Abstract
Purpose of Review
The purposes of this review were as follows: first, to provide an overview of the gut microbiota and its interactions with the gut and the central nervous system (the microbiota-gut-brain axis) in health, second, to review the relevance of this axis to the pathogenesis of neurodegenerative diseases, such as Parkinson’s disease, and, finally, to assess the potential for microbiota-targeted therapies.
Recent Findings
Work on animal models has established the microbiota-gut-brain axis as a real phenomenon; to date, the evidence for its operation in man has been limited and has been confronted by considerable logistical challenges. Animal and translational models have incriminated a disturbed gut microbiota in a number of CNS disorders, including Parkinson’s disease; data from human studies is scanty. While a theoretical basis can be developed for the use of microbiota-directed therapies in neurodegenerative disorders, support is yet to come from high-quality clinical trials.
Summary
In theory, a role for the microbiota-gut-brain axis is highly plausible; clinical confirmation is awaited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Not since the human genome project has an area of biomedical science generated such widespread interest in the general public and explosion in scientific and medical literature as the microbiome. Those not directly involved in the field could be excused for believing that the microbiome is going to provide long-awaited answers to all unsolved conundrums and yield new molecules that will cure all ills. Most of this interest has revolved around the most studied commensal bacterial community on or in the human body—the gut microbiome. Not only has the gut microbiome been invoked as a contributor to, if not the cause of, every gastrointestinal ailment but its influence has been extended far afield to impinge on the lungs, joints, endocrine organs, vascular system, and, the topic of this review, the nervous system [1,2,3,4,5].
Before we delve in to the intricacies of microbiota-brain interactions, let us briefly review some general aspects of the microbiome.
The Gut Microbiome—the Basics
The microbiome revolution, as some have termed it, is largely facilitated by the development and rapid evolution of a number of technologies that rapidly and with ever increasing detail and economy permit the thorough evaluation of the microbial inhabitants of our gastrointestinal tract, their biological activities and metabolic products [6,7,8,9]. With these technologies comes a new terminology:
Microbiota
The assemblage of microorganisms (bacteria, archaea, or lower eukaryotes...) present in a defined environment, such as the gastrointestinal tract. [WHAT IS THERE?]
Microbiome
The full complement of microbes (bacteria, viruses, fungi, and protozoa), their genes, and genomes (though strictly speaking different, the terms microbiome and microbiota are often used interchangeably).
Metagenomics
The study of the gene content and encoded functional attributes of the gut microbiome in healthy humans. [WHAT THEY COULD DO?]
Metabonomics
Quantitative measurement of the multiparametric (time-related) metabolic responses of complex systems to a pathophysiological stimulus or genetic modification; often used synonymously with metabolomics. [WHAT THEY PRODUCE?]
The term “flora” which dates from the time when bacteria were included in the plant kingdom, has now been largely abandoned and replaced by the term microbiota.
The Microbiome in Health: Development, Influences, and Functions
At the level of bacterial strains, the gut microbiota demonstrates tremendous diversity and variation between individuals. At higher levels of organization, however, some more overarching themes do emerge. Thus two phyla, Firmicutes and Bacteroidetes dominate in the human gut and it has also been proposed that populations can be classified based on the prominence of one of the following species: Prevotella, Bacteroides, or Ruminococcus [10]. These species have been referred to as enterotypes and it has been proposed that their relative prevalence is largely driven by diet [10, 11].
Though new evidence indicates that colonization of the infant’s gut may commence in utero from the placenta, most of the infant’s microbiota is acquired from the mother during birth and continues to be populated through feeding and contact with the external environment [12]. The infant microbiota evolves rapidly over the next 2–3 years to resemble that of the adult, its composition influenced by such factors as mode of delivery (vaginal birth vs. cesarean section), source of nutrients (breast milk vs. formula), geography, and exposure to antibiotics [12,13,14,15,16,17,18]. These years have come to be viewed as a vulnerable period in that perturbations may have far-reaching impacts on development (including that of the brain) [19,20,21••] and predilection to later disease [20••, 21, 22]. Stable through childhood, adolescence and adulthood, further changes in the microbiota are thought to occur in later life. Given that many neurodegenerative diseases occur in the elderly, the delineation of the normal older person’s microbiota is of critical importance [23•]. Diet may be a major factor in age-related changes in the elderly microbiota; if inadequate, it can lead to a reduction in microbial diversity, a phenomenon which has been linked to inflammation (“inflammaging”) in the elderly [24, 25]. It remains to be determined whether aging per se, independent of any external influences, alters the microbiota.
