Abstract
Purpose of Review
Fat embolism syndrome (FES) is a rare disorder with potentially devastating neurologic complications. This article reviews the history, pathophysiology, clinical features, diagnosis, and treatment of FES with a focus on its neurologic aspects.
Recent Findings
The neurologic complications of FES are more commonly recognized with current diagnostic testing and increase awareness of the disorder. FES may present initially with neurologic manifestations. Prompt diagnosis of FES and of its neurologic manifestations could be lifesaving. This includes respiratory support and management of neurological complications.
Summary
The classic clinical triad of pulmonary insufficiency, neurologic disturbances, and petechial skin rash typically presents 24 to 72 h following an initial insult, most commonly a traumatic long bone fracture. Early onset (< 24 h) and delayed onset (> 72 h) have been described. Neurologic manifestations may include ischemic/hemorrhagic strokes, retinal ischemia, seizures, autonomic dysfunction, and diffuse brain injury. Diagnosis remains clinical. Management consists mainly of supportive care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fat embolism syndrome (FES) is a rare disorder most commonly manifested with respiratory insufficiency following orthopedic trauma. The classical triad of FES consists of respiratory insufficiency/distress, neurologic impairment, and petechial skin rash. Neurologic involvement in FES is the result of a major cause of morbidity and mortality, migration of fat globules to the central nervous system (CNS). The term cerebral fat emboli (CFE) is used to imply FES affecting the brain. Neurologic complications of CFE include ischemic and hemorrhagic strokes, seizures, convulsive and non-convulsive status epilepticus, autonomic impairment, acute encephalopathy, and coma.
History
Fat embolism was first described in an animal model more than three centuries ago [1]. Mechanical obstruction of small blood vessels by fat globules and pulmonary edema was first demonstrated by Magendie in an animal study using intravenous oil.
The first human case of FES was confirmed by the post-mortem presence of fat globules in pulmonary capillaries [2]. Fracture immobilization as a plausible preventive measure for FES was first posited in 1914 by Tanton [1].
Pathophysiology
FES has been attributed to either mechanical or biochemical pathophysiologic mechanisms. The mechanical pathophysiology proposed by Gauss in 1924 had three essential components: (1) adipose tissue damage, (2) rupture of venous channels, and (3) passage of fat globules into open-ended venous channels [3]. A biochemical pathophysiology proposed by Lehman in 1927 suggested plasma intermediaries promoting fat mobilization from body storage with subsequent formation of fat globules into the intravascular compartment. Figure 1 illustrates the mechanical theory of FES.
Etiology
Orthopedic trauma is the most common cause of FES. Table 1 provides a list of the underlying causes of FES.
Traumatic
The reported risk of FES following orthopedic trauma ranges between < 1 and > 30%. Traumatic femoral shaft fracture (excluding isolated neck fractures) carries an almost inevitable risk for FES. FES is less common (< 1%) with isolated tibial or fibular fractures or with isolated fractures of the femur. Other fracture sites (e.g., pelvic and ribs) also appear to have a low incidence of FES [4].
Albeit rare, FES may complicate hip arthroplasty, revision of hip arthroplasty, knee arthroplasty, intraosseous procedures, intraosseous infusions as well as surgical interventions including percutaneous vertebroplasties [5,6,7,8,9,10].
Adipose tissue damage could be the initial trigger for the development of FES. Adipose tissue damage may follow a variety of procedures and interventions, including cosmetic procedures involving the gluteal region such as liposuction, cosmetic filler injections, autologous fat transfer, depot drug delivery, and implants. Dermal fillers or fat cosmetic injections may also result in ophthalmic artery embolism. High-risk regions of injection sites include the periosteum, the nasal dorsum, and the nasolabial fold [11].
Trauma without bony fractures, so-called non-orthopedic trauma, may also result in FES [12]. Some conditions where non-orthopedic trauma is thought to play a role in FES include lung transplantation, and coronary artery bypass graft (CABG). Off-pump CABG, as compared to on-pump techniques, appears to have a much lower risk of FES than on pump [13]. FES following multiple vertebral compression fractures has been described after lung transplantation [14]. FES has also been observed following renal transplantation [15].
Non-traumatic
Non-traumatic causes of FES are heterogeneous and include pancreatic diseases, boney or soft tissue infections, bone marrow disorders, hemoglobinopathy malignancies, and drugs [16,17,18,19,20,21,22]. Mechanisms for the development of FES vary in each of the listed conditions. In instances of renal angiomyolipomas, these rare tumors may invade the inferior vena cava resulting in FES; resection of renal angiomyolipomas could be an effective preventive treatment of FES [11].
