Abstract
Purpose of Review
For millennia, there has been interest in the use of cannabis for the treatment of epilepsy. However, it is only recently that appropriately powered controlled studies have been completed. In this review, we present an update on the research investigating the use of cannabidiol (CBD), a non-psychoactive component of cannabis, in the treatment of epilepsy.
Recent Findings
While the anticonvulsant mechanism of action of CBD has not been entirely elucidated, we discuss the most recent data available including its low affinity for the endocannabinoid receptors and possible indirect modulation of these receptors via blocking the breakdown of anandamide. Additional targets include activation of the transient receptor potential of vanilloid type-1 (TRPV1), antagonist action at GPR55, targeting of abnormal sodium channels, blocking of T-type calcium channels, modulation of adenosine receptors, modulation of voltage-dependent anion selective channel protein (VDAC1), and modulation of tumor necrosis factor alpha release. We also discuss the most recent studies on various artisanal CBD products conducted in patients with epilepsy in the USA and internationally. While a high percentage of patients in these studies reported improvement in seizures, these studies were either retrospective or conducted via survey. Dosage/preparation of CBD was either unknown or not controlled in the majority of these studies. Finally, we present data from both open-label expanded access programs (EAPs) and randomized placebo-controlled trials (RCTs) of a highly purified oral preparation of CBD, which was recently approved by the FDA in the treatment of epilepsy. In the EAPs, there was a significant improvement in seizure frequency seen in a large number of patients with various types of treatment-refractory epilepsy. The RCTs have shown significant seizure reduction compared to placebo in patients with Dravet syndrome and Lennox-Gastaut syndrome. Finally, we describe the available data on adverse effects and drug-drug interactions with highly purified CBD. While this product is overall well tolerated, the most common side effects are diarrhea and sedation, with sedation being much more common in patients taking concomitant clobazam. There was also an increased incidence of aspartate aminotransferase and alanine aminotransferase elevations while taking CBD, with many of the patients with these abnormalities also taking concomitant valproate. CBD has a clear interaction with clobazam, significantly increasing the levels of its active metabolite N-desmethylclobazam in several studies; this is felt to be due to CBD’s inhibition of CYP2C19. EAP data demonstrate other possible interactions with rufinamide, zonisamide, topiramate, and eslicarbazepine. Additionally, there is one case report demonstrating need for warfarin dose adjustment with concomitant CBD.
Summary
Understanding of CBD’s efficacy and safety in the treatment of TRE has expanded significantly in the last few years. Future controlled studies of various ratios of CBD and THC are needed as there could be further therapeutic potential of these compounds for patients with epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent anecdotal reports of possible efficacy of plant-derived cannabis extracts for treatment-refractory epilepsy (TRE) have rekindled the interest in studying them in detail [1, 2]. While the focus has been predominantly on cannabidiol (CBD), several observational studies have addressed the efficacy of artisanal products containing a potpourri of phytocannabinoids with typically high CBD and low THC content. Tremendous progress has been made in the last 3–4 years necessitating an update for practicing neurologists. In this review, we focus on the new findings regarding the mechanism of action (MOA), developments in artisanal products for the treatment of various epilepsies, reports of the expanded access programs (EAP), and, finally, results of the randomized clinical trials (RCTs).
Mechanism of Action
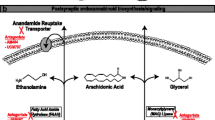
The MOA of CBD’s anticonvulsant effects has been studied and reviewed extensively. However, its exact MOA has been only partially elucidated [3]. Agonist activity at endocannabinoid receptors CB1 and CB2 has demonstrated an anticonvulsant effect that is seen with the psychoactive component of the cannabis plant, tetrahydrocannabinol (THC) [4]. CBD actually has low affinity for these endocannabinoid receptors [5]. A series of studies have demonstrated anticonvulsant properties of both THC and CBD; THC’s action was primarily due to agonist activity at the CB1 receptor while CBD’s action was not [6,7,8]. Additionally, there have been several studies that demonstrate that CBD has antagonist activity at the CB1 and CB2 receptors [9,10,11]. However, CBD has been shown to block anandamide (ANA) uptake and hydrolysis, effectively increasing its availability to activate the CB1 and CB2 receptors. As such, CBD may modulate the endocannabinoid system (ECS) indirectly [12]. Thus, while CBD is likely to modulate the ECS, its anticonvulsant MOA continues to be unclear. However, there is increasing evidence for ECS’ importance for the process of epileptogenesis [13•, 14].
