Abstract
Purpose of Review
GBA mutations are the most common known genetic cause of Parkinson’s disease (PD). Its biological pathway may be important in idiopathic PD, since activity of the enzyme encoded by GBA, glucocerebrosidase, is reduced even among PD patients without GBA mutations. This article describes the structure and function of GBA, reviews recent literature on the clinical phenotype of GBA PD, and suggests future directions for research, counseling, and treatment.
Recent Findings
Several longitudinal studies have shown that GBA PD has faster motor and cognitive progression than idiopathic PD and that this effect is dose dependent. New evidence suggests that GBA mutations may be important in multiple system atrophy. Further, new interventional studies focusing on GBA PD are described. These studies may increase the interest of PD patients and caregivers in genetic counseling.
Summary
GBA mutation status may help clinicians estimate PD progression, though mechanisms underlying GBA and synucleinopathy require further understanding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction—the Discovery of GBA Mutations in Parkinson’s Disease
Unlike most genetic findings in recent decades, which are generally based on linkage analysis, genome-wide association studies (GWAS), and whole exome sequencing studies, the discovery of the association between GBA mutations and Parkinson’s disease (PD) came from clinical observations [1, 2]. Initial reports on the frequency of GBA mutations in PD were inconsistent, until later studies unequivocally demonstrated a strong association between GBA mutations and PD [3,4,5]. The first case-control study was on Ashkenazi-Jews (AJ), in which GBA mutations are particularly common, and suggested that around 30% of AJ PD patients carry a GBA mutation [3]. Subsequently, follow-up studies from the USA and Israel reported that 15–20% of AJ PD carries a GBA mutation [6••, 7]. A large meta-analysis from 16 centers worldwide has since clearly demonstrated that GBA mutations are associated with PD [8], which was replicated in many other populations [4, 9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. In large-scale GWASs, the GBA locus was associated with the strongest risk for PD, driven by the p.E326K variant [31,32,33].
Gaucher’s disease (GD, OMIM #230800, #230900, and #231000) is caused by a recessively inherited deficiency of the lysosomal enzyme glucocerebrosidase (GCase), encoded by the GBA gene (OMIM #606463). Traditionally, GD is divided into three types according to increasing severity of the disease and the degree of neuronal involvement, where type 1 is non-neuronopathic, type 2 is acute neuronopathic, and type 3 is chronic neuronopathic [34].
We hereby review the current knowledge on the structure and function of the GBA gene, its protein product GCase, and their phenotypic correlations. We also discuss implications for diagnosis, counseling, and treatment and suggest future directions for research.
The Normal Structure and Function of the GBA Gene and Its Protein Product, Glucocerebrosidase
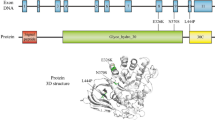
The GBA gene contains 11 exons spanning 7.6 kb on chromosome 1q21, in a gene-rich region that includes 9 genes and 2 pseudo-genes within a 100-kb-long sequence. The GBA promoter region includes putative TATA and CAAT-like boxes approximately 250 bp upstream to the ATG start site and lacks the GGCGGG motif [35]. The two pseudo-genes, GBAP and MTX1P, are located between the GBA and MTX1 genes and appear to have resulted from a duplication event which took place approximately 27–40 million years ago. These pseudo-genes are found in humans and primates, but not in other species [36, 37]. GBAP shares 96% sequence identity with GBA but spans only 5 kb due to intronic Alu sequences, a form of transposon that can relocate throughout the DNA during evolution, which are present in the GBA but not in the GBAP gene sequence. Additionally, a 55 bp deletion in exon 9 flanked by a short inverted repeat also distinguishes the pseudo-gene from the GBA gene [36].
The active protein transcribed by GBA, GCase, is a 497-amino acid (AA) lysosomal hydrolase. The main function of GCase is to degrade glucocerebroside into ceramide and glucose, but it also cleaves glucosylsphingosine and potentially other β-glucosides [38]. During its transport to the lysosome, GCase undergoes several modifications. Having two functional ATG initiation sites, GCase is transcribed as a 536 or 516 AA protein [39], which is further processed into the functional 497 AA enzyme. This cleavage of the 19 or 39 AA long leader peptide occurs while entering the endoplasmic reticulum (ER) [40], and it was suggested that the different leaders differentially affect transport into the ER [41]. Oligosaccharide modifications also occur, but they have no effect on the catalytic activity or the intracellular stabilization of GCase; therefore, their importance is not clear [42]. Several lines of evidence suggest that the transport of GCase from the ER to the lysosome is mannose-6-phosphate independent [37,38,39]. First, GCase is located within the lysosome in I-cell disease (ICD), which is caused by deficiency of the enzyme N-acetylglucosamine 1-phosphodiester N-acetylglucosaminidase [43]. In this disorder, lysosomal enzymes that are usually transported into the lysosome via the mannose-6-phosphate-dependent pathway cannot be targeted into the lysosome [44]. Second, by using cellular and animal models, it was shown that the lysosomal integral membrane protein type 2 (LIMP2/SCARB2) is a mannose-6-phosphate independent receptor which transfers GCase to the lysosome [45].
Within the lysosome, GCase is peripherally associated to the inner membrane [46, 47], where it exerts its activity together with Saposin C and negatively charged lipids that are essential for its proper function [48, 49]. X-ray studies showed that GCase has three tertiary structure domains: domain I (residues 1–27 and 383–414), which contains two disulfide bridges (residues 4–16 and 18–23), domain II (residues 30–75 and 431–497), an immunoglobulin-like domain, and domain III (residues 76–381 and 416–430), a TIM (triophosphate isomerase) barrel. The TIM barrel contains three free cysteines and the catalytic site (with glutamate residues in positions 235 and 340), which is covered by three loops (residues 312–319, 341–350, and 393–396) [50].
