Abstract
Purpose of Review
Whilst gait impairment is a main cause for disability in Parkinson’s disease (PD), its neural control remains poorly understood. We performed a systematic review and meta-analysis of neuroimaging studies of surrogate features of gait in PD.
Findings
Assessing the results from PET or SPECT scans after a period of actual walking as well as fMRI during mental imagery or virtual reality (VR) gait paradigms, we found a varying pattern of gait-related brain activity. Overall, a decrease in activation of the SMA during gait was found in PD compared to elderly controls. In addition, the meta-analysis showed that the most consistent gait-related activation was situated in the cerebellar locomotor region (CLR) in PD.
Summary
Despite methodological heterogeneity, the combined neuroimaging studies of gait provide new insights into its neural control in PD, suggesting that CLR activation likely serves a compensatory role in locomotion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gait impairment is one of the main causes for disability in the daily lives of patients with Parkinson’s disease (PD) [35]. Combined with postural instability, PD patients are at an exceptionally high risk for repeated falls, leading to a complex chain reaction of clinical consequences, including severe injuries, immobilisation and mortality [2, 4]. Even early in the disease course patients present with continuous gait problems, such as reduced step length with increased cadence and stride time variability, causing them to be at a higher risk for falls than their age-matched peers [21, 23]. Indeed, more than half of PD patients experience a fall within the first 3 years after clinical diagnosis [24]. As the disease progresses over time, often other more complex and episodic gait disorders appear, such as festination and freezing of gait [30], which further increase the risk of falling [23] and drastically reduce the quality of life for patients [40].
The pathophysiology underlying gait impairment in PD remains poorly understood. One of the main reasons is that gait control involves a complex interplay across multiple neural circuits that span cortical but also subcortical structures that cannot be directly imaged with ambulatory neuroimaging techniques. Some approaches such as electroencephalography (EEG) and near-infrared spectroscopy (NIRS) have provided some insights into gait disturbances [1•, 6, 8, 25, 32], but they lack neuroanatomical localisation. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are nuclear imaging techniques that provide whole brain activity coverage by measuring radiotracer uptake using gamma rays. The strength of these techniques is that they can assess the brain activations associated with actual gait tasks [19, 26, 37]. These techniques can also be used to study the neural control of gait in patients with metal implants, such as deep brain stimulators in PD [41]. Yet, PET and SPECT require the recording of activity usually after a period of gait has been undertaken, resulting in an ‘estimate’ of changes that might have been present during the task. Magnetic resonance imaging (MRI) is a safe technique that provides whole-brain coverage with the best spatial resolution and without requiring radioactive tracers. It can be used to study structural alterations in the brain (i.e. grey matter volumes and white matter connectivity) or changes in blood level oxygen level (BOLD) during the resting state or while performing functional tasks. Functional MRI (fMRI) has therefore often been the technique of choice for studying the neural correlates underlying complex human behaviours. However, the key difficulty of MRI is that subjects have to be in a supine position and lie absolutely still, thereby precluding the study of actual gait [8, 25].
The evidence that can be provided with resting state fMRI or structural MRI can only be indirectly related to gait by comparing groups with and without gait impairment or by correlating the imaging findings with gait measures obtained outside the scanner. However, in the last decade, researchers have developed methods that allow for the investigation of the neural mechanisms underlying surrogate measures of gait during fMRI [1•, 8, 25]. These include action simulation (i.e. motor imagery or action observation) of gait or motor execution of the lower limbs using various paradigms [1•, 5•, 8, 25, 29]. Action simulation can give rise to a comprehensive experience of gait that, despite the supine position, likely activates areas involved with postural control [33, 36]. On the downside, only proxy measures of gait-related output can be generated from action simulation paradigms to ascertain if subjects are truly engaged in action simulation of gait and to compare performance across subjects and groups. Thus, researchers started to study the neural correlates of virtual reality (VR) gait paradigms, which utilise foot tapping movements to navigate a three-dimensional environment during fMRI, under the assumption that bilateral coordination of the lower limbs and ankle dorsi- and plantar flexion are integral features of gait control [16, 29, 34]. The benefit of VR paradigms is that behavioural data of the subject’s stepping performance can be generated and directly linked to the BOLD data obtained. With the addition of VR environments, the immersion in a gait-like experience can be further enhanced [5•]. The virtual environments also engage sensorimotor feedback mechanisms implicated in gait control. Finally, virtual environments can be adjusted to simulate the visual feedback obtained during a variety of common gait conditions. This is of particular interest for PD, as the virtual environments can simulate situations in which gait control often breaks down in real-life, such as during turning [15] or passing through doorways [27]. However, similar to other MR imaging paradigms, foot tapping in a supine position does not engage the postural and balance control circuits that are integrally involved in real-life walking [32] and impaired in PD [3].