Another proposed MOA of CBD is activation of the transient receptor potential (TRP) of vanilloid type-1 (TRPV1), which is also a target for ANA and may modulate calcium channels [3, 12, 15, 16]. One recent animal study showed CBD to attenuate seizures and EEG activity in the pentylenetetrazole (PTZ) model of epilepsy; its effects on seizure latency and duration were reversed individually by CB1, CB2, and TRPV1 antagonists [15]. TRPV1 activation has been shown to increase calcium influx and, thus, neural activity and glutamate release. TRPV1 blockade has been shown to abolish anticonvulsant activity which makes notion that CBD’s agonism of TRPV1 results in anticonvulsant activity somewhat contradictory [16]. However, these authors suggest that the TRPV1 channels actually become desensitized after an agonist (i.e., CBD) binds to them and the desensitization that eventually results in anticonvulsant activity [15].
CBD also has antagonist action on the lipid activated G protein coupled receptor GPR55; this receptor is considered a “novel cannabinoid receptor” whose antagonism could have anticonvulsant effects [17]. GPR55 is expressed in the hippocampus, pyramidal cells, and the interneurons in the pyramidal cell layer and has been shown to modulate hippocampal synaptic plasticity [18]. A recent animal study showed that antagonism of GPR55 occluded CBD’s actions on seizures and mimicked CBD’s enhancement of inhibitory transmission in mouse dentate granule cells [19].
Many other actions of CBD could plausibly contribute to its anticonvulsant activity. CBD can preferentially target abnormal/mutant sodium channels, which would be of interest in, e.g., Dravet syndrome [20]. CBD blocks human T-type calcium channels—similar action is seen with other anti-seizure drugs (ASD) [21]. Other possibilities include modulation of adenosine receptors, voltage-dependent anion selective channel protein (VDAC1), and tumor necrosis factor alpha release [22,23,24]. CBD also appears to have an anti-inflammatory effect in the nervous system by decreasing pro-inflammatory functions and signaling in astrocytes to prevent the increase in inflammatory cytokines (IL-6) in animal models of epilepsy; however, the role neural inflammation plays in seizure initiation and maintenance continues to be under investigation [15, 25]. Finally, while CBD has affinity for serotonin receptors 5-HT1A and 5-HT2A, a recent PTZ model animal study showed that pre-treatment with serotonin receptor antagonists did not block CBD’s anticonvulsant effect; thus, this possible MOA is less plausible [26].
Artisanal Products
We recently reviewed data available prior to 2014 and reported a calculated open-label efficacy of ~ 61% (any improvement) [2]. Since then (Table 1), one study described experiences from California, Maine, and Washington State [27•]. Their retrospective data collection summarized the efficacy and adverse events in the combined 272 patients with various clinical diagnoses including Lennox-Gastaut syndrome (LGS), Dravet syndrome, and Rett syndrome. However, majority of their patients had epilepsy of unknown etiology. Overall, 37 (14%) found cannabis ineffective while 45 (17%) experienced a 51–75% reduction in seizures, 75 (28%) had 76–99% reduction in seizures, and 26 (10%) achieved seizure freedom. The products utilized in the study were different between sites—California patients used predominantly high CBD/low THC products (ratio ranging from 15:1 to 27:1), Washington State patients used untested products with some of them self-medicating with homemade preparations; content was tested using HPLC in patients from Maine. Four cases from Maine were presented in addition to the 272 cases discussed above in whom various low-dose CBD-enriched combinations of CBD, tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), THC, and tetrahydrocannabivarin (THCV) were used with various success. The calculated overall seizure responder rate (RR) was ~ 54%.
