surgical survivors often have good recovery with 40% of patients regaining functional independence [56]. In contrast to supratentorial and MCA infarct, a cerebellar infarction volume predictive of the need for decompression has not been established.
Post-acute Follow-Up Neuroimaging (24–48 H)
Follow-up neuroimaging with CT or MRI is usually performed approximately 24 h after thrombolysis or thrombectomy to assess for haemorrhagic transformation. Although large parenchymal haematoma are uncommon among clinically stable patients, asymptomatic PHs and HI2 can be present in 1.8% and 6.0% of patients, respectively [9]. This is clinically relevant when deciding when to commence antithrombotics after an acute infarction and is especially applicable for patients undergoing EVT as the clinical improvement gained from tissue salvage may mask symptom progression from concurrent ICH development [11]. In addition, follow-up neuroimaging at this time point also provides important prognostic information and is a quality assurance measure after reperfusion treatment.
Infarction Visualisation and Quantification
A fundamental role of follow-up neuroimaging is to visualise the infarcted tissue to confirm the diagnosis and vascular distribution of cerebral ischaemia. IVT decision-making is commonly based on a clinical diagnosis of acute ischaemic stroke. When CT perfusion is not routinely performed, patients with stroke mimics not infrequently receive thrombolysis with a mimic rate of 15% in a recent series [57] and 17% reported in the NOR-TEST trial [58]. Securing the diagnosis of cerebral infarction on follow-up neuroimaging is therefore clinically important for the purpose of prognosticating neurological recovery, predicting risk of future stroke recurrence and guiding the need of stroke work-up and secondary prophylaxis. For example, identifying multi-territory infarction would suggest an embolic or systemic aetiology that is likely to require dedicated comprehensive cardiac investigations.
Infarct Evolution
Infarct volume assessment at 24 h is an important prognostic marker and is used as a radiological endpoint in research [59•, 60••]. Regions of restricted diffusion appearing hyperintense on B1000 diffusion-weighted imaging general reflect irreversible tissue injury and are highly sensitive. However, infarct evolution is a dynamic process and DWI lesions may grow or temporarily, and in some cases permanently, recede in the acute period (Table 2).
The frequency, extent and clinical significance of DWI lesion reversal (DLR) has been a matter of ongoing debate. While earlier meta-analysis reported DLR to be frequent and present up to a quarter of patients, true DLR has been shown to be uncommon, small in volume and unlikely to be clinically relevant after adjusting for infarct atrophy [61, 62•]. In general, DLR is associated with reperfusion and may be transient, as discussed above, where restriction abnormalities temporarily normalise in the first 24 h before later re-appearing, or sustained where the abnormal restriction permanently normalises [63•, 64•]. Sustained DLR tends to be less hypoperfused at presentation, smaller, higher in apparent diffusion coefficient value (ADC) and is located in cortical regions [65]. Permanent reversal of diffusion lesions may be more frequent with rapid endovascular reperfusion. However, animal data suggest that ischaemia sufficient to cause even brief periods of diffusion restriction that fully reverse lead to neuronal loss [66•, 67]. It is possible that there is a continuum of infarct severity rather than the conventional binary approach to infarct delineation that presumes that radiologically abnormal, infarcted tissue is uniformly damaged and radiologically normal non-infarcted tissue fully preserved [68].
The underlying mechanism of DLR is also unclear. Transient DLR has been speculated to reflect salvage followed by secondary injury [69]. However, DLR changes have not been shown to correlate clinically with the expected neurological recovery and subsequent deterioration that this hypothesis might suggest [70].
Similarly, the ischaemic lesion continues to evolve after 24 h. ADC values typically increase in the days following stroke due to progressive ionic and vasogenic oedema and cell lysis in a phenomenon termed “ADC pseudo-normalisation” [71]. T2-weighted fluid-attenuated inversion recovery (FLAIR) hyperintensity is used instead to evaluate the infarct lesion after the first few days of stroke.
Assessing the infarct lesion at this subacute time frame is challenging as this coincides with maximal cerebral oedema which can substantially overestimate infarct volume [72]. Infarct lesions measured at 5 days have been shown to be up to 77.7% larger than initial acute DWI lesion measured on the first day and 115% larger than the follow-up infarct lesion measured at 3 months [73•]. New DWI abnormalities developing in a different vascular territory and anatomical distortion in adjacent tissue have been used as a surrogate marker of true infarct growth and cerebral oedema, respectively [74, 75]. However, there is no established method available currently to precisely differentiate acute infarction from secondary oedema.