GBA Mutations and Their Effect on Glucocerebrosidase Structure and Function
Approximately 300 mutations and gene re-arrangements in the GBA gene have been described [40], which are classified according to the type of GD (I, II, or III) that might develop [51]. Severe (including null) GBA mutations are those that when inherited from both parents result in the severe types of GD (types II or III), while mild mutations are those that if inherited in a homozygous or compound heterozygous manner, cause the mild type of GD (type I) [51]. In addition, there are variants which have an unclear role in GD but are clearly risk factors for PD, the most notable example being E326K. The following is a review of the possible effects of different GBA mutations on the structure and function of GCase.
Effects of GBA Mutations on GCase Enzymatic Activity
Different GBA mutations differentially affect the enzymatic activity of GCase, as some mutations result in almost no residual activity whereas others show only reduced activity. In several cases, the level of enzymatic activity does not correlate with the severity of GD [34], and the enzymatic activity range of severe and mild GBA mutations can overlap. For instance, the measured enzymatic activity of GCase with the p.N370S mutation, which is always associated with type I GD, may be lower than the measured enzymatic activity of GCase with severe mutations such as p.L444P, p.G390R, p.N382K, and others [38, 52, 53•]. One clinical example of such lack of correlation between residual enzymatic activity and severity of disease was shown in an infant with very severe neuropathic type II GD, who was homozygous for the p.G202R mutation, but had “only slightly reduced activity” of GCase [54]. Ideally, GCase activity should be measured within the lysosome only, which may better reflect the true function of GCase. Additionally, factors other than the residual GCase activity, whether genetic/biologic or environmental, may determine the severity of the disease.
Effects of GBA Mutations on GCase Traffic, Binding, and Interaction Properties
In the infant homozygous to the severe p.G202R mutation described above [54], the degree of ER retention of GCase was examined, and it was shown that the mutated GCase was not transported to the lysosome and was retained in the ER. It was therefore possible that the severity of the disease was not only due to the enzymatic level of activity, but also due to the fact that GCase could not exert its function where it belongs, within the lysosome. Subsequent studies demonstrated that the transport of GCase with the severe p.D409H and p.L444P mutations to the lysosome is also restricted and that the transport of GCase with the mild p.N370S mutation was partial [55]. Similarly, the degree of ER retention of GCase was determined in seven patients with type I GD and four with types II or III. Generally, ER retention was higher in GD patients with the severe type of the disease, carrying mutations such as p.P415R, p.L444P, and p.D409H, with the exception of one patient with type III GD that had a comparable degree of ER retention to that of type I GD patients [56]. These studies suggest that although mutated GCase may have residual enzymatic activities in cellular assays, they may not reach the lysosome in vivo, and therefore cannot exert their function. This will result in a de facto severe deficiency of GCase. Indeed, it is possible that impaired transportation of GCase to the lysosome may also lead to PD. GWASs and other genetic studies have identified variants around SCARB2, the transporter of GCase from the ER to the lysosome, as a genetic risk factor for PD [31, 57,58,59]. An association with SCARB2 was also suggested in dementia with Lewy bodies (DLB) [60]. These data may indicate that even when GCase is functioning based on enzymatic activity assays, perturbed transport to the lysosome may increase the risk for synucleinopathies.
GBA mutations may also cause structural effects that can influence the function of GCase. Experiments in human fibroblasts containing the mutated p.N370S GCase demonstrated a reduced capacity of the enzyme to interact with its activator, saposin C, and with anionic phospholipids that are necessary for its proper function [61]. Supporting this observation, in a structural model of the interaction between GCase and saposin C, the p.N370S mutation was mapped to the interacting surface of the two proteins, and so was the severe mutation p.L444P [62]. In addition, it was suggested that the GBA p.N370S mutation may affect the stability of the helical turn conformation of loop 1 [63].
Potential Mechanisms of GBA Parkinson's Disease
Several pathways were suggested to be involved in GBA-associated neurodegeneration. Mazzulli and colleagues demonstrated that the substrate of GCase, glucosylceramide, may lead to α-synuclein accumulation, and inversely, α-synuclein accumulation may lead to reduced GCase activity [64]. This relationship has been supported by several lines of research: overexpression of α-synuclein led to decreased GCase activity in mice and cell models [65], and reduced GCase levels have been shown in brain tissue [66,67,68], CSF [69], and peripheral blood of PD patients compared to controls [53•], independent of GBA mutation carrier status. Further, a recent study found that in human-induced pluripotent stem-derived neuronal models (iPSn), treatment with GCase inhibitors led to increased α-synuclein aggregation [70]. This aggregation was reversible by treating GD and PD patient iPSns with a glucosylceramide synthase inhibitor. These studies suggest a vicious, neurotoxic cycle between α-synuclein and GCase that may partially explain the mechanism underlying GBA PD, but the vulnerability of specific neuron types is still not understood. Further, no study to date has shown elevated concentration of glucosylceramide in GBA heterozygotes.
Another mechanism by which GBA mutations may result in PD is ER-associated degradation (ERAD) impairment and ER stress-related cell death. α-synuclein accumulation may cause ER stress, impair degradation of ERAD substrates, and inhibit ER to Golgi traffic [71]. Supporting this observation is the role of some PD-associated genes, such as PARK2, in ERAD [72, 73]. Taken together, it is possible that ERAD and ER stress could be important in PD pathogenesis. Along this line, the ER retention detected in experiments with some of the mutated forms of GCase [54,55,56] may suggest that ER stress is also involved in the pathogenesis of PD in carriers of some GBA mutations. It was also shown that mutated GCase interacts with parkin, which promotes the accumulation of GCase in aggresome-like structures [74].