Medications and interventions associated with FES include corticosteroids, cis-platinum, cyclosporine solvent, lipid infusion, and cell-salvage devices [23,24,25]. Cell salvage is the collection of a patient’s own blood during surgery. The continuous cell salvage device removes fat to a higher extent as compared to discontinuous devices. Of particular interest to neurologists is the risk of FES with chronic corticosteroid use. Duchenne muscular dystrophy (DMD) also poses an increased risk for FES [26••]. This is thought to be secondary to osteopenia with associated non-traumatic fractures secondary to chronic corticosteroid use. Thus, FES should be considered among the plausible causes of respiratory insufficiency in patients with DMD.
Epidemiology
Trauma, in particular, long bone fractures, carries the highest risk for FES. FES is more common among men between 10 and 40 years of age. Elderly patients with high body mass index (BMI) or high bone marrow fat content appear to be at increased risk for pulmonary fat embolism during total knee arthroplasty [27].
Clinical Features
The most common manifestation of FES is respiratory distress and usually occurs 24 to 72 h following initial insult. Early onset FES (< 24 h) usually occurs within 12 to 24 h following the initial insult [28]. Delayed onset (> 72 h) has been described post-CABG and as a late complication of trauma-related FES [29].
Pulmonary Manifestations
Respiratory distress is usually the first and most common clinical manifestation of FES. Patients may develop dyspnea, tachypnea, hypoxemia, or a full-blown acute respiratory distress syndrome (ARDS)-like picture.
Dermatologic Manifestations
Most commonly, a petechial rash involves the head, neck, axilla, anterior thorax, and subconjunctiva [30]. Dermatologic manifestations occur on average in one-third of patients.
Miscellaneous Manifestations
It may include fever, anemia, thrombocytopenia, lipiduria, cardiomyopathy, and arterial hypotension [31].
Neurologic Manifestations
Neurologic manifestations typically follow the pulmonary manifestations and may include cerebral and/or spinal cord ischemia, hemorrhagic stroke, seizures, autonomic dysfunction, and diffuse brain injury leading to acute encephalopathy or coma. In addition, ocular manifestations, such as retinal ischemia, could be considered as part of neurologic manifestations.
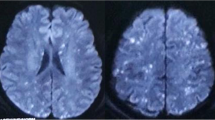
CFE is a rare cause of ischemic and hemorrhagic stroke [32]. Aside for traumatic orthopedic and other causes mentioned above for FES, CFE may also occur following reconstructive eye surgery [33]. FES should be considered in patients with sickle cell disease and thalassemia presenting with multifocal cerebral embolism. Findings include a star field pattern on brain MRI [34]. Susceptibility-weighted images (SWI) are of particular value for the suspicion of FES in patients with sickle cell disease and other hemoglobinopathies [35, 36].
AC best rare, paraplegia due to spinal cord infarction (SCI), could be the initial presentation of FES [37, 38•].
Focal seizures with or without secondary generalization usually follow other manifestations of FES. FES may ensue after seizures complicating long bone fractures, as well as seizures without associated orthopedic trauma [39]. The mechanism of post-seizure FES without boney fractures is thought to be secondary to soft tissue trauma. Refractory status epilepticus and non-convulsive status epilepticus could be the main neurologic manifestations of FES [40••].
Paroxysmal sympathetic hyperactivity syndrome (PSHS) characterized by the sudden occurrence of arterial hypertension, tachycardia, diaphoresis, and fever may be associated with FES [41]. Episodes may be manifested spontaneously or induced by stimulation (e.g., turning, bathing, aspiration of secretions, and pain). PSHS is commonly underdiagnosed and delayed recognition is associated with increased morbidity [41].
Fat emboli may invade the subarachnoid space in patients with FES [42]. Subarachnoid fat droplets may occur without FES as a sequela of ruptured dermoid cysts [43].
Ocular fat embolism syndrome occurs most commonly when fat globules result in retinal ischemia [44]. Furthermore, iris segmental infarction has been described following autologous fat injection into the lower eyelid tissues [45].
Unresponsiveness could be the initial manifestation of FES [46]. FES should be considered in the differential diagnosis of rapid onset of coma following long bone fracture [47]. Delayed coma could also be the initial clinical manifestation of FES in trauma patients with head injury [48•].