The true extent of delayed infarct growth developing after 24 h has not been well studied. As cerebral oedema starts to resolve in the latter subacute period, cerebral atrophy begins to develop with a net reduction in infarct lesion size as time progresses. While earlier reports suggested infarct growth beyond 24 h is negligible with follow-up infarct volume measured at 1 and 3 months being consistently smaller than volume measured at 24-h [59•, 73•], recent data from the third Endovascular Therapy Following Imaging Evaluation of Ischaemic Stroke trial (DEFUSE 3) suggest that target mismatch (at-risk penumbral tissue) may be present up to 48 h after stroke onset and potentially contribute to delayed infarct expansion in the subacute period [76].
The prognostic significance of infarct volume measured at the 24-h time point has also been challenged recently. A pooled analysis of seven randomised multicentre randomised clinical trials investigating the efficacy of EVT has shown follow-up infarct volume measured at 24-h only explained 12% of the benefit of treatment on mediation analysis [77••]. The study concluded that 24-h infarct volume is not a valid proxy for estimating treatment effect in acute ischaemic stroke. The reason for this observation is unclear and may be due to additional benefits conferred by EVT in addition to infarct volume reduction, the potential for prolonged hypoperfusion with delayed infarct growth and the unaccounted variance of infarct topography and intralesional heterogeneity of cellular injury within the infarct lesion.
To date, the available data on infarct evolution have predominantly been derived from studies on patients receiving thrombolysis. Future studies on EVT patients where the exact timing and extent of reperfusion can be characterised may help clarify the temporal profile and determinants of DWI lesion dynamics.
Emerging Neuroimaging Techniques and Potential Application
Post-treatment Perfusion Imaging
Perfusion imaging on CT (CTP) or MR perfusion (MRP) is often performed at presentation to guide hyperacute reperfusion treatment decision-making. Perfusion imaging predicts treatment response by quantifying the extent of potentially salvageable and irreversible tissue [3, 7, 8, 78]. Occasionally, perfusion imaging is acquired post-treatment to evaluate the extent of reperfusion when angiographic assessment is not available. While resolution of perfusion abnormality in the ischaemic territory typically follows successful treatment and is a strong predictor of favourable outcome, perfusion abnormalities can persist even after recanalisation of the primary occlusion [79].
Post-ischaemic hyperperfusion following recanalisation (Fig. 1f) has been observed in up to 30–40% of patients on CTP, dynamic susceptibility contrast perfusion MRI as well as arterial spin labelling MRI [80,81,82,83•]. Studies to date have shown conflicting results regarding the nature of hyperperfusion. While some have demonstrated an association with haemorrhagic transformation and infarct growth within the hyperperfused region, other studies have shown an association with neurological improvement [84]. The mechanism of hyperperfusion is also unclear and loss of cerebrovascular autoregulation in severely injured cerebral tissue has been postulated as the underlying mechanism.
In contrast, hypoperfusion may also persist despite successful recanalisation (Fig. 1f). This “no-reflow” phenomenon was first described in animal histological studies and is hypothesised to be due to microvascular obstruction from microemboli, luminal cellular trapping and endothelial swelling [85•]. In the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE), 4/19 (21%) patients with major vessel recanalisation following IVT did not show reperfusion on follow-up PWI MRI while similar proportion of patients (4 out of 18 patients) in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) trial similarly showed persistent hypoperfusion [86, 87]. In another study using CTP, 38% of thrombolysis patient had persistent hypoperfusion at 24 h [88]. However, in these human studies it is difficult to differentiate persistent perfusion abnormalities due to residual distal occlusions that may be beyond the resolution of noninvasive angiography from true no-reflow phenomenon within infarcted regions. Interestingly, recent data using transcranial Doppler ultrasound to infer microvascular dysfunction based on flow resistance have suggested that no-reflow may be present regardless of final infarct volume and hence may not be an epiphenomenon of infarcted tissue [89].
To date, the prognostic significance of persistent altered tissue perfusion following reperfusion treatment is unclear. Whether hyperperfusion may help identify a subset of patients with high risk of symptomatic haemorrhage is uncertain. It is possible that patients with persistent hypoperfusion may benefit if regional cerebral perfusion can be augmented in addition to recanalisation [85•]. Further research is needed to address whether they are markers of secondary injury and potential therapeutic targets that may help maximise outcome following reperfusion treatment.