However, the mechanisms suggested above are challenged by the fact that null GBA mutations which do not result in a protein product [51, 52, 54], such as 84GG, IVS2+1, R359X [51], and others, also increase the risk of developing PD [4, 75]. If the protein does not exist, it cannot accumulate or assist in fibrillization. It is possible that the ER stress observed in models with GBA mutations may be due to α-synuclein accumulation rather than accumulation of GCase itself. It is also likely that GBA mutations increase susceptibility to PD in more ways than one and that both suggested mechanisms contribute to disease development.
The ceramide metabolism pathway is also a mechanism that may be involved in PD pathogenesis. Since LBs are the pathologic hallmark of PD, but are also found in other diseases, it was noted that some of the genes underlying these diseases (such as GBA, SMPD1, ASAH1, GALC, PANK2, and PLA2G6) have a significant role in the ceramide metabolism pathway [76,77,78], and recent studies suggested the involvement of SMPD1, ASAH1, and GALC in PD [31, 77,78,79]. Ceramide may play a role in some PD-related mechanisms such as stress-induced cell death [80] or inflammation [81]. Moreover, ceramide binds cathepsin D and triggers its cleavage to the catalytic form [82], which is one of the main lysosomal enzymes responsible for α-synuclein degradation [83]. Therefore, the ceramide metabolism pathway may be related to LB formation in GBA PD [76].
Genotype-Phenotype Correlations in GBA Parkinson's Disease
The initial reports on GBA-PD reported atypical parkinsonism with seizures and dementia among Gaucher patients who are homozygous GBA mutation carriers and a phenotype similar to idiopathic PD (iPD) in GBA heterozygotes [34]. Subsequently, a wealth of information was collected on GBA PD. Similar to idiopathic PD, phenotype is heterogeneous and symptomatology can vary (Table 1). On an individual level, GBA heterozygotes or homozygotes with PD are indistinguishable from idiopathic PD patients. However, rate of motor progression is faster in GBA PD compared to idiopathic PD [84••]. In addition, GBA mutation carriers are more likely to manifest non-motor symptoms which are prevalent in iPD including cognitive impairment [89], REM sleep behavior disorder (RBD) [93], hyposmia [85], and autonomic dysfunction [89, 90]. The rate of cognitive change is faster in GBA PD than in idiopathic PD, and therefore, patients tend to have faster motor and cognitive progression than iPD (Table 2). In addition, multiple studies suggest a “dose effect”, where Gaucher PD (PD with homozygous or compound heterozygous GBA mutations) is associated with earlier age at onset and more cognitive changes than GBA PD (PD with heterozygous mutations) [85, 97]. Among GBA PD cases, those who carry severe mutations (e.g., L444P) have faster motor progression as measured by the UPDRS, faster rate of dementia and younger age-at-death than carriers of mild mutations (e.g., N370S) [6••, 84••, 86•, 91•, 98]. Referral to deep brain stimulation may happen earlier in GBA PD [95], but has also been associated with early cognitive impairment after DBS.
GBA Mutations and Other Synucleinopathies
GBA in REM Sleep Behavior Disorder
Most individuals with RBD are likely to develop one of the synucleinopathies: PD, DLB, or MSA [99]. Interestingly, when comparing the clinical presentation of GBA PD and RBD-associated PD, there are many similar characteristics. PD patients with GBA mutations and PD patients with RBD are both likely to have non-motor symptoms: autonomic dysfunction [89, 100], cognitive decline, and faster progression to dementia [89, 101, 102]. Furthermore, both are also associated with similar motor characteristics: rapid motor progression [89, 103] and the postural-instability-gait-dysfunction phenotype [104, 105]. Therefore, it is no surprise to find that GBA mutations were associated with RBD in a cohort of idiopathic RBD patients [93] and in subsequent studies [106, 107]. This association was even stronger than the association with PD in a similar population [108], suggesting that GBA mutations are more specifically associated with the RBD subtype of PD. Furthermore, in clinical PD patients screened with an RBD questionnaire, GBA mutations were associated with probable RBD [93]. It was also demonstrated that among biallelic GBA mutation carriers, as well as among heterozygous carriers who did not have PD, RBD scores were significantly worse than non-carriers [109]. From a pathological point of view, both RBD-associated PD and GBA PD probably have a more diffused spread of α-synuclein accumulation compared to idiopathic PD [110, 111]. Overall, these studies strongly support the association between GBA and RBD.
GBA in Dementia with Lewy Bodies
Several case control studies demonstrated an increased proportion of GBA mutation carriers among DLB patients compared to controls, ranging in frequency from 3.5 to 31% depending on the cohort [17, 112]. The largest multicenter study to date compared 721 clinically diagnosed DLB cases and 1962 controls and found that 7.49% of DLB patients carried a GBA mutation (odds ratio of 8.28 compared to controls) [113]. This relationship has been further supported by studies on neuropathologically confirmed DLB [10, 110, 114], which show increased frequency of GBA mutation carriers among DLB patients compared to controls, and a reverse association between GBA mutations and severity of Alzheimer’s pathology [10, 115]. This suggests that GBA mutations specifically facilitate the development of synucleinopathies, although the mechanism for this relationship remains unclear. However, lysosomal dysfunction is likely a contributing factor [60, 116], as several lysosomal genes have been associated with DLB, including SCARB2, SMPD1, and MCOLN1 [60, 116].
GBA Mutations and Multiple System Atrophy
The association between GBA mutations and MSA is less consistent, possibly because MSA is a rarer disease. In total, eight studies examined the association between GBA variants and MSA [110, 117,118,119,120,121,122,123]. Six studies have found no association between GBA variants and MSA. However, two studies reported an association between GBA and MSA, including the largest international MSA clinical study to date [122], and a study at Columbia University which was enriched for Ashkenazi Jews in both MSA cases and controls [123].