Taken together, there has been a recent surge of evidence on the neural mechanisms underlying gait control in people with PD, which has great potential to guide treatment developments [17]. However, heterogeneity between task conditions and subjects across studies results in variable outcomes making it difficult to pinpoint the precise activation patterns that characterize altered gait control in PD. The aim of the present review was therefore to systematically summarize the outcomes of any task-based fMRI experiment and conduct an exploratory activation of likelihood estimation (ALE) meta-analysis to assess whether there are any convergent activation patterns that characterize gait control in PD, in comparison to gait control in healthy older adults.
Literature Searches
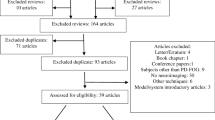
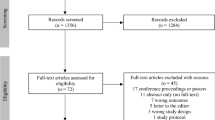
A systematic search without language or date restrictions was conducted in the electronic databases of Pubmed, Medline, Embase and Web of Science to identify relevant studies published until March 22nd 2019. For PD, the following search terms and their common abbreviations were used: (Parkinson disease AND (gait OR walking OR stepping OR freezing AND (Magnetic Resonance Imaging OR Positron-Emission Tomography OR Tomography, Emission-Computed, Single-Photon)), yielding a total of 892 non-duplicate hits. For healthy older adults, a similar search string was used after replacing “Parkinson disease” with (Aged AND (Healthy OR Community dwelling)), yielding a total of 365 non-duplicate hits. Titles, abstracts and full-texts were subsequently screened for inclusion and exclusion criteria.
Inclusion Criteria for the Systematic Overview
Peer-reviewed published articles in any language using PET, SPECT or fMRI were included if they studied the neural underpinnings of gait impairment in patients with idiopathic Parkinson’s disease or healthy older adults (mean age ≥ 55 years). This included (i) studies using action simulation paradigms, such as motor imagery and action observation or studies investigating motor execution of the bilateral lower limbs with or without cueing or virtual reality paradigms; (ii) studies assessing radiotracer uptake after an actual gait task performed outside the scanner (PET, SPECT) or during scanning (SPECT); and (iii) studies using the above-named techniques to assess the effects of an intervention on the neural correlates of gait in PD.
Exclusion Criteria for the Systematic Overview
Non-peer reviewed articles, conference abstracts and reviews of the literature with or without meta-analysis, as well as animal studies were excluded. Studies using structural MRI, resting state fMRI, EEG, NIRS or non-invasive brain stimulation (e.g. transcranial magnetic stimulation), as well as resting state PET or SPECT studies without inclusion of the techniques described above to model gait, were also excluded. Finally, passive movements of the lower limbs or unilateral foot tapping, as well as changes in optic flow without inclusion of the techniques described above were not considered as gait-specific and thus excluded from the review and meta-analysis.
The above-described screening led to a final inclusion of 23 studies on PD (Table 1) and 7 additional studies on healthy older adults (Supplementary materials). Key findings of this analysis are reported in Table 1, showing a great variety of gait-related brain activations, incorporating the frontal and parietal cortices and the cerebellum across groups. Furthermore, an altered activation of the SMA during gait was a consistent finding in PD. However, the great variety of gait-related brain activations hampers drawing firm conclusions. Therefore, a meta-analysis was performed to determine brain regions consistently activated during gait-like tasks across studies.
Inclusion and Exclusion Criteria for the Exploratory ALE Meta-analysis
From our initial systematic search, we selected experiments from studies that reported whole brain peak voxel coordinates in standard stereotactic space (i.e. MNI or Talaraich) for within-group comparisons, for both PD and controls, whereby ‘gait’ was taken as the condition of interest (e.g. motor imagery of gait > visual imagery of gait, foot tapping > rest). Contrasts between two similar gait conditions were included if the condition of interests was hypothesised to still engage gait-related areas. For intervention studies, only the baseline contrasts prior to the intervention were included. The number of included subjects in each experiment had to be reported as the ALE analysis controlled for the number of subjects in each experiment. Between-subject contrasts were excluded as to prevent comparisons across groups of unequal size and to limit between-subject variability [20••]. Finally, studies that only reported the outcomes of a region-of-interest analysis were also excluded.