Blood-Brain Barrier Disruption
Radiological evaluation of BBB integrity is performed by detecting abnormal leakage of blood product or contrast material as a marker of increased BBB permeability. On neuroimaging, this presents as iodine-contrast extravasation on non-contrast CT immediately after endovascular therapy, gadolinium contrast-enhancing parenchymal hyperintensities on T1-weighted MRI and CSF-space hyperintensities on post-contrast fluid-attenuated inversion recovery (FLAIR) MRI. The latter has been termed “hyperintense acute reperfusion marker” (HARM) and is associated with HT and poor clinical recovery after thrombectomy [90,91,92]. In a study of 60 patients treated with EVT, HARM was detected in 52% of patients. Not infrequently, vessel wall injury may also present as focal enhancement of the target vessel after EVT [93]. Both findings have been attributed to mechanical injury from thrombectomy causing endothelial and BBB disruption.
In recent years, varying techniques to quantify of parenchymal BBB permeability have also been developed. BBB permeability can be assessed on CT using CTP-derived relative permeability-surface area product value while MRI permeability quantitative evaluation can be achieved by T1-based dynamic contrast-enhanced (DCE) or T2*-based dynamic susceptibility contrast (DSC) PWI [94, 95]. On quantitative BBB permeability analysis, increased permeability is shown to be associated with increased risk of ICH in a dose-dependent manner as well as poor clinical outcome [96••, 97, 98]. Emerging data have further suggested that the degree of BBB disruption varies over time and may be reversible with reperfusion.
Overall, data regarding post-treatment BBB disruption is scarce. The determinants of increased BBB permeability and whether BBB permeability can be modulated or potentially normalised to prevent ICH are unexplored areas to date.
Conclusion
Follow-up neuroimaging after thrombolysis and thrombectomy is important and should be incorporated into routine clinical practice to guide ongoing management of acute ischaemic stroke patients. Although current understanding of and therapeutic options for secondary reperfusion injury are limited, emerging techniques in neuroimaging may help advance our understanding of the phenomenon and pathophysiology in the post-reperfusion period of acute ischaemic stroke.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–35.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Campbell BCV, Majoie C, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18(1):46–55.
Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611–22.
Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795–803.
Campbell BCV, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, et al. Extending thrombolysis to 4.5-9 hours and wake-up stroke using perfusion imaging: a meta-analysis of individual patient data from EXTEND, ECASS4-EXTEND and EPITHET. Lancet. 2019. https://doi.org/10.1016/S0140-6736(19)31053-0.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–18.
George AJ, Boehme AK, Dunn CR, Beasley T, Siegler JE, Albright KC, et al. Trimming the fat in acute ischemic stroke: an assessment of 24-h CT scans in tPA patients. Int J Stroke. 2015;10(1):37–41.
Guhwe M, Utley-Smith Q, Blessing R, Goldstein LB. Routine 24-hour computed tomography brain scan is not useful in stable patients post intravenous tissue plasminogen activator. J Stroke Cerebrovasc Dis. 2016;25(3):540–2.
Campbell BC, Parsons MW. Repeat brain imaging after thrombolysis is important. Int J Stroke. 2015;10(2):E18.
Zaidat OO, Lazzaro MA, Liebeskind DS, Janjua N, Wechsler L, Nogueira RG, et al. Revascularization grading in endovascular acute ischemic stroke therapy. Neurology. 2012;79(13 Suppl 1):S110–6.
Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T, et al. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36(11):2400–3.
Qureshi AI, Siddiqui AM, Kim SH, Hanel RA, Xavier AR, Kirmani JF, et al. Reocclusion of recanalized arteries during intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2004;25(2):322–8.
Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–37.
Manning NW, Chapot R, Meyers PM. Endovascular stroke management: key elements of success. Cerebrovasc Dis. 2016;42(3–4):170–7.
Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa MA, Fugate JE, et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke. 2013;44(9):2509–12.
•• Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433–8 Analysis of eTICI outcomes.
• Balami JS, White PM, McMeekin PJ, Ford GA, Buchan AM. Complications of endovascular treatment for acute ischemic stroke: Prevention and management. Int J Stroke. 2018;13(4):348–61 Summary of thrombectomy complications.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
Chueh JY, Puri AS, Wakhloo AK, Gounis MJ. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J Neurointerv Surg. 2016;8(2):197–202.