Clinical Implications—Diagnosis, Counseling, and Treatment
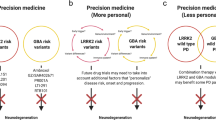
The vast research data obtained in the past decades regarding GBA PD has yet to significantly impact patient care. Genetic testing is not currently standard practice in PD treatment, even though genetic information could potentially inform diagnosis, prognosis, and treatment [124, 125]. In addition, patient knowledge regarding genetics in PD is limited, with two reports suggesting that the majority of patients think genetic testing would be useful in PD while only a minority are familiar with GBA or LRRK2 [126, 127]. Though these attitudes can differ across cultures [128], initiatives to improve genetic counseling access for GBA PD patients are warranted.
It is very likely that the launch of precision medicine clinical trials targeting GBA mutation carriers will change patient interest in receiving genotype information. Patients can now be screened for a multicenter randomized clinical trial assessing a glucosylceramide synthase inhibitor for treatment of GBA PD (clinicaltrials.gov identifier: NCT02906020). Another potential therapy is ambroxol hydrochloride, a small molecular chaperone that has been shown to increase brain GCase activity in mouse and primate models [129]. Originally identified through a library screen of FDA-approved compounds [130], ambroxol is now being tested in clinical trials for PD (clinicaltrials.gov identifier: NCT02941822) and PD with dementia (clinicaltrials.gov identifier: NCT02914366). These trials suggest a promising future of precision medicine approaches to genetic PD, though the efficacy of these compounds for improving symptoms remains to be seen. Nonetheless, ascertaining eligibility for these trials is a compelling reason to want to know one’s genotype, and the growing development of such targeted initiatives will likely lead to increased demand for genetic counseling regarding GBA and other genetic risk factors for PD.
The counseling of GBA mutation carriers is complicated by the incomplete penetrance of GBA, where the majority of GBA mutation carriers will not go on to develop synucleinopathy. Despite the clear relationship between GBA and PD, no formal genetic counseling guidelines address how to approach this topic with GBA mutation carriers [131], particularly with family members of patients with GBA PD. Some GBA mutation carriers may also find out their genetic status when undergoing prenatal testing or when their newborns are tested for Gaucher disease, a practice that is becoming increasingly common. One study has suggested that most prospective parents do want to be informed of their PD risk prior to genetic testing for Gaucher [132]. Navigating this relationship will continue to be an issue given the rise of direct-to-consumer genetic testing, which may or may not offer genetic counseling for results.
Future Challenges and Directions for Research
Much has been learned about the genetics of PD over the last 20 years, but there is still more to be uncovered. The mechanistic link between glucocerebrosidase and α-synuclein remains unsolved, and understanding this relationship will be instrumental in designing more targeted therapies for GBA PD. This has not stopped development of the first clinical trial for GBA PD (clinicaltrials.gov identifier: NCT02906020), but more research is needed to develop truly targeted therapies, especially given the phenotypic heterogeneity of disease. It also remains unclear how GBA is related to RBD, and other synucleinopathies such as DLB and MSA. Future studies will need to further investigate these relationships and explore whether alternate mechanisms are at play.
GBA PD patients have a more rapid disease progression than non-carrier PD patients, and there is a dose effect on the rate of disease progression [6••, 84••, 85]. However, the clinical utility of genetic testing in PD is still debated. Some clinicians argue that genotype information is useful for prognostication, and therefore symptom prevention [124]. With the advent of genotype-specific clinical trials, genetic testing is also useful for determining eligibility for experimental therapies. Yet, there are several drawbacks, especially with regards to pre-symptomatic at-risk individuals, including the lack of preventative treatment, the ambiguity of negative results, and the limited ethnic populations in whom genetic testing can identify risk mutations [125]. Tackling these issues will be increasingly important as direct-to-consumer genetic testing becomes cheaper and more widely used. More research is needed to determine how knowing genetic status influences clinical outcomes and to better elucidate barriers to genetic testing in clinical settings. To further improve upon prognostication, better phenotyping of specific GBA mutations may also be warranted.
Conclusion
There has been a breadth of research on GBA since it was identified as a genetic risk factor for synucleinopathies. The mechanism of this relationship remains unclear, though several lines of study suggest interactions with α-synuclein, lysosomal dysfunction, ER stress, and ceramide metabolism. Phenotypically, GBA mutation carriers can present with Parkinson’s disease or dementia with Lewy bodies, though the relationship with multiple system atrophy is still uncertain. In PD patients, GBA mutation carriers are more likely than non-carriers to have younger age of onset, cognitive impairment, and RBD and tend to have quicker motor progression and cognitive decline. Further, there appears to be a dose effect where carriers of severe mutations have quicker progression than carriers of mild mutations, and homozygotes or compound heterozygotes have quicker progression than heterozygotes. Yet, the vast majority of GBA mutation carriers will never develop a synucleinopathy, which complicates genetic counseling for carriers without PD. Now that clinical trials are underway specifically targeting GBA PD, further research is warranted to identify barriers to genetic testing and counseling and to improve upon mutation-based prognostication. Further research is also still needed to elucidate how GBA mutations contribute to disease pathology.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89(9):691–4.
Van Bogaert L, Froehlich A. Un cas de maladie de Gaucher de l'adulte avec syndrome de Raynaud, pigmentation, et rigidite du type extrapyrajidal aux membres inferieurs. Ann Med. 1939;45:57–70.
Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004;351(19):1972–7.
Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70(24):2277–83.