Two studies with overlapping populations summarized Colorado experiences. The first focused on the response to oral cannabis extracts [28]. These authors retrospectively reviewed charts of children and adolescents with TREs who utilized cannabis products. Of the 75 patients, 57% reported improvement in seizure frequency with RR of 33%. Of interest is the fact that the responder rate varied by residence status. Of patients or families who relocated to Colorado to obtain cannabis extracts for the treatment of epilepsy, 47% were considered responders compared to only 22% who were long-standing Colorado residents indicating high placebo (or expectation of efficacy) response that is very common in neurological disorders [44]. The responses also varied by epilepsy syndrome: Dravet—23%, Doose—0%, and Lennox-Gastaut syndrome (LGS)—88.9%. An expanded follow-up study reported that of the 119 patients, 71% terminated the use of the cannabis product within ± 11.7 months (range 0.3–57 months). This reflects overall a very low retention rate—part of this is likely driven by lack of treatment response and part by the cost of treatment since artisanal products are not covered by medical insurance. The perceived seizure benefit was the only factor associated with longer duration of treatment. Again, relocation to CO was associated with perceived benefit in 65% of users vs. 38% in patients who were long-standing residents of the State. In this cohort, only 24% of participants were considered responders.
An online survey of 117 parents of children with epilepsy focusing on children with epileptic spasms and LGS examined the perceived efficacy and tolerability of CBD-enriched cannabis preparations [29]. The results were similar in etiologic groups with 85% of respondents reporting improvement in seizures and 14% reporting seizure freedom when utilizing median dose of CBD of 4.3 mg/kg/day (minority of patients were able to calculate the utilized dose of CBD). Of parents who knew the CBD:THC content, most indicated the ratio of 15:1. The RR in this study was not provided.
Two studies reported experiences from Israel. The first study presented data from 74 children with epilepsy ages 1–18 years [30]. They used 20:1 CBD:THC ratio with the CBD dose ranging from 1 to 20 mg/kg/day titrated based on seizure response and adverse events. The treatment resulted in significant decrease in seizures in 66/74 (89%) of children with a RR of 51%. A recent second report from Israel utilized artisanal 20:1 CBD:THC ratio plant-derived product in 46 children and adults with TRE [31]. While the product was obtained from local dispensary, it was extensively tested for content and proportion of CBD:THC. Overall, participants who received doses of CBD higher than 11 mg/kg/day fared better than those who received lower doses (80% improvement vs. 50% respectively; p = 0.043). Lowering doses of concomitant ASDs did not affect the response to the cannabis product. Of further interest is the addition of a vaporized form of cannabis to the treatment in patients who either did not tolerate the oral version or wanted additional supplementation (N = 17); while the chemical composition of the vaporized product was not provided, 6/17 had further improvement. Overall, 56% of patients in this study were considered responders.
Two Australian studies are also available. In one nationwide survey of patient experiences with cannabis products, of the 976 responses that met the inclusion criteria, 15% of adults and 13% of children with epilepsy were currently using, or had previously used various cannabis products to treat epilepsy. Ninety percent of adults and 71% of parents reported improved seizure frequency while taking these products but stratification by responder rate was not documented (their questionnaire included dichotomized response) [32]. The number of previously tried ASDs was a significant predictor of medicinal cannabis use in both adults and children with epilepsy in uni- and multi-variate analyses indicating that many had TRE. In another study by the same group, the authors conducted an in-person interview of families (N = 41) that used cannabis products for the treatment of epilepsy [33]. Some families co-administered more than one cannabis product; of the 51 products tested, 38/51 were considered effective by their users. Of interest is that most of the tested cannabis products, contrary to expectation, contained low CBD concentration while THC was present in almost all tested samples supporting the previous notion of low accuracy of the artisanal cannabis product labelling [45].