Data Extraction and ALE Meta-analysis
In short, ALE is a coordinate-based meta-analysis technique that determines whether peak voxel activation coordinates (i.e. foci) across different experiments overlap at a statistical level (greater than expected by chance) by modelling them as 3D Gaussian probability distributions centred at the respective coordinates [10, 38]. The exploratory meta-analyses were performed using the revised version of the ALE algorithm that takes the number of subjects in each experiment into account [10, 11, 38]. We followed the methodology as described in detail by Hardwick et al. [20••]. For each experiment, the sample size, mean age, sex distribution of the subjects, contrast of interest and whole-brain peak-voxel coordinates in MNI or Talaraich space were extracted. Meta-analyses across all subjects, one across PD subjects only and one across healthy older adults were performed. In addition, contrasts between the resultant meta-analyses of PD and controls were conducted using random effects ALE subtraction analysis [11], as per Hardwick et al. [20••] and anatomically labelled in MNI space using the xjView software.
ALE-Sample Characteristics
Sixteen gait-related within-subject experiments totalling 161 peak voxel coordinates (i.e. foci) were included for PD and also 16 gait-related within-subject experiments, totalling 264 foci, were included for controls (Table 2). The outcomes of the meta-analysis are based on a total of 178 PD subjects (16.2 subjects on average per experiment; mean (SD) of the mean age = 65.5 (4.1); %males = 67.1%) and a total of 208 healthy older adults (16.0 subjects on average per experiment; mean (SD) of mean age = 67.3 (6.3), %males = 43.8%). There was no significant difference between the two cohorts on the average number of subjects per experiment (independent t test: t = 0.07, p = 0.942) or average age of the subjects in each experiment (t = − 0.75, p = 0.460). A significant difference in sex distribution was found (Chi-square test: χ2 = 19.0, p < 0.01) with significantly more females in the control cohort. As we included < 20 experiments, which is condsidered the minimal number required to achieve sufficient power for moderate effects [12••], for both PD and controls, our ALE meta-analysis must be considered exploratory.
Exploratory ALE-Results
First, the spatial distribution of ALE z-scores across both groups was plotted without further statistical inference to ensure that a motor network would appear. The ALE scores represent the sum of modelled activations, whereby each foci has been replaced with three-dimensional Gaussian distributions with a width that was set according to the number of subjects in each experiment [10, 20••]. This exploration revealed an expected gait-related pattern of activations across the primary and pre-motor cortices, the visual cortex, basal ganglia, and the cerebellar and brainstem locomotor regions (Fig. 1a).
Exploratory ALE meta-analysis results. a Spatial distribution of ALE z-scores across both PD and healthy older adults, uncorrected. b Significant gait-related activations found across both groups, cFWE corrected. c Significant CLR activations found in PD patients compared to healthy older adults, cFWE corrected. d Significant SMA and M1 activations found in healthy older adults as compared to PD patients, cFWE corrected. Abbreviations: ALE = activation of likelihood estimation; PD = Parkinson’s disease; cFWE = cluster-wise family error corrected for multiple comparisons; L = left; R = right; SMA = supplementary motor area; M1 = primary motor cortex; CLR = cerebellar locomotor region; CBM-VI = lobule VI of the cerebellum
A cluster-level family wise error (cFWE) correction for multiple comparisons was applied when conducting the actual meta-analyses [12••]. Across both PD and healthy older adults, significant gait-related activations were found in the midline supplementary motor area (SMA, 281 voxels, peak voxel: − 2/− 4/66), the midline leg area of the primary motor cortex (M1, 359 voxels, peak voxel: − 8/− 32/66), the cerebellar locomotor region (CLR, 313 voxels, peak voxel: − 1/− 50/− 10) and right lateral cerebellar lobule VI/Crus-I (221 voxels, peak voxel at: 36/− 54/− 32) (Fig. 1b). Within the PD cohort, the most consistent activation was found in the CLR (194 voxels, peak voxel: − 2/− 52/− 8) and within the control group the most consistent activation was found in the midline SMA (181 voxels, peak voxel: 2/− 4/64) and leg area of M1 (94 voxels, peak voxel: 6/− 34/74). The same areas also survived the random effects ALE subtraction analysis (see Fig. 1c, d).