Zimran A, Neudorfer O, Elstein D. The glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2005;352(7):728–31. author reply 728-31
•• Gan-Or Z, Amshalom I, Kilarski LL, Bar-Shira A, Gana-Weisz M, Mirelman A, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84(9):880–7. This meta-analysis showed differential odds ratios for PD between mild and severe GBA mutation carriers (2.2 and 10.3, respectively), as well as differential age of PD onset, with severe GBA mutation carriers having earlier age of onset than mild GBA mutation carriers (53.1 vs. 58.1, respectively).
Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Ross BM, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67(9):1116–22.
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361(17):1651–61.
Bras J, Paisan-Ruiz C, Guerreiro R, Ribeiro MH, Morgadinho A, Januario C, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol Aging. 2009;30(9):1515–7.
Clark LN, Kartsaklis LA, Wolf Gilbert R, Dorado B, Ross BM, Kisselev S, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66(5):578–83.
Clark LN, Nicolai A, Afridi S, Harris J, Mejia-Santana H, Strug L, et al. Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson's disease in subjects of Jewish ethnicity. Mov Disord. 2005;20(1):100–3.
Clark LN, Ross BM, Wang Y, Mejia-Santana H, Harris J, Louis ED, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69(12):1270–7.
De Marco EV, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in southern Italy. Mov Disord. 2008;23(3):460–3.
Eblan MJ, Nguyen J, Ziegler SG, Lwin A, Hanson M, Gallardo M, et al. Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord. 2006;21(2):282–3.
Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120(5):641–9.
Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452(2):87–9.
Mata IF, Samii A, Schneer SH, Roberts JW, Griffith A, Leis BC, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008;65(3):379–82.
Mitsui J, Mizuta I, Toyoda A, Ashida R, Takahashi Y, Goto J, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009;66(5):571–6.
Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132(Pt 7):1783–94.
Nichols WC, Pankratz N, Marek DK, Pauciulo MW, Elsaesser VE, Halter CA, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72(4):310–6.
Sato C, Morgan A, Lang AE, Salehi-Rad S, Kawarai T, Meng Y, et al. Analysis of the glucocerebrosidase gene in Parkinson's disease. Mov Disord. 2005;20(3):367–70.
Emelyanov A, Boukina T, Yakimovskii A, Usenko T, Drosdova A, Zakharchuk A, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in Russia. Mov Disord. 2012;27(1):158–9.
Huang CL, Wu-Chou YH, Lai SC, Chang HC, Yeh TH, Weng YH, et al. Contribution of glucocerebrosidase mutation in a large cohort of sporadic Parkinson's disease in Taiwan. Eur J Neurol. 2011;18(10):1227–32.
Lesage S, Condroyer C, Hecham N, Anheim M, Belarbi S, Lohman E, et al. Mutations in the glucocerebrosidase gene confer a risk for Parkinson disease in North Africa. Neurology. 2011;76(3):301–3.
Moraitou M, et al. beta-Glucocerebrosidase gene mutations in two cohorts of Greek patients with sporadic Parkinson's disease. Mol Genet Metab. 2011;104(1–2):149–52.
Socal MP, Bock H, Michelin-Tirelli K, Hilbig A, Saraiva-Pereira ML, Rieder CRM, et al. Parkinson's disease and the heterozygous state for glucocerebrosidase mutations among Brazilians. Parkinsonism Relat Disord. 2009;15(1):76–8.
Spitz M, Rozenberg R, da Veiga Pereira L, Reis Barbosa E. Association between Parkinson's disease and glucocerebrosidase mutations in Brazil. Parkinsonism Relat Disord. 2008;14(1):58–62.
Tan EK, Tong J, Fook-Chong S, Yih Y, Wong MC, Pavanni R, et al. Glucocerebrosidase mutations and risk of Parkinson disease in Chinese patients. Arch Neurol. 2007;64(7):1056–8.
Wu YR, Chen CM, Chao CY, Ro LS, Lyu RK, Chang KH, et al. Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry. 2007;78(9):977–9.
Ziegler SG, Eblan MJ, Gutti U, Hruska KS, Stubblefield BK, Goker-Alpan O, et al. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol Genet Metab. 2007;91(2):195–200.
Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet. 2017;49(10):1511–6.
Nalls MA, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet. 2014;46(9):989–93.
Pankratz N, Beecham GW, DeStefano AL, Dawson TM, Doheny KF, Factor SA, et al. Meta-analysis of Parkinson disease: identification of a novel locus, RIT2. Ann Neurol. 2012;71(3):370–84.
Sidransky E. Gaucher disease: complexity in a "simple" disorder. Mol Genet Metab. 2004;83(1–2):6–15.
Reiner O, Wigderson M, Horowitz M. Structural analysis of the human glucocerebrosidase genes. DNA. 1988;7(2):107–16.
Horowitz M, Wilder S, Horowitz Z, Reiner O, Gelbart T, Beutler E. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics. 1989;4(1):87–96.
Martinez-Arias R, et al. Sequence variability of a human pseudogene. Genome Res. 2001;11(6):1071–85.
Liou B, Kazimierczuk A, Zhang M, Scott CR, Hegde RS, Grabowski GA. Analyses of variant acid beta-glucosidases: effects of Gaucher disease mutations. J Biol Chem. 2006;281(7):4242–53.
Sorge JA, West C, Kuhl W, Treger L, Beutler E. The human glucocerebrosidase gene has two functional ATG initiator codons. Am J Hum Genet. 1987;41(6):1016–24.
Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat. 2008;29(5):567–83.
Pasmanik-Chor M, et al. Overexpression of human glucocerebrosidase containing different-sized leaders. Biochem J. 1996;317(Pt 1):81–8.