Diet looms large as a major influence on the microbiota throughout all phases of life and is, undoubtedly, a major confounder in studies of the gut microbiota in disease [26]. The overall features of the diet (i.e., total calories, whether highly processed or vegetable and fruit-based) [24, 27,109,26,28,29], as well as individual components such as carbohydrate [30, 31], protein [32], fat [33], fiber [34,35,36], and vitamins [37] all influence the composition of the microbiota. Though diet-related changes are most likely to occur over time, alterations in microbiota composition can also occur in the short term, if the dietary change is sufficiently drastic [10, 11, 23•, 25, 26, 30, 38, 39]. In evaluating microbial patterns which are proposed to be linked to a given neurodegenerative disorder, these dietary influences must be remembered and, if possible, controlled for, given the sometimes drastic limitations on nutrient intake that occur in such patients be they imposed by swallowing difficulties, cognitive impairment, or gastrointestinal dysfunction. Given their usual age profile, other physical co-morbidities are also prevalent in the patient with neurodegenerative disease and, therefore, the likelihood that they may be prescribed medications that alter microbiota composition: antibiotics [40•, 41], proton pump inhibitors [42, 43], and metformin [44]. It is likely that other prescription and over-the-counter drugs also impact on gut bacterial communities.
The role of the gut microbiota in homeostasis and health continues to be revealed; suffice it to say that an intact microbiota is essential for gut and bodily well-being. Well-documented roles of the microbiota include the development and maturation of the mucosal immune system [5, 45, 46], maintaining the integrity of the gut barrier (proposed to play a key role in certain neurological diseases) [47], modulating gut neuromuscular functions [48,49,50], and performing a number of key metabolic functions [51, 52]. The latter could, of course, lead to the elaboration of molecules that influence brain function.
The Microbiome-Gut-Brain Axis—an Introduction
The concept of the brain-gut axis, a bidirectional channel of communication between the “big brain” in the cranium and the “little brain” (i.e., the enteric nervous system) in the abdomen linked by neurons of the sympathetic and parasympathetic nervous systems, as well as by circulating hormones and other neuromodulatory molecules, is far from new and has long been visualized as the mediator of stress-related gastrointestinal symptoms. Interactions between brain and gut extend well beyond the bounds of the stressed, anxious, or depressed gut and should also encompass situations where the brain, the gut, and their linkage, through the autonomic nervous system, are affected by the same pathologic process, as in Parkinson’s disease, those instances where neurologic symptoms are a consequence of a primarily gastrointestinal pathology, as in the malabsorption syndromes, and, finally, to a host of common gastrointestinal symptoms (or syndromes) that reflect dysfunction somewhere along the gut-brain axis, such as irritable bowel syndrome (IBS) [53]. This axis has now been extended to include the microbiota (the microbiota-gut-brain axis) and tantalizing evidence to suggest that bacteria resident in the gut could impact on the “big brain” has emerged [54]; consequently, the microbiome has emerged as a potential diagnostic and therapeutic target in disorders as diverse as Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, autism, stroke, depression, and drug addiction [54].
It needs to be remembered that the microbiota-gut-brain axis is far from a novel concept and was clearly described over 60 years ago when relationships between gut bacteria, their metabolic products, and hepatic coma and the alleviation of the syndrome of hepatic encephalopathy by the administration of antibiotics were first described [55,56,57]. In these studies the importance of small intestinal overgrowth (SIBO) with coliforms, in particular, was emphasized and these same bacteria and the inflammatory response that they evoke have since been incriminated in the pathophysiology of portal hypertension and other complications of chronic liver disease as spontaneous bacterial peritonitis, systemic sepsis, and hemostatic failure [58, 59]. Indeed, there are several resonances between microbiota interactions with the liver and those with the central nervous system; SIBO, an abnormal microbiota, impaired gut barrier function, a pro-inflammatory state, and the appearance in the systemic circulation of neuroactive molecules generated by bacterial metabolism are postulated to play important roles in the actual pathogenesis of a number of common liver diseases [60, 61].