Darkhabani Z, Nguyen T, Lazzaro MA, Zaidat OO, Lynch JR, Fitzsimmons BF, et al. Complications of endovascular therapy for acute ischemic stroke and proposed management approach. Neurology. 2012;79(13 Suppl 1):S192–8.
Mokin M, Fargen KM, Primiani CT, Ren Z, Dumont TM, Brasiliense LBC, et al. Vessel perforation during stent retriever thrombectomy for acute ischemic stroke: technical details and clinical outcomes. J Neurointerv Surg. 2017;9(10):922–8.
Haussen DC, Ferreira IM, Barreira C, Grossberg JA, Diana F, Peschillo S, et al. Active reperfusion hemorrhage during thrombectomy: angiographic findings and real-time correlation with the CT “spot sign”. Interv Neurol. 2018;7(6):370–7.
Engelhorn T, Struffert T, Richter G, Doelken M, Ganslandt O, Kalender W, et al. Flat panel detector angiographic CT in the management of aneurysmal rupture during coil embolization. AJNR Am J Neuroradiol. 2008;29(8):1581–4.
Gupta R. Arterial vasospasm during mechanical thrombectomy for acute stroke. J Neuroimaging. 2009;19(1):61–4.
Nishino W, Tajima Y, Inoue T, Hayasaka M, Katsu B, Ebihara K, et al. Severe vasospasm of the middle cerebral artery after mechanical thrombectomy due to infective endocarditis: an autopsy case. J Stroke Cerebrovasc Dis. 2017;26(9):e186–e8.
Akins PT, Amar AP, Pakbaz RS, Fields JD, Investigators S. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices. AJNR Am J Neuroradiol. 2014;35(3):524–8.
Ohta H, Nakano S, Yokogami K, Iseda T, Yoneyama T, Wakisaka S. Appearance of early venous filling during intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: a predictive sign for hemorrhagic complications. Stroke. 2004;35(4):893–8.
Cartmell SCD, Ball RL, Kaimal R, Telischak NA, Marks MP, Do HM, et al. Early cerebral vein after endovascular ischemic stroke treatment predicts symptomatic reperfusion hemorrhage. Stroke. 2018;49(7):1741–6.
Salehi Omran S, Boddu SR, Gusdon AM, Kummer B, Baradaran H, Patel P, et al. Angiographic blush after mechanical thrombectomy is associated with hemorrhagic transformation of ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27(11):3124–30.
• Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis. 2012;34(2):106–14 Review of classification criteria of intracranial hemorrhage after reperfusion treatment.
• Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S52–7 Summary of blood-brain barrier disruption.
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018:163–164:144–71.
Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274(13):1017–25.
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30(11):2280–4.
•• von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–6 Recent revised classification of post-thrombectomy intracranial hemorrhage.
The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28(11):2109–18.
• Phan CM, Yoo AJ, Hirsch JA, Nogueira RG, Gupta R. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol. 2012;33(6):1088–94 First description of dual-energy head CT.
Yedavalli V, Sammet S. Contrast extravasation versus hemorrhage after thrombectomy in patients with acute stroke. J Neuroimaging. 2017;27(6):570–6.
Nikoubashman O, Jablawi F, Dekeyzer S, Oros-Peusquens AM, Abbas Z, Lindemeyer J, et al. MRI appearance of intracerebral iodinated contrast agents: is it possible to distinguish extravasated contrast agent from hemorrhage? AJNR Am J Neuroradiol. 2016;37(8):1418–21.
Morales H, Lemen L, Samaratunga R, Nguyen P, Tomsick T. Effects of iodinated contrast on various magnetic resonance imaging sequences and field strength: implications for characterization of hemorrhagic transformation in acute stroke therapy. World J Radiol. 2016;8(6):588–93.
Bang JS, Oh CW, Jung C, Park SQ, Hwang KJ, Kang HS, et al. Intracranial stent placement for recanalization of acute cerebrovascular occlusion in 32 patients. AJNR Am J Neuroradiol. 2010;31(7):1222–5.
Brogan ME, Manno EM. Treatment of malignant brain edema and increased intracranial pressure after stroke. Curr Treat Options Neurol. 2015;17(1):327.