Van Weely S, et al. Function of oligosaccharide modification in glucocerebrosidase, a membrane-associated lysosomal hydrolase. Eur J Biochem. 1990;191(3):669–77.
Aerts JM, et al. Glucocerebrosidase, a lysosomal enzyme that does not undergo oligosaccharide phosphorylation. Biochim Biophys Acta Gen Subj. 1988;964(3):303–8.
Dierks T, Schlotawa L, Frese MA, Radhakrishnan K, von Figura K, Schmidt B. Molecular basis of multiple sulfatase deficiency, mucolipidosis II/III and Niemann-pick C1 disease - lysosomal storage disorders caused by defects of non-lysosomal proteins. Biochim Biophys Acta. 2009;1793(4):710–25.
Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131(4):770–83.
Erickson AH, Ginns EI, Barranger JA. Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem. 1985;260(26):14319–24.
Imai K. Characterization of beta-glucosidase as a peripheral enzyme of lysosomal membranes from mouse liver and purification. J Biochem. 1985;98(5):1405–16.
Morimoto S, Kishimoto Y, Tomich J, Weiler S, Ohashi T, Barranger JA, et al. Interaction of saposins, acidic lipids, and glucosylceramidase. J Biol Chem. 1990;265(4):1933–7.
Vaccaro AM, Tatti M, Ciaffoni F, Salvioli R, Maras B, Barca A. Function of saposin C in the reconstitution of glucosylceramidase by phosphatidylserine liposomes. FEBS Lett. 1993;336(1):159–62.
Dvir H, Harel M, McCarthy AA, Toker L, Silman I, Futerman AH, et al. X-ray structure of human acid-beta-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 2003;4(7):704–9.
Beutler E, Gelbart T, Scott CR. Hematologically important mutations: Gaucher disease. Blood Cells Mol Dis. 2005;35(3):355–64.
Montfort M, Chabás A, Vilageliu L, Grinberg D. Functional analysis of 13 GBA mutant alleles identified in Gaucher disease patients: pathogenic changes and "modifier" polymorphisms. Hum Mutat. 2004;23(6):567–75.
• Alcalay RN, Levy OA, Waters CH, Fahn S, Ford B, Kuo SH, et al. Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations. Brain. 2015;138(Pt 9):2648–58. The authors show that GCase activity was significantly different between PD patients and controls, even among non-carriers of GBA or LRRK2 mutations, suggesting that the biological pathway of GBA is important to idiopathic PD. Further, higher GCase activity was associated with longer disease duration, suggesting a milder disease course.
Zimmer KP, le Coutre P, Aerts HMFG, Harzer K, Fukuda M, O'Brien JS, et al. Intracellular transport of acid beta-glucosidase and lysosome-associated membrane proteins is affected in Gaucher's disease (G202R mutation). J Pathol. 1999;188(4):407–14.
Schmitz M, Alfalah M, Aerts JMFG, Naim HY, Zimmer KP. Impaired trafficking of mutants of lysosomal glucocerebrosidase in Gaucher's disease. Int J Biochem Cell Biol. 2005;37(11):2310–20.
Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14(16):2387–98.
Michelakakis H, Xiromerisiou G, Dardiotis E, Bozi M, Vassilatis D, Kountra PM, et al. Evidence of an association between the scavenger receptor class B member 2 gene and Parkinson's disease. Mov Disord. 2012;27(3):400–5.
Alcalay RN, Levy OA, Wolf P, Oliva P, Zhang XK, Waters CH, et al. SCARB2 variants and glucocerebrosidase activity in Parkinson's disease. NPJ Parkinsons Dis. 2016;2:16004.
Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7(6):e1002141.
Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet. 2014;23(23):6139–46.
Salvioli R, Tatti M, Scarpa S, Moavero SM, Ciaffoni F, Felicetti F, et al. The N370S (Asn370-->Ser) mutation affects the capacity of glucosylceramidase to interact with anionic phospholipid-containing membranes and saposin C. Biochem J. 2005;390(Pt 1):95–103.
Atrian S, López-Viñas E, Gómez-Puertas P, Chabás A, Vilageliu L, Grinberg D. An evolutionary and structure-based docking model for glucocerebrosidase-saposin C and glucocerebrosidase-substrate interactions - relevance for Gaucher disease. Proteins. 2008;70(3):882–91.
Lieberman RL, Wustman BA, Huertas P, Powe AC, Pine CW, Khanna R, et al. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3(2):101–7.
Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52.
Yang J, Hertz E, Zhang X, Leinartaité L, Lundius EG, Li J, et al. Overexpression of α-synuclein simultaneously increases glutamate NMDA receptor phosphorylation and reduces glucocerebrosidase activity. Neurosci Lett. 2016;611:51–8.
Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, et al. Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson's disease. Brain. 2014;137(Pt 3):834–48.
Chiasserini D, Paciotti S, Eusebi P, Persichetti E, Tasegian A, Kurzawa-Akanbi M, et al. Selective loss of glucocerebrosidase activity in sporadic Parkinson's disease and dementia with Lewy bodies. Mol Neurodegener. 2015;10:15.
Gegg ME, Burke D, Heales SJR, Cooper JM, Hardy J, Wood NW, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72(3):455–63.
Parnetti L, Paciotti S, Eusebi P, Dardis A, Zampieri S, Chiasserini D, et al. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in parkinson's disease patients. Mov Disord. 2017;32(10):1423–31.
Zunke F, et al. Reversible Conformational Conversion of a-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron. 97(1):92–107. e10
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313(5785):324–8.
Shimura H, Hattori N, Kubo SI, Mizuno Y, Asakawa S, Minoshima S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25(3):302–5.
Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson's disease. Antioxid Redox Signal. 2007;9(5):553–61.