Evaluation and Diagnosis
Diagnosis of FES is primarily clinical. FES should be suspected among high-risk patients before all manifestations are evident. Tables 2, 3 and 4 provide the diagnostic criteria proposed by Gurds, Schonfeld’s, and Lindeque.
The classic clinical triad usually does not occur simultaneously and the petechial rash is present in less than half of the cases. The hemodynamic status should be closely monitored since patients with FES may develop acute arterial hypotension. Funduscopy may show evidence of retinal scotomata (Purtscher’s retinopathy).
Laboratory studies may show evidence of anemia and thrombocytopenia. Chest roentgenogram and CT of the chest are usually normal in most patients. However, on occasions, air space disease, ground-glass opacities, or centrilobular nodules may be present.
MRI of the brain may show diffuse hyperintense punctate lesions on DWI that tend to correlate with hypointense signal changes on SWI/GE. Diagnostic criteria have been developed to support the clinical diagnosis. Transcranial Doppler ultrasound may predict the development of neurologic symptoms in patients with long bone fractures; the presence of microembolic signals to the brain has been noted to be more frequent among patients with cardiac right to left shunts [51]. A hypodense middle cerebral artery on CT could serve for diagnostic suspicion for cerebral fat embolism [52, 53].
Ancillary tests support but do not confirm FES diagnosis. Autopsy may not be necessarily confirmatory of FES as fat may be seen incidentally at autopsy. Hence, the clinical significance of this finding is uncertain. Nonetheless, a high percentage of fat in alveolar macrophages on bronchoalveolar lavage, high amount fat globules on wedged pulmonary artery sample, or fat globules during autopsy could settle the diagnostic suspicion of FES.
Differential Diagnosis
Main differential diagnoses include ARDS and pulmonary embolism. Neurological differential diagnoses depend on the initial manifestation of disease. Most importantly is to investigate and exclude other causes of cerebral embolism. Embolization syndromes that could present with respiratory distress and CNS involvement include amniotic fluid embolism syndrome, tumor embolism, air embolism, foreign body embolism, food embolism, and vasculitic cutaneous disorders [54,54,56].
Management, Prevention, and Therapeutic Considerations
Appropriate therapy requires prompt recognition and supportive care. Most clinicians advocate early correction of long bone fractures to prevent FES, and also during established FES in order to prevent recurrent embolization.
Supportive care is aimed at respiratory and neurologic care. Patients may need immediate mechanical respiratory support. Neurocritical care, including management of increased intracranial pressure (ICP), may be warranted. In addition, hemodynamic and cardiac interventions (vasopressors, extracorporeal membrane oxygenation etc.) may be necessary.
It is unclear if systemic corticosteroid administration is beneficial or in patients with FES. Corticosteroids could be potentially beneficial. However, the use of prophylactic corticosteroids in high-risk patients with long bone fractures remains controversial. A metanalysis of 104 studies concluded that corticosteroids may be beneficial in preventing FES and hypoxia without increasing the risk of infection. However, there was no impact on mortality [57]. At present, there is no high level of evidence supporting corticosteroid use for the management or prevention of FES.
Heparin may increase lipid clearance from the intravascular compartment. However, the routine administration of heparin for FES is not recommended.
High-dose statins following CFE may improve outcomes [58]. However, more data are needed to validate the potential efficacy of statin in the management of CFE.
There is no clear indication to screen for the presence of patent foramen ovale (PFO), prior to lower extremities orthopedic surgeries [59].
Prognosis
Most patients with FES have spontaneous full recovery. A case report of nine patients reported a mortality rate of 22% at approximately 5 years of follow-up, and minor or no neurologic impairment in the remainder [60••].
Conclusions
Neurologists should be familiar with the clinical triad of FES (pulmonary insufficiency, neurologic disturbances, and petechial rash). Physicians are commonly aware of FES in high-risk patients mostly following long bone fractures. Neurologists should be mindful of atypical causes of FES as well as initial neurologic insult (e.g., stroke) as the initial manifestation of this disease. Prompt therapeutic interventions, including neurocritical care, could be lifesaving.
Resources
For patients with Muscular Dystrophy: https://www.parentprojectmd.org/care/care-guidelines/by-area/bone-and-joint-care/fat-embolism-syndrome/
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Talbot M, Schemitsch EH. Fat embolism syndrome: history, definition, epidemiology. Injury. 2006;37(Suppl 4):S3–7 Erratum in: Injury. 2007 Oct;38(10):1224.