Open-label Studies of Pharmaceutical Grade CBD
The first published data on pharmaceutical grade CBD’s (Epidiolex®) efficacy for TREs come from an open-label study conducted at 11 epilepsy centers across the USA via EAPs (Table 1) [34]. Participants were adults and children who were started on CBD at a dose of 2–5 mg/kg/day. The dose could be titrated by 2–5 mg/kg a week until intolerance or a maximum dose of 25 mg/kg/day was reached (however, at some sites, the maximum dose was 50 mg/kg/day). In 137 participants, the median decrease in total seizures was 34.6%. Decreases in seizure frequency were also analyzed by seizure type: focal seizures (n = 42, 55.0%), atonic seizures (n = 32, 54.3%), tonic seizures (n = 65, 36.5%), and tonic-clonic seizures (n = 89, 16.0). RR for all seizure types was 37%, 22% had reduction in seizure frequency of ≥ 70%, and 8% had a reduction of ≥ 90%. In a post hoc analysis, RR was not different between patients with Dravet syndrome or LGS and other participants. Follow-up analysis on data from 25 EAPs was recently published [35•]. Six hundred seven adult and pediatric patients (mean age 18, range 1–61) with TRE were enrolled with 580 included in the efficacy analysis. CBD was started at 2–10 mg/kg/day depending on the study site and was titrated to a maximum dose of 25–50 mg/kg/day. After 12 weeks of treatment, the median monthly frequency of convulsive seizures was reduced by 51% and the frequency of total seizures was reduced by 48%; the observed reductions were not affected by dropouts. RRs for total seizures were 49%, 30% had ≥ 75% reduction, and 6% were seizure-free. RRs remained relatively stable for convulsive and total seizures during the 12–96-week analysis period.
Separate analysis conducted at a single EAP investigated CBD’s effect on both seizure frequency and severity [36]. In 132 adult and pediatric participants, the mean reduction in all seizure types was 63.6% (p = 0.01) at 12-week follow-up, with sustained seizure frequency reduction at 24- and 48-week follow-up visits. Seizure severity assessed via the Chalfont Seizure Severity Scale (CSSS) showed improvement from a total score of 80.7 at enrollment to 39.3 at week 12 (p < 0.0001) with continued stable CSSS scores at 24 and 48 weeks.
Several manuscripts have described CBD’s effects on seizures in certain disease states; most of these studies are sub-analyses from EAPs. One study investigated the effects of CBD on 18 patients with tuberous sclerosis complex; median seizure frequency reduction was 48.8% after 3 months of treatment [37]. Patients taking concomitant clobazam with CBD had increased seizure frequency reduction (53.2% with concomitant clobazam vs. 36.4% without clobazam). Seizure frequency response to CBD in patients with various types of epileptic encephalopathies (CDKL5 deficiency disorder, n = 20; Aicardi syndrome, n = 19; Dup15q syndrome, n = 8; Doose syndrome, n = 8) enrolled in various EAPs was also presented [38]. Mean seizure frequency was reduced by 51.4% at 12 weeks, with sustained reduction at 48 weeks. A case series of 7 children with refractory seizures due to febrile infection-related epilepsy syndrome (FIRES) received CBD in the acute (n = 2) or chronic (n = 5) phase of the illness [39]. In the 2 patients in the acute phase, 1 had cessation of status epilepticus and the other patient (who died due to multi-organ failure felt to be due to prolonged isofluorane exposure) had only stimulus-induced seizures after CBD treatment. The 5 patients in the chronic phase had a mean seizure frequency reduction of 90.9% at 4 weeks and 65.3% at 48 weeks compared to baseline. Finally, a recently published study prospectively examined the efficacy of 50:1 CBD/THC pharmaceutical grade product [40]. These authors were able to show 70.6% median motor seizure reduction in patients with DS with a RR of 63%. The dose of CBD and THC in this study ranged from 2 to 16 mg/kg/day and from 0.14 to 0.32 mg/kg/day, respectively.
Randomized Controlled Trials of Pharmaceutical Grade CBD
Currently, there are no randomized controlled trials of artisanal cannabis products. Some of the initial studies included in our 2014 review were small randomized trials with CBD extracts of cannabis plant with unclear purity that likely included some amounts of THC [2]. However, the results of these trials were inconclusive as to the efficacy of cannabis products for the treatment of epilepsy [41]. In the last 2 years, four randomized controlled trials of pharmaceutical grade CBD have been completed and one is currently ongoing (CBD for seizures in tuberous sclerosis complex; NCT02544763).