Ron I, Rapaport D, Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet. 2010;19(19):3771–81.
Sunwoo M-K, Kim SM, Lee S, Lee PH. Parkinsonism associated with glucocerebrosidase mutation. J Clin Neurol. 2011;7(2):99–101.
Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275(23):5767–73.
Gan-Or Z, Ozelius LJ, Bar-Shira A, Saunders-Pullman R, Mirelman A, Kornreich R, et al. The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology. 2013;80(17):1606–10.
Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson's disease. Brain. 2017;140(12):3191–203.
Gan-Or Z, Orr-Urtreger A, Alcalay RN, Bressman S, Giladi N, Rouleau GA. The emerging role of SMPD1 mutations in Parkinson's disease: implications for future studies. Parkinsonism Relat Disord. 2015;21(10):1294–5.
Grassmé H, Riethmüller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46(3):161–70.
Kitatani K, Sheldon K, Anelli V, Jenkins RW, Sun Y, Grabowski GA, et al. Acid β-glucosidase 1 counteracts p38δ-dependent induction of Interleukin-6 possible role for ceramide as an anti-inflammatory lipid. J Biol Chem. 2009;284(19):12979–88.
Heinrich M, Wickel M, Schneider-Brachert W, Sandberg C, Gahr J, Schwandner R, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18(19):5252–63.
Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47(36):9678–87.
•• Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson's disease: the mutation matters. Ann Neurol. 2016;80(5):662–73. The authors compared PD patients with and without GBA mutations longitudinally, and showed that mutation carriers had a greater risk for dementia (hazard ratio = 3.16) and death (hazard ratio = 1.85) than noncarriers, and that carriers of severe mutations had greater risk for dementia than carriers of mild mutations but similar mortality risk.
Thaler A, Gurevich T, Bar Shira A, Gana Weisz M, Ash E, Shiner T, et al. A "dose" effect of mutations in the GBA gene on Parkinson's disease phenotype. Parkinsonism Relat Disord. 2017;36:47–51.
• Brockmann K, Srulijes K, Pflederer S, Hauser AK, Schulte C, Maetzler W, et al. GBA-associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. 2015;30(3):407–11. This study examines a cohort of PD patients with and without GBA mutations over 3 years, finding that GBA PD patients had earlier age of onset, faster motor progression, and reduced survival rates compared to non-carriers.
Jesus S, et al. GBA variants influence motor and non-motor features of Parkinson's disease. PLoS One. 2016;11(12):e0167749.
Chahine LM, Qiang J, Ashbridge E, Minger J, Yearout D, Horn S, et al. Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol. 2013;70(7):852–8.
Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, Gasser T, et al. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011;77(3):276–80.
Li Y, Sekine T, Funayama M, Li L, Yoshino H, Nishioka K, et al. Clinicogenetic study of GBA mutations in patients with familial Parkinson's disease. Neurobiol Aging. 2014;35(4):935.e3–8.
• Liu G, Boot B, Locascio JJ, Jansen IE, Winder-Rhodes S, Eberly S, et al. Specifically neuropathic Gaucher's mutations accelerate cognitive decline in Parkinson's. Ann Neurol. 2016;80(5):674–85. A total of 2,304 PD patients from 7 cohorts were followed for up to 12.8 years, showing that GBA mutation carriers were more likely to develop cognitive impairment, as measured by the mini-mental state exam, than non-carriers. Cognitive decline was faster in carriers of severe mutations in GBA compared to mild ones.
Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, van Deerlin VM, et al. GBA variants are associated with a distinct pattern of cognitive deficits in Parkinson's disease. Mov Disord. 2016;31(1):95–102.
Gan-Or Z, Mirelman A, Postuma RB, Arnulf I, Bar-Shira A, Dauvilliers Y, et al. GBA mutations are associated with rapid eye movement sleep behavior disorder. Ann Clin Transl Neurol. 2015;2(9):941–5.
Parkkinen L, Neumann J, O'Sullivan SS, Holton JL, Revesz T, Hardy J, et al. Glucocerebrosidase mutations do not cause increased Lewy body pathology in Parkinson's disease. Mol Genet Metab. 2011;103(4):410–2.
Angeli A, Mencacci NE, Duran R, Aviles-Olmos I, Kefalopoulou Z, Candelario J, et al. Genotype and phenotype in Parkinson's disease: lessons in heterogeneity from deep brain stimulation. Mov Disord. 2013;28(10):1370–5.
Pal GD, Hall D, Ouyang B, Phelps J, Alcalay R, Pauciulo MW, et al. Genetic and clinical predictors of deep brain stimulation in young-onset Parkinson's disease. Mov Disord Clin Pract. 2016;3(5):465–71.
Alcalay RN, Dinur T, Quinn T, Sakanaka K, Levy O, Waters C, et al. Comparison of parkinson risk in ashkenazi jewish patients with gaucher disease and gba heterozygotes. JAMA Neurol. 2014;71(6):752–7.
Arkadir D, Dinur T, Mullin S, Mehta A, Baris HN, Alcalay RN, et al. Trio approach reveals higher risk of PD in carriers of severe vs. mild GBA mutations. Blood Cells Mol Dis. 2018;68:115–6.
Hogl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat Rev Neurol. 2018;14(1):40–55.
Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. 2008;23(12):1665–72.
Anang JB, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83(14):1253–60.
Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78(18):1434–40.
Fereshtehnejad SM, Romenets SR, Anang JBM, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 2015;72(8):863–73.
Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79(10):1117–21.
Kumar KR, Ramirez A, Göbel A, Kresojević N, Svetel M, Lohmann K, et al. Glucocerebrosidase mutations in a Serbian Parkinson's disease population. Eur J Neurol. 2013;20(2):402–5.