• Thoren M, Azevedo E, Dawson J, Egido JA, Falcou A, Ford GA, et al. Predictors for cerebral edema in acute ischemic stroke treated with intravenous thrombolysis. Stroke. 2017;48(9):2464–71 Analysis of edema following thrombolysis.
Dong MX, Hu QC, Shen P, Pan JX, Wei YD, Liu YY, et al. Recombinant tissue plasminogen activator induces neurological side effects independent on thrombolysis in mechanical animal models of focal cerebral infarction: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158848.
• Irvine HJ, Ostwaldt AC, Bevers MB, Dixon S, Battey TW, Campbell BC, et al. Reperfusion after ischemic stroke is associated with reduced brain edema. J Cereb Blood Flow Metab. 2018;38(10):1807–17 Analyasis of edema following thrombectomy.
Cheripelli BK, Huang X, MacIsaac R, Muir KW. Interaction of recanalization, intracerebral hemorrhage, and cerebral edema after intravenous thrombolysis. Stroke. 2016;47(7):1761–7.
•• Kimberly WT, Dutra BG, Boers AMM, Alves H, Berkhemer OA, van den Berg L, et al. Association of reperfusion with brain edema in patients with acute ischemic stroke: a secondary analysis of the MR CLEAN Trial. JAMA Neurol. 2018;75(4):453–61 Recent post-hoc analysis of prospective thrombectomy trial MR CLEAN on relationship between thrombectomy and edema.
Kasner SE, Demchuk AM, Berrouschot J, Schmutzhard E, Harms L, Verro P, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32(9):2117–23.
• Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–38 National guidelines on cerebellar infarction surgical options.
Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42(5):1270–5.
Oppenheim C, Samson Y, Manai R, Lalam T, Vandamme X, Crozier S, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31(9):2175–81.
Sah RG, d'Esterre CD, Hill MD, Hafeez M, Tariq S, Forkert ND, et al. Diffusion-weighted MRI stroke volume following recanalization treatment is threshold-dependent. Clin Neuroradiol. 2019;29(1):135–41.
•• Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110 National guidelines on the indications of decompressive craniectomy for anterior circulation stroke.
Pfefferkorn T, Eppinger U, Linn J, Birnbaum T, Herzog J, Straube A, et al. Long-term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke. 2009;40(9):3045–50.
Tsivgoulis G, Zand R, Katsanos AH, Goyal N, Uchino K, Chang J, et al. Safety of intravenous thrombolysis in stroke mimics: prospective 5-year study and comprehensive meta-analysis. Stroke. 2015;46(5):1281–7.
Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Ronning OM, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781–8.
• Campbell BC, Tu HT, Christensen S, Desmond PM, Levi CR, Bladin CF, et al. Assessing response to stroke thrombolysis: validation of 24-hour multimodal magnetic resonance imaging. Arch Neurol. 2012;69(1):46–50 Data on the validity of 24hour follow-up scan post-treatment.
•• Warach SJ, Luby M, Albers GW, Bammer R, Bivard A, Campbell BC, et al. Acute stroke imaging research roadmap III imaging selection and outcomes in acute stroke reperfusion clinical trials: consensus recommendations and further research priorities. Stroke. 2016;47(5):1389–98 Current up-to-date guidelines on the use of imaging post reperfusion treatment.
Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am J Neuroradiol. 2009;30(6):1206–12.
• Campbell BC, Purushotham A, Christensen S, Desmond PM, Nagakane Y, Parsons MW, et al. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab. 2012;32(1):50–6 Earlier imaging analysis of DWI reversal.
• Soize S, Tisserand M, Charron S, Turc G, Ben Hassen W, Labeyrie MA, et al. How sustained is 24-hour diffusion-weighted imaging lesion reversal? Serial magnetic resonance imaging in a patient cohort thrombolyzed within 4.5 hours of stroke onset. Stroke. 2015;46(3):704–10 Earlier imaging analysis of DWI reversal.
• Inoue M, Mlynash M, Christensen S, Wheeler HM, Straka M, Tipirneni A, et al. Early diffusion-weighted imaging reversal after endovascular reperfusion is typically transient in patients imaged 3 to 6 hours after onset. Stroke. 2014;45(4):1024–8 Earlier imaging analysis of DWI reversal.