Zenker F. Beitrage zur normalen und pathologischen anatomic der lunge. J Braunsdorf. 1862;31.
Gauss H. The pathology of fat embolism. Arch Surg. 1924;9:592–605.
Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472–7. https://doi.org/10.1097/MAJ.0b013e318172f5d2.
Ritter MA, Harty LD. Fat embolism in revision total hip arthroplasty. J Arthroplast. 2002;17(8):1063–5.
Christensen L, Brandt E, Gibson LI. Cerebral fat emboli after elective total hip replacement. Ugeskr Laeger. 2015;177(41):V05150414.
Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239–42. https://doi.org/10.4021/jocmr1251w.
Rubal BJ, Meyers BL, Kramer SA, Hanson MA, Andrews JM, DeLorenzo RA. Fat Intravasation from intraosseous flush and infusion procedures. Prehosp Emerg Care. 2015;19(3):376–90. https://doi.org/10.3109/10903127.2014.980475.
Byrick RJ. Pulmonary fat embolism and intraosseous infusion. Pediatr Crit Care Med. 2001;2(2):184–5.
Ahmadzai H, Campbell S, Archis C, Clark WA. Fat embolism syndrome following percutaneous vertebroplasty: a case report. Spine J. 2014;14(4):e1–5. https://doi.org/10.1016/j.spinee.2013.09.021.
Wu S, Pan L, Wu H, Shi H, Zhao Y, Ji Y, et al. Anatomic study of ophthalmic artery embolism following cosmetic injection. J Craniofac Surg. 2017;28(6):1578–81. https://doi.org/10.1097/SCS.0000000000003674.
Digby J, Lewis JV. A case of traumatic fat embolism syndrome without long bone fracture. Tenn Med. 1999;92(8):308–9.
Ajzan A, Modine T, Punjabi P, Ganeshalingam K, Philips G, Gourlay T. Quantification of fat mobilization in patients undergoing coronary artery revascularization using off-pump and on-pump techniques. J Extra Corpor Technol. 2006;38(2):116–21.
Day JD, Walden SM, Stuart SR, Hutchins GM, Hruban RH. Fatal fat embolism syndrome after numerous vertebral body compression fractures in a lung transplant recipient. J Heart Lung Transplant. 1994;13(5):785–90.
Scott J, Collin N, Baker R, Ravanan R. Fat embolism: a rare cause of perioperative renal transplant dysfunction. BMJ Case Rep. 2017. https://doi.org/10.1136/bcr-2017-221829.
Guardia SN, Bilbao JM, Murray D, Warren RE, Sweet J. Fat embolism in acute pancreatitis. Arch Pathol Lab Med. 1989;113(5):503–6.
Alves Júnior A, Coelho AM, Sampietre SN, Kubrusly MS, Molan NA, Leite KR, et al. Physiopathology of lung injury in acute pancreatitis. Rev Hosp Clin Fac Med Sao Paulo. 1996;51(6):232–8 Review. Portuguese.
Broder G, Ruzumna L. Systemic fat embolism following acute primary osteomyelitis. JAMA. 1967;199(13):150–2.
Garza JA. Massive fat and necrotic bone marrow embolization in a previously undiagnosed patient with sickle cell disease. Am J Forensic Med Pathol. 1990;11(1):83–8.
Lipton JH, Russell JA, Burgess KR, Hwang WS. Fat embolization and pulmonary infiltrates after bone marrow transplantation. Med Pediatr Oncol. 1987;15(1):24–7.
Gangaraju R, Reddy VV, Marques MB. Fat embolism syndrome secondary to bone marrow necrosis in patients with hemoglobinopathies. South Med J. 2016;109(9):549–53. https://doi.org/10.14423/SMJ.0000000000000520.
Menendez LR, Bacon W, Kempf RA, Moore TM. Fat embolism syndrome complicating intraarterial chemotherapy with cis-platinum. Clin Orthop Relat Res. 1990;254:294–7.
Buskens CJ, Gratama JW, Hogervorst M, van Leeuwen RB, Rommes JH, Spronk PE. Encephalopathy and MRI abnormalities in fat embolism syndrome: a case report. Med Sci Monit. 2008;14(11):CS125–9.
Estebe JP, Malledant Y. Fat embolism after lipid emulsion infusion. Lancet. 1991;337(8742):673.