Gamez-Valero A, et al. Glucocerebrosidase gene variants are accumulated in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2018;50:94–8.
Barber TR, Lawton M, Rolinski M, Evetts S, Baig F, Ruffmann C, et al. Prodromal parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. 2017;40(8)
Noreau A, Rivière JB, Diab S, Dion PA, Panisset M, Soland V, et al. Glucocerebrosidase mutations in a French-Canadian Parkinson's disease cohort. Can J Neurol Sci. 2011;38(5):772–3.
Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AHV. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72(2):201–8.
Nishioka K, Ross OA, Vilariño-Güell C, Cobb SA, Kachergus JM, Mann DMA, et al. Glucocerebrosidase mutations in diffuse Lewy body disease. Parkinsonism Relat Disord. 2011;17(1):55–7.
Postuma RB, Adler CH, Dugger BN, Hentz JG, Shill HA, Driver-Dunckley E, et al. REM sleep behavior disorder and neuropathology in Parkinson's disease. Mov Disord. 2015;30(10):1413–7.
Shiner T, Mirelman A, Gana Weisz M, Bar-Shira A, Ash E, Cialic R, et al. High frequency of GBA gene mutations in dementia with Lewy bodies among Ashkenazi Jews. JAMA Neurol. 2016;73(12):1448–53.
Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70(6):727–35.
Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology. 2012;79(19):1944–50.
Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16(1):55–65.
Clark LN, Chan R, Cheng R, Liu X, Park N, Parmalee N, et al. Gene-wise association of variants in four lysosomal storage disorder genes in neuropathologically confirmed Lewy body disease. PLoS One. 2015;10(5):e0125204.
Goker-Alpan O, Giasson BI, Eblan MJ, Nguyen J, Hurtig HI, Lee VMY, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67(5):908–10.
Segarane B, Li A, Paudel R, Scholz S, Neumann J, Lees A, et al. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology. 2009;72(13):1185–6.
Jamrozik Z, Lugowska A, Slawek J, Kwiecinski H. Glucocerebrosidase mutations p.L444P and p.N370S are not associated with multisystem atrophy, progressive supranuclear palsy and corticobasal degeneration in polish patients. J Neurol. 2010;257(3):459–60.
Sun QY, Guo JF, Han WW, Zuo X, Wang L, Yao LY, et al. Genetic association study of glucocerebrosidase gene L444P mutation in essential tremor and multiple system atrophy in mainland China. J Clin Neurosci. 2013;20(2):217–9.
Srulijes K, Hauser AK, Guella I, Asselta R, Brockmann K, Schulte C, et al. No association of GBA mutations and multiple system atrophy. Eur J Neurol. 2013;20(4):e61–2.
Mitsui J, Matsukawa T, Sasaki H, Yabe I, Matsushima M, Dürr A, et al. Variants associated with Gaucher disease in multiple system atrophy. Ann Clin Transl Neurol. 2015;2(4):417–26.
Sklerov M, Kang UJ, Liong C, Clark L, Marder K, Pauciulo M, et al. Frequency of GBA variants in autopsy-proven multiple system atrophy. Mov Disord Clin Pract. 2017;4(4):574–81.
Giladi N, et al. A personalized approach to Parkinson’s disease patients based on founder mutation analysis. Front Neurol. 2016;7:71.
Sokol LL, Young MJ, Jankovic J. Counseling at-risk Parkinson’s disease cohorts: integrating emerging evidence. Curr Genet Med Rep. 2017;5(2):100–7.
Sakanaka K, Waters CH, Levy OA, Louis ED, Chung WK, Marder KS, et al. Knowledge of and interest in genetic results among Parkinson disease patients and caregivers. J Genet Couns. 2014;23(1):114–20.
Falcone DC, Wood EMC, Xie SX, Siderowf A, van Deerlin VM. Genetic testing and Parkinson disease: assessment of patient knowledge, attitudes, and interest. J Genet Couns. 2011;20(4):384–95.
Tan EK, Lee J, Hunter C, Shinawi L, Fook-Chong S, Jankovic J. Comparing knowledge and attitudes towards genetic testing in Parkinson's disease in an American and Asian population. J Neurol Sci. 2007;252(2):113–20.
Migdalska-Richards A, Daly L, Bezard E, Schapira AHV. Ambroxol effects in glucocerebrosidase and alpha-synuclein transgenic mice. Ann Neurol. 2016;80(5):766–75.
Maegawa GH, et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009;284(35):23502–16.
Cook L, Schulze J. Connecting Gaucher and Parkinson disease: considerations for clinical and research genetic counseling settings. J Genet Couns. 2017;26(6):1165–72.
Mulhern M, Bier L, Alcalay RN, Balwani M. Patients' opinions on genetic counseling on the increased risk of Parkinson disease among Gaucher disease carriers. J Genet Couns. 2018;27(3):675–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ziv Gan-Or is supported by research grants from the Michael J. Fox Foundation, the Canadian Consortium on Neurodegeneration in Aging (CCNA), the Canadian Glycomics Network (GlycoNet), and the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains for Healthy Lives (HBHL) program. Dr. Gan-Or is consulting for Sanofi and for Lysosomal Therapeutics Inc. (LTI).
Christopher Liong declares no potential conflicts of interest.
Roy N. Alcalay is supported by the Parkinson’s Disease Foundation, the Michael J. Fox Foundation, and the National Institutes of Health.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Gan-Or, Z., Liong, C. & Alcalay, R.N. GBA-Associated Parkinson’s Disease and Other Synucleinopathies. Curr Neurol Neurosci Rep 18, 44 (2018). https://doi.org/10.1007/s11910-018-0860-4
Published:
DOI: https://doi.org/10.1007/s11910-018-0860-4