Methods Used to Study the Neural Control of Gait in PD
The pros and cons of the different neuroimaging methods used to study gait in the included studies have been extensively reviewed by others (e.g. see [1•, 20••, 25]). As expected, most studies resulting from our systematic search used either MI of gait or bilateral foot tapping during fMRI. One noteworthy recent development is that studies started to combine foot tapping paradigms with cognitive dual tasking to model the neural correlates underlying gait automaticity impairments in PD [29] and healthy elderly [7•]. Perhaps the most prominent feature of gait in PD is that it progressively fails to fall under automatic control, thus forcing patients to increasingly rely on compensatory circuits to control their steps [16]. The loss of motor automaticity is linked to the loss of dopaminergic innervation in the posterior striatum [43], but how exactly patients achieve compensatory stepping and the clinical impact of when these compensatory circuits fail remains largely unknown [16, 29]. Interestingly, Bürki et al. [7•] used two different cognitive tasks during foot tapping in the elderly, namely a verbal fluency task and a serial subtraction task inside as well as outside the scanner, the latter while walking on a GaitRite mat. This allowed them to investigate differential effects of distraction on foot tapping performance as well as on actual gait [7•]. Similar studies could be performed in PD that systematically increase the load on the attentional compensatory circuits that PD presumably rely on to perform otherwise automatic foot tapping. This would also help to map out the attentional compensatory circuits which are likely to converge across all task loads (i.e. both the cognitive and motor task). More studies using dual tasking combined with real gait and action simulation paradigms are needed to better map the failing automaticity and compensatory circuits that underpin gait in PD [29].
So far, only two studies by Hanakawa et al. [18, 19] used SPECT to assess the brain activations during actual treadmill gait in PD [18, 19]. Surprisingly, this methodology has never since been replicated by others, possibly due to increasing utility of PET over SPECT in medical imaging. A total of four studies utilized PET to compare the radiotracer uptake following a period of walking vs. rest. Two of these administered H215O [26, 41] and one18FDG isotopes [37] to study the general brain activation patterns resulting from neuronal oxygen and glucose consumption, respectively. One PET study used [11C]-CFT to map changes in dopamine transporter availability (DAT) in the striatum and extra-striatal regions following gait in PD [31].
Although the following studies did not make the inclusion criteria for the present review, their novel methodologies are worth a mention. Firstly, van der Hoorn et al. [39] used changes in optic flow to simulate a visual sensation of forward progression similar to that experienced during gait [39]. By suddenly changing the speed of the optic stimuli, a sensation of gait cessation could be induced. It can be envisioned that the combination of such changes in optic flow and foot tapping in a virtual environment could perhaps induce the sensation of near-falls to map the neural correlates of gait adaptation and fear of falling in PD and the elderly. Secondly, de Lima-Pardini et al. [9•] developed a unique apparatus with MRI-compatible force sensors that allowed for the simulation of anticipatory postural adjustments (APA) that are integral to the initiation of the first step [9•]. PD patients often show multiple APA’s during gait initiation [22], and such novel methodologies hold strong potential to provide insights into these challenging gait epiphenomena.
Interpreting the Main Outcomes
One of the most consistent finding across studies was an altered involvement of the SMA during gait in PD. This is supported by our exploratory meta-analysis showing consistent SMA activation in healthy elderly controls, but not in PD. The SMA is part of the mesial premotor loop and highly connected to the putamen where it likely aids in the selection of appropriate motor sequences [28]. The lower SMA activation seen in PD may thus be the result of lower striatal activations following dopaminergic denervation. Structural white matter changes have also been shown in the SMA pathways [14]. Taken together, reduced SMA activation is likely a hallmark of impaired gait control in PD.
Another outcome that was supported by our exploratory meta-analysis was that gait in PD was consistently associated with increased activations across the cerebellum, and in particular the CLR. Interestingly, a recent study by Fasano et al. [13••] showed that the majority of brain lesions that result in freezing of gait are located in areas that have strong functional connections with the CLR in almost exactly the same peak location as seen in our exploratory analysis [13••]. Together, this indicates that PD patients are heavily reliant on the CLR to modulate their gait, perhaps due to the lack automatic control from the basal ganglia. In contrast, [43] found evidence based on PET-data that the cerebellum was involved in the pathophysiology of gait and balance problems in PD, as decreased levels of acetylcholinesterase were found in the midbrain and the cerebellum. Wu and Hallett [42] put forward a model in which both pathological and compensatory processes of the cerebellum could play a role in various symptoms of PD, including gait. This interplay of compensatory and pathological effects was projected to change with disease progression. The compensatory role of the cerebellum provides merit to develop interventions that attempt to modulate the cerebellar input during gait, such as split-belt training interventions [45]. Recently, excitatory theta burst stimulation applied to the cerebellar hemispheres induced gait speed improvements, although freezing of gait was not alleviated [46]. However, several studies on healthy older adults also found increased activations in the cerebellum, so the exact specificity of CLR activation to PD gait cannot be concluded from this exploratory meta-analysis. More data is required to support our preliminary findings and provide more insight into the complex role of the CLR.