Albach FN, Brunecker P, Usnich T, Villringer K, Ebinger M, Fiebach JB, et al. Complete early reversal of diffusion-weighted imaging hyperintensities after ischemic stroke is mainly limited to small embolic lesions. Stroke. 2013;44(4):1043–8.
• Li F, Liu KF, Silva MD, Omae T, Sotak CH, Fenstermacher JD, et al. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats : correlation with histopathology. Stroke. 2000;31(4):946–54 Experimental data on DWI reversal.
Ringer TM, Neumann-Haefelin T, Sobel RA, Moseley ME, Yenari MA. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke. 2001;32(10):2362–9.
Tourdias T, Dousset V, Sibon I, Pele E, Menegon P, Asselineau J, et al. Magnetization transfer imaging shows tissue abnormalities in the reversible penumbra. Stroke. 2007;38(12):3165–71.
Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52(6):698–703.
Chemmanam T, Campbell BC, Christensen S, Nagakane Y, Desmond PM, Bladin CF, et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology. 2010;75(12):1040–7.
Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology. 1997;49(1):113–9.
Bucker A, Boers AM, Bot JCJ, Berkhemer OA, Lingsma HF, Yoo AJ, et al. Associations of ischemic lesion volume with functional outcome in patients with acute ischemic stroke: 24-hour versus 1-week imaging. Stroke. 2017;48(5):1233–40.
• Gaudinski MR, Henning EC, Miracle A, Luby M, Warach S, Latour LL. Establishing final infarct volume: stroke lesion evolution past 30 days is insignificant. Stroke. 2008;39(10):2765–8 Longitudinal comparison of infarct volume over time.
Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45(12):3643–8.
Harston GWJ, Carone D, Sheerin F, Jenkinson M, Kennedy J. Quantifying infarct growth and secondary injury volumes: comparing multimodal image registration measures. Stroke. 2018;49(7):1647–55.
Christensen S, Mlynash M, Kemp S, Yennu A, Heit JJ, Marks MP, et al. Persistent target mismatch profile >24 hours after stroke onset in DEFUSE 3. Stroke. 2019;50(3):754–7.
•• Boers AMM, Jansen IGH, Brown S, Lingsma HF, Beenen LFM, Devlin TG, et al. Mediation of the relationship between endovascular therapy and functional outcome by follow-up infarct volume in patients with acute ischemic stroke. JAMA Neurol. 2019;76(2):194–202 Recent HERMES meta-analysis of prognostic value of follow-up infarct volume following thrombectomy or thrombolysis.
van Seeters T, Biessels GJ, Kappelle LJ, van der Schaaf IC, Dankbaar JW, Horsch AD, et al. The prognostic value of CT angiography and CT perfusion in acute ischemic stroke. Cerebrovasc Dis. 2015;40(5–6):258–69.
Lin L, Cheng X, Bivard A, Levi CR, Dong Q, Parsons MW. Quantifying reperfusion of the ischemic region on whole-brain computed tomography perfusion. J Cereb Blood Flow Metab. 2017;37(6):2125–36.
Nguyen TB, Lum C, Eastwood JD, Stys PK, Hogan M, Goyal M. Hyperperfusion on perfusion computed tomography following revascularization for acute stroke. Acta Radiol. 2005;46(6):610–5.
Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, et al. Diffusion-perfusion MRI characterization of post-recanalization hyperperfusion in humans. Neurology. 2001;57(11):2015–21.
Yu S, Liebeskind DS, Dua S, Wilhalme H, Elashoff D, Qiao XJ, et al. Postischemic hyperperfusion on arterial spin labeled perfusion MRI is linked to hemorrhagic transformation in stroke. J Cereb Blood Flow Metab. 2015;35(4):630–7.
• Okazaki S, Yamagami H, Yoshimoto T, Morita Y, Yamamoto H, Toyoda K, et al. Cerebral hyperperfusion on arterial spin labeling MRI after reperfusion therapy is related to hemorrhagic transformation. J Cereb Blood Flow Metab. 2017;37(9):3087–90 Recent clinical data on post-treatment hyperperfusion in relation to hemorrhagic transformation.
Shimonaga K, Matsushige T, Hosogai M, Hashimoto Y, Mizoue T, Ono C, et al. Hyperperfusion after endovascular reperfusion therapy for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(5):1212–8.
• Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab. 2012;32(12):2091–9 Comprehensive discussion on the potential implications of post-treatment perfusion changes.
Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–17.