The first study reported on the use of CBD (Epidiolex®) for the treatment of convulsive seizures in patients with Dravet syndrome (DS; Table 1) [46•]. In this double-blind, placebo-controlled study, patients were randomized to receive either CBD at 20 mg/kg/day or placebo in addition to their standard ASDs. The primary outcome measure was the change in convulsive seizures over a 14-week treatment period compared to a 4-week baseline. The authors were able to show a significant decrease in convulsive seizures per month from 12.4 to 5.9 with CBD vs. 14.9 to 14.1 with placebo (p = 0.01 after adjusting for baseline differences). The responder rate of convulsive seizures in this study was 43% for CBD vs. 27% for placebo (p = 0.08). The authors also reported on the overall seizure frequency (all seizure types) which has improved in the CBD group (p = 0.03). However, there was no significant improvement in the non-convulsive seizures. There was an overall improvement in the Caregiver Global Impression of Change scale in 62% of the CBD compared to 34% of the patients treated with placebo (p = 0.02). In the second Dravet syndrome study, patients were randomized to receive placebo or 5 mg/kg/day, 10 mg/kg/day, or 20 mg/kg/day of CBD [42]. Since this was a pharmacokinetic (PK) study (no efficacy data were provided), we discuss this study in the “Interactions” section below.
Of the two LGS studies, the first study included 171 patients with drop seizures who were randomized to receive either placebo or CBD (Epidiolex®) at 20 mg/kg/day after a 4-week baseline; the primary endpoint was change from baseline in drop seizure frequency. After 14 weeks of treatment, the median percentage reduction in drop seizure frequency per month from baseline was 43.9% in the CBD group and 21.8% in the placebo group (p = 0.0135). Forty-four percent of patients were considered responders in the treatment phase and 46% in the maintenance phase of the study with respect to a reduction of drop seizures. The second randomized and placebo-controlled study also evaluated the efficacy of CBD in LGS with the primary endpoint being change in the rate of drop seizures [43•]. In this dose ranging study, patients were randomized to placebo, 10 mg/kg/day or 20 mg/kg/day of CBD with response measured at 14 weeks when compared to 4-week baseline. Of the 225 enrolled patients, 41.9% in the 20 mg/kg/day CBD group, 37.2% in the 10 mg/kg/day CBD group, and 17.2% in the placebo group responded to therapy with comparisons between treatment and placebo groups being significant. Responder rates for drop seizures were 39%, 36%, and 14% in the 20 mg/kg/day, 10 mg/kg/day, and placebo groups, respectively.
Of note is that two recently completed randomized controlled trials failed to show efficacy of CBD and CBDV for the treatment of epilepsy. In a study by Zynerba Pharmaceuticals (zynerba.com), a transdermal delivery of CBD did not produce statistically significant reduction of seizures in patients with refractory epilepsy with focal seizures. This trial was conducted in 188 patients who were randomized to receive either 195 mg of ZYN002 4.2% gel every 12 h, 97.5 mg of ZYN002 4.2% gel every 12 h, or placebo. Overall, patients on the low dose of ZYN002 had 18.4% seizure reduction vs. 14% on high dose vs. 8.7% seizure reduction for placebo. Another recently completed RCT showed lack of efficacy of CBDV vs. placebo for the treatment of focal onset epilepsy in 162 adults; both the placebo and active arm showed ~ 40% seizure reduction from baseline (GW Pharmaceuticals; gwpharm.com).
Adverse Effects
Adverse effects of artisanal products have been reported. In one study, in addition to standard adverse events of increased seizures and fatigue/somnolence, the authors reported positive effects of improved behavior/alertness (33%), improved language (10%), and improved motor skills (10%) [28]. Another study reported increased appetite while positive adverse effects included improved sleep, alertness, and mood which were reported in > 50% of participants [29]. One of the studies from Israel reported aggressive behavior and worsening of seizures; some patients had somnolence, fatigue, gastrointestinal disturbances, and irritability [30]. Finally, 46% of participants reported adverse events including somnolence in 14% in the second study from Israel [31].