Seyfried TF, Haas L, Gruber M, Breu A, Loibl M, Hansen E. Fat removal during cell salvage: a comparison of four different cell salvage devices. Transfusion. 2015;55(7):1637–43. https://doi.org/10.1111/trf.13035.
•• Murphy LD, Yabrodi M, Lutfi R. Fat embolism syndrome in Duchenne muscular dystrophy patients: early recognition and aggressive therapy. Case Rep Crit Care. 2018;2018:3686470. https://doi.org/10.1155/2018/3686470 eCollection 2018. A high index of suspicion for fat embolism syndrome is needed in patients with Duchene muscular dystrophy.
Lu K, Xu M, Li W, Wang K, Wang D. A study on dynamic monitoring, components, and risk factors of embolism during total knee arthroplasty. Medicine (Baltimore). 2017;96(51):e9303. https://doi.org/10.1097/MD.0000000000009303.
Cronin KJ, Hayes CB, Moghadamian ES. Early-onset fat embolism syndrome: a case report. JBJS Case Connect. 2018;8(2):e44. https://doi.org/10.2106/JBJS.CC.17.00175.
Kellogg RG, Fontes RB, Lopes DK. Massive cerebral involvement in fat embolism syndrome and intracranial pressure management. J Neurosurg. 2013;119(5):1263–70. https://doi.org/10.3171/2013.7.JNS13363.
Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52–5.
Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435–9.
Rafik R, Hachimi MA, Ouarssani A, Atoini F, Rouimi A. A rare cause of cerebral ischemic stroke: cerebral fat embolism. Rev Neurol (Paris). 2012;168(3):298–9. https://doi.org/10.1016/j.neurol.2011.06.007 French.
Toledano S, Zyss J, Gerber S, Rodallec M, Zuber M. Fat emboli responsible for ischemic stroke in reconstructive eye surgery. J Neurol. 2010;257(11):1927–8. https://doi.org/10.1007/s00415-010-5621-5.
Kang JH, Hargett CW, Sevilis T, Luedke M. Sickle cell disease, fat embolism syndrome, and “starfield” pattern on MRI. Neurol Clin Pract. 2018;8(2):162–4. https://doi.org/10.1212/CPJ.0000000000000443.
Medina FJ, Marquez JC, Castillo M. Cerebral fat embolism detection with susceptibility-weighted images in sickle cell disease. Neuroradiol J. 2012;25(4):411–4.
AMELIN AZ. Hemorrhagic stroke following fat embolism. Arkh Patol. 1964;26:69–71 Russian.
Peters ST, Witvliet MJ, Vennegoor A, Ten Tusscher B, Boden B, Bloemers FW. The fat embolism syndrome as a cause of paraplegia. SAGE Open Med Case Rep. 2018;6:2050313X18789318.
• Kearsley R, Galbraith J, Dalton D, Motherway C. Spinal cord infarction as a rare complication of fat embolism syndrome following bilateral intramedullary nailing of femur fractures. BMJ Case Rep. 2016;2016. https://doi.org/10.1136/bcr-2016-215690 Spinal cord infarction secondary to fat embolism syndrome should be considered in high risk population (e.g. femur fractures).
Kaufman HD, Finn R, Bourdillon RE. Fat embolism following an epileptic seizure. Br Med J. 1966;1(5495):1089.
•• Chatterjee R, et al. Nonconvulsive status in the trauma centre: Think of cerebral fat embolism. Neurol India. 2017. https://doi.org/10.4103/0028-3886.217950 Fat embolism syndrome should be suspected in trauma patients with nonconvulsive status epilepticus.
Godoy DA, Orquera J, Rabinstein AA. Paroxysmal sympathetic hyperactivity syndrome caused by fat embolism syndrome. Rev Bras Ter Intensiva. 2018. https://doi.org/10.5935/0103-507X.20180035.
Yamamoto K, Kushimoto S. Subarachnoidal fat droplet deposition and fat embolism syndrome. BMJ Case Rep. 2017, 2017. https://doi.org/10.1136/bcr-2017-221493.
Woo JK, Malfair D, Vertinsky T, Heran MK, Graeb D. Intracranial transthecal subarachnoid fat emboli and subarachnoid haemorrhage arising from a sacral fracture and dural tear. Br J Radiol. 2010;83(985):e18–21. https://doi.org/10.1259/bjr/66268641.