Conclusions
Recent advances in neuroimaging allow for the investigation of the neural mechanisms underlying gait impairments in PD. Here, we show for the first time that different imaging methodologies complement each other and that their outcomes can be used to reveal the most consistent whole brain activations that are gait-related. This knowledge will aid in our focus to develop interventions, targeting the most prominent changes in the neural control of gait in PD. It is projected that with the inclusion of more future studies, such meta-analysis techniques will be able to reveal neural signatures that are specific to certain gait impairments in subtypes of PD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Allali G, Blumen HM, Devanne H, Pirondini E, Delval A, Van De Ville D. Brain imaging of locomotion in neurological conditions. Neurophysiol Clin. 2018;48:337–59 Review of the literature on brain imaging of locomotion in PD and other neurological conditions with pros and cons of different neuroimaging techniques highlighted.
Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson's disease: a systematic review. Parkinsons Dis. 2013;2013:906274–16.
Bekkers EMJ, Dijkstra BW, Heremans E, Verschueren SMP, Bloem BR, Nieuwboer A. Balancing between the two: are freezing of gait and postural instability in Parkinson's disease connected? Neurosci Biobehav Rev. 2018;94:113–25.
Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–84.
• Bluett B, Bayram E, Litvan I. The virtual reality of Parkinson's disease freezing of gait: a systematic review. Parkinsonism Relat Disord. 2018. Systematic review on the different utilities of virtual reality to study freezing of gait in PD, including its ability to be combined with neuroimaging to study the neural underpinnings of freezing.
Bohnen NI, Jahn K. Imaging: what can it tell us about parkinsonian gait? Mov Disord. 2013;28:1492–500.
• Bürki CN, Bridenbaugh SA, Reinhardt J, Stippich C, Kressig RW, Blatow M. Imaging gait analysis: An fMRI dual task study. Brain Behav. 2017;7:e00724 Healthy older participants in this study performed two cognitive dual-tasks while simultaneously tapping their feet inside the fMRI scanner. Such paradigms could be used to study the neural basis underlying gait automaticity impairments in PD.
Crémers J, D'Ostilio K, Stamatakis J, Delvaux V, Garraux G. Brain activation pattern related to gait disturbances in Parkinson's disease. Mov Disord. 2012;27:1498–505.
• de Lima-Pardini AC, de Azevedo Neto RM, Coelho DB, Boffino CC, Shergill SS, de Oliveira Souza C, et al. An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Sci Rep. 2017;7:43088 The authors present a new apparatus that allows for the investigation of anticipatory postural adjustments during fMRI.
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26.
Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–61.
•• Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70–85 Methodological paper by the developers of the ALE meta-analysis technique, presenting simulations on thresholding methods and sample-size requirements.
•• Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol. 2017;81:129–41 This neuroanatomical study showed that the large majority of brain lesions that result in freezing of gait are located in areas that have strong functional connections with the cerebellar locomotor regions.
Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014;9:e100291.
Gilat M, Shine JM, Walton CC, O'Callaghan C, Hall JM, Lewis SJG. Brain activation underlying turning in Parkinson's disease patients with and without freezing of gait: a virtual reality fMRI study. Nat Partner J Parkinsons Dis. 2015; 1. Available from: http://www.nature.com/articles/npjparkd201520. Accessed 12 Mar 2018
Gilat M, Bell PT, Ehgoetz Martens KA, Georgiades MJ, Hall JM, Walton CC, et al. Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson's disease. Neuroimage. 2017;152:207–20.
Gilat M, Lígia Silva de Lima A, Bloem BR, Shine JM, Nonnekes J, Lewis SJG. Freezing of gait: promising avenues for future treatment. Parkinsonism Relat Disord. 2018;52:7–16.
Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson's disease. Ann Neurol. 1999a;45:329–36.
Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, et al. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain. 1999b;122 (Pt 7:1271–82.
•• Hardwick RM, Caspers S, Eickhoff SB, Swinnen SP. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci Biobehav Rev. 2018;94:31–44 Systematic review with ALE meta-analysis assessing the neural basis of motor imagery, action observation and motor execution in young healthy adults as derived from fMRI or PET, including locomotor tasks.
Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson's disease. Eur J Neurosci. 2007;26:2369–75.
Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–41.
Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–24.
Lord S, Galna B, Yarnall AJ, Coleman S, Burn D, Rochester L. Predicting first fall in newly diagnosed Parkinson's disease: insights from a fall-naïve cohort. Mov Disord. 2016;31:1829–36.
Maillet A, Pollak P, Debû B. Imaging gait disorders in parkinsonism: a review. J Neurol Neurosurg Psychiatry. 2012;83:986–93.
Maillet A, Thobois S, Fraix V, Redouté J, Le Bars D, Lavenne F, et al. Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson's disease: a kinesthetic imagery approach. Hum Brain Mapp. 2015;36:959–80.
Matar E, Shine JM, Gilat M, Ehgoetz Martens KA, Ward PB, Frank MJ, et al. Identifying the neural correlates of doorway freezing in Parkinson's disease. Hum Brain Mapp. 2019;40:2055–64.
Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, et al. Dopaminergic modulation of motor network dynamics in Parkinson's disease. Brain. 2015;138:664–78.
Nieuwhof F, Bloem BR, Reelick MF, Aarts E, Maidan I, Mirelman A, et al. Impaired dual tasking in Parkinson's disease is associated with reduced focusing of cortico-striatal activity. Brain. 2017;140:1384–98.
Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44.
Ouchi Y, Kanno T, Okada H, Yoshikawa E, Futatsubashi M, Nobezawa S, et al. Changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson's disease. Brain. 2001;124:784–92.
Peterson DS, Horak FB. Neural control of walking in people with parkinsonism. Physiology (Bethesda). 2016;31:95–107.
Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait-related brain activity in people with Parkinson disease with freezing of gait. PLoS One. 2014;9:e90634.
Shine JM, Matar E, Ward PB, Bolitho SJ, Gilat M, Pearson M, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson's disease. Brain. 2013;136:1204–15.
Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23:790–6.
Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain. 2011;134:59–72.
Tard C, Delval A, Devos D, Lopes R, Lenfant P, Dujardin K, et al. Brain metabolic abnormalities during gait with freezing in Parkinson's disease. Neuroscience. 2015;307:281–301.
Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80.
van der Hoorn A, Renken RJ, Leenders KL, de Jong BM. Parkinson-related changes of activation in visuomotor brain regions during perceived forward self-motion. PLoS One. 2014;9:e95861.
Walton CC, Shine JM, Hall JM, O'Callaghan C, Mowszowski L, Gilat M, et al. The major impact of freezing of gait on quality of life in Parkinson's disease. J Neurol. 2015;262:108–15.
Weiss PH, Herzog J, Pötter-Nerger M, Falk D, Herzog H, Deuschl G, et al. Subthalamic nucleus stimulation improves parkinsonian gait via brainstem locomotor centers. Mov Disord. 2015;30:1121–5.
Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136(3):696–709.
Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiol. Dis. 2015;82:226–34.
Gilman S, Koeppe RA, Nan B, Wang C-N, Wang X, Junck L, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsoni an syndromes. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. 2010;74:1416–23.
Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J. Neurosci. Society for Neuroscience. 2006;26:9107–16.
Janssen AM, Munneke MAM, Nonnekes J, van der Kraan T, Nieuwboer A, Toni I, et al. Cerebellar theta burst stimulation does not improve freezing of gait in patients with Parkinson's disease. J. Neurol. Springer Berlin Heidelberg. 2017;264:963–72.
Acknowledgements
The authors would like to thank Dr. Robert Hardwick for assisting with the ALE meta-analysis.
Funding
MG is supported by a Postdoctoral Mandate of the KU Leuven Internal Fund; AN and BWD are supported by Flanders Research Funds (G086715N), ND is supported by Jacques & Gloria Gossweiler Foundation, SJGL is supported by a NHMRC–Australia Research Council dementia fellowship (#1110414).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Moran Gilat, Bauke W. Dijkstra, Nicholas D’Cruz, Alice Nieuwboer and Simon JG Lewis each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuroimaging
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Gilat, M., Dijkstra, B.W., D’Cruz, N. et al. Functional MRI to Study Gait Impairment in Parkinson’s Disease: a Systematic Review and Exploratory ALE Meta-Analysis. Curr Neurol Neurosci Rep 19, 49 (2019). https://doi.org/10.1007/s11910-019-0967-2
Published:
DOI: https://doi.org/10.1007/s11910-019-0967-2