Reported side effects in both the open-label EAPs and the RCTs of highly purified CBD (Epidiolex®) have been similar. In the most recent update from the EAPs [35•], diarrhea (29.2% of all patients) and somnolence (22.4%) were the most commonly reported adverse events; other less commonly reported AEs included upper respiratory infection (12.4%), decreased appetite (12.4%), convulsion (16.8%), vomiting (11.4%), fatigue (10.7%), pyrexia (10.4%), status epilepticus (7.4%), and pneumonia (6.8%). Somnolence and diarrhea appeared to be dose-related, and somnolence was much more common in patients taking concomitant clobazam (38% of those taking clobazam vs. 14% not taking clobazam). Abnormal liver function tests (LFTs; alanine aminotransferase/aspartate aminotransferase elevations > 3 times the upper limit of normal) were seen in 10% of patients, but 75% were taking concomitant valproate. In this study, 5.2% (n = 31/607) of patients discontinued CBD due to AEs.
In the first published RCT for Dravet syndrome [46•], patients were assigned to either a CBD dose of 20 mg/kg/day or placebo; thus, no dose-related effects could be ascertained. In this study, AEs were similar to the EAP data, with diarrhea (31% in the treatment group vs. 10% in the placebo group) and somnolence (36% in the treatment group vs. 10% in the placebo group) were the most common side effects; again, the majority (18/22) of patients reporting sedation were also taking clobazam. LFT abnormalities led to withdrawal of 3 patients in the treatment group and 1 patient in the placebo group; all of these patients were taking valproate. In 9 patients who had LFT elevations and continued in the trial, the levels returned to normal while they continued to receive CBD. In the safety trial in Dravet syndrome [42], rash was more frequently reported (5 in the CBD group vs. 1 in the placebo group), with a diffuse maculopapular rash, localized rash, papular rash, viral rash, and concomitant rash and hives seen in the CBD group and diaper rash seen in the 1 patient in the placebo group.
In the LGS trial [47•], reported side effects in patients receiving CBD were the same and included diarrhea, somnolence, decreased appetite, and vomiting. Of the patients who had adverse events, the events resolved by the end of the trial in 45 (61%) of patients receiving CBD and 38 (64%) of patients in the placebo group. Three patients had to withdraw from the study due to LFT elevations. Additionally, 1 patient withdrew from this study due to each of the following side effects: diarrhea, vomiting, acute hepatic failure, viral infection, increased concentration of another ASD, convulsion, lethargy, restlessness, acute respiratory distress syndrome, hypercapnia, hypoxia, pneumonia aspiration, and rash. All patients who had respiratory distress were all taking concomitant clobazam.
Interactions
Few studies have addressed drug-drug interactions with CBD. In particular, several studies have identified a pharmacokinetic interaction with clobazam. In a study of 13 children taking concomitant clobazam with purified CBD, the mean clobazam and N-desmethylclobazam plasma levels were increased after treatment with CBD compared to pre-CBD baseline, though the clobazam level changes were smaller and not statistically significant [48]. These increased levels led to reduction of clobazam dose due to reports of sedation. This interaction was felt to be caused by CBD’s potent inhibition of CYP2C19, that is, the enzyme responsible for metabolizing N-desmethylclobazam [49].
In the RCT dose ranging study for DS, changes in ASD levels with CBD treatment were reported [42]. The authors measured levels of clobazam, N-desmethylclobazam, valproate, stiripentol, and levetiracetam. Again, there was a significant increase in N-desmethylclobazam levels in all dose groups. They did not observe significant changes to levels of clobazam, valproate, stiripentol, and levetiracetam. The numbers of patients in each group were relatively small as compared to another study that showed clear interactions with several standard ASDs [50•]. This study also focused on pharmacokinetics; testing included serum levels of CBD and its multiple metabolites including 6-OH-CBD, 7-OH-CBD, and 7-COOH-CBD and levels of ASDs [42]. Of note is that of the 6/22 patients receiving valproate developed elevated transaminases—this is similar to another recently reported study [35]. However, none of these elevations met criteria for drug-induced liver injury and all patients were reported to recover.