Nentwich MM, Remy M, Schaller UC. Ocular fat embolism syndrome. Int Ophthalmol. 2011;31(1):15–6. https://doi.org/10.1007/s10792-010-9378-8.
Kim J, Kim SK, Kim MK. Segmental ischaemic infarction of the iris after autologous fat injection into the lower eyelid tissue: a case report. BMC Ophthalmol. 2017;17. https://doi.org/10.1186/s12886-017-0599-8.
Mustanoja S, Sundararajan S, Strbian D. Unconscious patient after elective bilateral total knee arthroplasty. Stroke. 2014;45(3):e38–9. https://doi.org/10.1161/STROKEAHA.113.004011.
Mijalski C, Lovett A, Mahajan R, Sundararajan S, Silverman S, Feske S. Cerebral fat embolism: a case of rapid-onset coma. Stroke. 2015;46(12):e251–3. https://doi.org/10.1161/STROKEAHA.115.011440.
• Metting Z, Rödiger LA, Regtien JG, van der Naalt J. Delayed coma in head injury: consider cerebral fat embolism. Clin Neurol Neurosurg. 2009;111(7):597–600. https://doi.org/10.4103/0972-5229.169358 Consider cerebral fat embolism in patients with traumatic brain injury with prolonged coma, particularly in patients with long bone fractures. The classical clinical triad for fat embolism syndrome may not be present present in these patients. Brain MRI is valuable for diagnosis of fat embolism syndrome leading to delayed coma..
Tanton J. L’embolie graisseuse traumatique. J de Chir. 1914;12:287–96.
Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg (Br). 1987;69(1):128–31.
Forteza AM, Koch S, Campo-Bustillo I, Gutierrez J, Haussen DC, Rabinstein AA, et al. Transcranial Doppler detection of cerebral fat emboli and relation to paradoxical embolism: a pilot study. Circulation. 2011;123(18):1947–52. https://doi.org/10.1161/CIRCULATIONAHA.110.950634.
Abend NS, Levine JM. Hypodense middle cerebral artery with fat embolus. Neurocrit Care. 2007;6(2):147–8.
Saleh M, Juan E. Cerebral fat emboli syndrome: do not miss the transcranial Doppler findings. BMJ Case Rep. 2015;2015. https://doi.org/10.1136/bcr-2015-210445.
Reynolds P, Walker FO, Eades J, Smith JD, Lantz PE. Food embolus. J Neurol Sci. 1997;149(2):185–90.
Woo YS, Hong SC, Park SM, Cho KH. Ischemic stroke related to an amniotic fluid embolism during labor. J Clin Neurosci. 2015;22(4):767–8. https://doi.org/10.1016/j.jocn.2014.10.024.
Navi BB, Kawaguchi K, Hriljac I, Lavi E, DeAngelis LM, Jamieson DG. Multifocal stroke from tumor emboli. Arch Neurol. 2009;66(9):1174–5. https://doi.org/10.1001/archneurol.2009.172.
Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009;52(5):386–93 Review.
Whalen LD, Khot SP, Standage SW. High-dose rosuvastatin treatment for multifocal stroke in trauma-induced cerebral fat embolism syndrome: a case report. Pediatr Neurol. 2014;51(3):410–3. https://doi.org/10.1016/j.pediatrneurol.2014.04.025.
Gai N, Lavi R, Jones PM, Lee H, Naudie D, Bainbridge D. The use of point-of-care ultrasound to diagnose patent foramen ovale in elective hip and knee arthroplasty patients and its association with postoperative delirium. Can J Anesth. 2018;65:619–26. https://doi.org/10.1007/s12630-018-1073-7.
•• Dunkel J, Roth C, Erbguth F, Dietrich W, Hügens-Penzel M, Ferbert A. Cerebral fat embolism: clinical presentation, diagnostic steps and long-term follow-up. Eur Neurol. 2017;78(3–4):181–7. https://doi.org/10.1159/000479002 Most patients with fat embolism syndrome have good outcome after long term follow up.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sarkis Morales-Vidal declares no potential conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This Article is part of the Topical Collection on Neurology of Systemic Diseases
Rights and permissions
About this article
Cite this article
Morales-Vidal, S.G. Neurologic Complications of Fat Embolism Syndrome. Curr Neurol Neurosci Rep 19, 14 (2019). https://doi.org/10.1007/s11910-019-0928-9
Published:
DOI: https://doi.org/10.1007/s11910-019-0928-9