De Silva DA, Fink JN, Christensen S, Ebinger M, Bladin C, Levi CR, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET). Stroke. 2009;40(8):2872–4.
Soares BP, Tong E, Hom J, Cheng SC, Bredno J, Boussel L, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. 2010;41(1):e34–40.
Ng FC, Coulton B, Chambers B, Thijs V. Persistently elevated microvascular resistance postrecanalization. Stroke. 2018;49(10):2512–5.
Desilles JP, Rouchaud A, Labreuche J, Meseguer E, Laissy JP, Serfaty JM, et al. Blood-brain barrier disruption is associated with increased mortality after endovascular therapy. Neurology. 2013;80(9):844–51.
Knight RA, Barker PB, Fagan SC, Li Y, Jacobs MA, Welch KM. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke. 1998;29(1):144–51.
Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56(4):468–77.
Renu A, Laredo C, Lopez-Rueda A, Llull L, Tudela R, San-Roman L, et al. Vessel wall enhancement and blood-cerebrospinal fluid barrier disruption after mechanical thrombectomy in acute ischemic stroke. Stroke. 2017;48(3):651–7.
Villringer K, Sanz Cuesta BE, Ostwaldt AC, Grittner U, Brunecker P, Khalil AA, et al. DCE-MRI blood-brain barrier assessment in acute ischemic stroke. Neurology. 2017;88(5):433–40.
Simpkins AN, Dias C, Leigh R. National Institutes of Health Natural History of Stroke I. Identification of reversible disruption of the human blood-brain barrier following acute ischemia. Stroke. 2016;47(9):2405–8.
•• Leigh R, Jen SS, Hillis AE, Krakauer JW, Barker PB, Stir, et al. Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke. 2014;45(7):2030–5 Recent clinical data on blood-brain barrier disruption in thrombectomy patients.
Leigh R, Christensen S, Campbell BC, Marks MP, Albers GW, Lansberg MG, et al. Pretreatment blood-brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology. 2016;87(3):263–9.
Nadareishvili Z, Simpkins AN, Hitomi E, Reyes D, Leigh R. Post-stroke blood-brain barrier disruption and poor functional outcome in patients receiving thrombolytic therapy. Cerebrovasc Dis. 2019:1–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Felix C. Ng and Bruce C.V. Campbell each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stroke
Glossary
- IVT
-
Intravenous thrombolysis
- LVO
-
Large vessel occlusion
- EVT
-
Endovascular thrombectomy
- NCCT
-
Non-contrast computed tomography
- MRI
-
Magnetic resonance imaging
- DSA
-
Digital subtraction angiography
- AOL
-
Arterial occlusion lesion
- IMS
-
Interventional Management of Stroke study
- RCT
-
Randomised control trials
- mTICI
-
Modified treatment in cerebral ischaemia score
- HERMES
-
Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials
- sICH
-
Symptomatic intracerebral haemorrhage
- BBB
-
Blood-brain barrier
- HT
-
Haemorrhagic transformation
- ECASS
-
European Cooperative Acute Stroke Study
- HI
-
Haemorrhagic infarction
- PH
-
Parenchymal haematoma
- DECT
-
Dual-energy CT
- GRE
-
Gradient echo
- SWI
-
Susceptibility-weighted imaging
- CTP
-
CT perfusion
- MCA
-
Middle cerebral artery
- MLS
-
Midline shift
- DWI
-
Diffusion-weighted imaging
- DLR
-
Diffusion lesion reversal
- ADC
-
Apparent diffusion coefficient
- MRP
-
MR perfusion
- DEFUSE
-
Diffusion and perfusion imaging evaluation for understanding stroke evolution study
- EPITHET
-
Echoplanar Imaging Thrombolytic Evaluation Trial
- FLAIR
-
Fluid-attenuated inversion recovery
- HARM
-
Hyperintense acute reperfusion marker
- DCE
-
Dynamic contrast-enhanced MRI
- DSC
-
Dynamic susceptibility contrast MRI
Rights and permissions
About this article
Cite this article
Ng, F.C., Campbell, B.C.V. Imaging After Thrombolysis and Thrombectomy: Rationale, Modalities and Management Implications. Curr Neurol Neurosci Rep 19, 57 (2019). https://doi.org/10.1007/s11910-019-0970-7
Published:
DOI: https://doi.org/10.1007/s11910-019-0970-7