Finally, one EAP measured blood levels of all ASDs taken by 39 adult and 42 pediatric patients in the study prior to starting treatment with CBD and at every study follow-up visit [50•]. With increasing CBD dose, there were statistically significant increases in levels of clobazam, N-desmethylclobazam, rufinamide, and topiramate seen in all patients with increasing CBD dose. In adults only, there were also increases seen in eslicarbazepine and zonisamide levels. However, the mean changes in levels exceeded normal therapeutic range for clobazam and N-desmethylclobazam only. Additionally, patients taking concomitant valproate had statistically significant changes in mean ALT and AST levels compared to patients not taking valproate, though valproate levels did not change significantly from baseline. This program also reported an interaction between CBD and warfarin, necessitating adjustments in warfarin dosing [51]. In one study, the presence or absence of ASDs that interacted with CBD did not affect the response to CBD [52].
Conclusions
Understanding of CBD’s efficacy and safety in the treatment of TRE has expanded significantly in the last few years. The results of the published RCTs have led to FDA approval of a highly purified formulation of CBD. It appears CBD has a novel MOA which makes it a desirable option for patients with TRE who have failed standard ASDs. While there are potential adverse effects and drug-drug interactions with CBD, the published studies indicate it is well tolerated and only certain drug level and liver function monitoring needs to be performed. While these data are promising, it is important to note that these data cannot be generalized to all available CBD and cannabis products. Future controlled studies of various ratios of CBD and THC are needed as there could be further therapeutic potential of these compounds for patients with epilepsy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55:783–6.
Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy--from receptors to clinical response. Epilepsy Behav. 2014;41:277–82.
Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017;70:313–8.
Pertwee RG, Cascio MG. Known pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In: Pertwee RG, editor. Handbook of cannabis. Oxford: Oxford University Press; 2014. p. 115.
Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215.
Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–37.
Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452:295–301.
Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428:51–7.
McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–53.
Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res. 2017;7:61–76.
Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–23.
Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52.
• Cleeren E, Casteels C, Goffin K, et al. Positron emission tomography imaging of cerebral glucose metabolism and type 1 cannabinoid receptor availability during temporal lobe epileptogenesis in the amygdala kindling model in rhesus monkeys. Epilepsia. 2018;59:959–70. This study documents the relationship between epileptogenesis and endocannabinoid system through all stages of epilepsy development.
Szaflarski J. The highs and lows of the endocannabinoid system – Another piece to the epilepsy puzzle? Epilepsy Curr. 2018;in print.
Vilela LR, Lima IV, Kunsch EB, Pinto HPP, de Miranda AS, Vieira ÉLM, et al. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. 2017;75:29–35.
Naziroglu M. TRPV1 channel: a potential drug target for treating epilepsy. Curr Neuropharmacol. 2015;13:239–47.
Ryberg E, Larsson N, Sjogren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–101.
Hurst K, Badgley C, Ellsworth T, Bell S, Friend L, Prince B, et al. A putative lysophosphatidylinositol receptor GPR55 modulates hippocampal synaptic plasticity. Hippocampus. 2017;27:985–98.
Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017;114:11229–34.
Patel RR, Barbosa C, Brustovetsky T, Brustovetsky N, Cummins TR. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain J Neurol. 2016;139:2164–81.
Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J Biol Chem. 2008;283:16124–34.
Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802.
During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–24.
Rimmerman N, Ben-Hail D, Porat Z, Juknat A, Kozela E, Daniels MP, et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013;4:e949.
Kozela E, Juknat A, Vogel Z. Modulation of astrocyte activity by cannabidiol, a nonpsychoactive cannabinoid. Int J Mol Sci. 2017;18
Pelz MC, Schoolcraft KD, Larson C, Spring MG, Lopez HH. Assessing the role of serotonergic receptors in cannabidiol’s anticonvulsant efficacy. Epilepsy Behav. 2017;73:111–8.
• Sulak D, Saneto R, Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017;70:328–33. While open label, this study discusses various aspects of treating epilepsy with artisanal cannabis products in various settings.
Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49–52.
Hussain SA, Zhou R, Jacobson C, Weng J, Cheng E, Lay J, et al. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: a potential role for infantile spasms and Lennox-Gastaut syndrome. Epilepsy Behav. 2015;47:138–41.
Tzadok M, Uliel-Siboni S, Linder I, Kramer U, Epstein O, Menascu S, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure. 2016;35:41–4.
Hausman-Kedem M, Menascu S, Kramer U. Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents - an observational, longitudinal study. Brain and Development. 2018;40:544–51.
Suraev AS, Todd L, Bowen MT, Allsop DJ, McGregor IS, Ireland C, et al. An Australian nationwide survey on medicinal cannabis use for epilepsy: history of antiepileptic drug treatment predicts medicinal cannabis use. Epilepsy Behav. 2017;70:334–40.
Suraev A, Lintzeris N, Stuart J, Kevin RC, Blackburn R, Richards E, et al. Composition and use of cannabis extracts for childhood epilepsy in the Australian community. Sci Rep. 2018;8:10154.
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–8.
• Szaflarski JP, Bebin EM, Comi AM, et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia 2018;59:1540–1548. This open-label study documents the sustained response to cannabidiol over the duration of exposure.
Szaflarski JP, Bebin E, Cutter GR, et al. Cannabidiol improves seizure frequency and severity and reduces adverse events in an open-label prospective study. Epilepsy Behav. 2018; Accepted; In Press.
Hess EJ, Moody KA, Geffrey AL, Pollack SF, Skirvin LA, Bruno PL, et al. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1617–24.
Devinsky O, Verducci C, Thiele EA, Laux LC, Patel AD, Filloux F, et al. Open-label use of Highly* purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–7.
Gofshteyn JS, Wilfong A, Devinsky O, Bluvstein J, Charuta J, Ciliberto MA, et al. Cannabidiol as a potential treatment for febrile infection-related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol. 2017;32:35–40.
McCoy B, Wang L, Zak M, et al. A prospective open-label trial of CBD/THC cannabis oil in Dravet syndrome. Ann Clin Transl Neurol. in print.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3:CD009270.
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–11.
• Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888–97. RCT of cannabidiol for the treatment of seizures associated with Lennox-Gastaut syndrome
Espay AJ, Norris MM, Eliassen JC, Dwivedi A, Smith MS, Banks C, et al. Placebo effect of medication cost in Parkinson disease: a randomized double-blind study. Neurology. 2015;84:794–802.
Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. Jama. 2015;313:2491–3.
• Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20. RCT of cannabidiol for the treatment of seizures associated with Dravet syndrome.
• Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–96. RCT of cannabidiol for the treatment of seizures associated with Lennox-Gastaut syndrome.
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51.
Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28:332–8.
• Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58:1586–92. Detailed analysis of interactions of cannabidiol with common anti-seizure drugs.
Grayson L, Vines B, Nichol K, Szaflarski JP, Program UC. An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav Case Rep. 2018;9:10–1.
Gaston TE, Liu Y, Cutter GR, Bebin E, Grayson LE, Szaflarski JP. Effect of pharmaceutical formulation of cannabidiol (CBD) on seizure frequency and severity does not appear to be dependent on drug-drug interactions with other anti-epileptic drugs. American Academy of Neurology Annual Meeting; 2018 25 April 2018; Los Angeles, CA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Szaflarski reports personal fees from GW Pharmaceuticals; grants and personal fees from Serina Therapeutics, Inc., during the conduct of the study; personal fees from SK Life Sciences; personal fees from LivaNova Inc.; personal fees from Lundbeck; personal fees from NeuroPace Inc.; personal fees from Upsher-Smith Laboratories, Inc.; grants and personal fees from SAGE Pharmaceuticals; grants from UCB Pharma; grants from Biogen; and grants from Eisai Inc., outside the submitted work.
Dr. Gaston reports personal fees from GW Pharmaceuticals, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Epilepsy
Rights and permissions
About this article
Cite this article
Gaston, T.E., Szaflarski, J.P. Cannabis for the Treatment of Epilepsy: an Update. Curr Neurol Neurosci Rep 18, 73 (2018). https://doi.org/10.1007/s11910-018-0882-y
Published:
DOI: https://doi.org/10.1007/s11910-018-0882-y