The Gut Microbiome and the Development of the Central Nervous System
The reader will not be surprised to learn that the gut microbiome and the host immune response play important roles in the development and maturation of the “little brain,” the enteric nervous system (ENS) [62••]. Sheer proximity would, at first sight, support the plausibility of this concept, yet, even here, a number of critical questions need to be addressed: do bacteria and/or their products gain direct access to the ENS, or, are bacterial-neural interactions in the gut mediated through intermediaries? These are questions that will come into sharper relief when we consider microbiota-CNS communication.
Distant though they may be from each other, the ENS and CNS have many morphological, physiological, and pharmacological features in common, suggesting that if bacteria can influence the ENS, they could similarly impact on the CNS, if they, or their messengers, could reach there [63].
Many of the observations on the role of the gut microbiota in neurodevelopment come from experiments in germ-free animals [21••]. These experiments have demonstrated the impact of the microbiome on the morphological and functional development of various parts of the brain; effects which translate into observable alterations in behavior [64,65,66]. In the seminal studies of Diaz Heijtz and colleagues, for example, germ-free mice demonstrated decreased expression of the important neurotrophic factor, brain-derived neurotrophic factor (BDNF) in the cortex, hippocampus, and amygdala [65]. In line with these observations, others have demonstrated impaired neurogenesis in the hippocampus [67•] and altered neural morphology in the amygdala [68] in germ-free animals. Similar effects have been noted in response to another experimental strategy; suppressing the microbiota through the administration of broad-spectrum antibiotics [69]. That antibiotic administration in early life can, not only profoundly impact on the microbiota, but also predispose to the development of inflammatory and metabolic disorders later in life, has been well demonstrated in relation to obesity [70, 71].
The Gut Microbiota in Neurodegenerative Disorders—the Theory
Though it must be emphasized that the overwhelming majority of the supportive evidence comes from animal models, a hypothesis has emerged to link gut microbes to a number of neurodegenerative disorders ranging from Parkinson’s disease (PD) and Alzheimer’s disease (AD) to multiple sclerosis and amyotrophic lateral sclerosis. This is summarized in Figs. 1 and 2 and links gut bacteria with immune activation through a defective gut barrier, thereby leading to a systemic inflammatory response which, in turn, impairs the blood-brain barrier and promotes neuro-inflammation and, ultimately, neural injury and degeneration [21••, 72•, 73,74,75,76,77,78]. This aberrant microbiota-to-CNS pathway is thought to result in the deposition of β-amyloid in AD [72•, 77, 78] as well as in the characteristic neuropathological features of PD [73, 74, 76, 79, 80], including misfolding and aggregation of α-synuclein [81]. In some instances, specific microbial populations have been incriminated, such as periodontal, oral, and nasal communities in AD and PD [76, 82, 83] and that global inhabitant of the gastric mucus layer, Helicobacter pylori, in PD [84]. Certain factors may interact with and/or potentiate these effects. Among these, aging, with its attendant changes in the microbiota, as well as in immune, gut barrier, and blood-brain barrier functions, may be a critical player [77] and is especially relevant in this context given the age profile of many neurodegenerative disorders. Diet is another critical factor (and, indeed, confounder). In the elderly, poor diet has been associated with lower microbiota diversity, inflammation, and disability [24]; the sufferer from a neurodegenerative disorder is prey to nutritional deficiency given the frequent concurrence of dysphagia and other gastrointestinal issues. It is also interesting to note that some of the other socio-personal factors that have been linked to PD, such as smoking, have also been linked to inflammatory bowel disease, a disease where gut microbe-host interactions are thought to play a pivotal role [85]. Interactions between the microbiota and diet may also be beneficial and may, for instance, contribute to the benefits of pomegranates [86] and grape seed polyphenols [87] in AD.
Gut origins of inflammation in neurodegenerative disorders—a hypothesis. Bacterial signals from the oral cavity, stomach (Helicobacter pylori), small intestine (SIBO), or colon (“dysbiosis”) resulting in or associated with a disrupted gut barrier lead to a local immune response that generates pro-inflammatory cytokines that, in turn, lead to systemic inflammation.
The convergence of the gut-derived inflammatory response, aging and poor diet in generating neuro-inflammation—a proposed schema. Circulating pro-inflammatory cytokines (and, perhaps, bacteria-derived molecules, such as lipopolysaccharide) impair blood-brain barrier function and initiate a neuro-inflammatory response. These effects are exacerbated by the pro-inflammatory effects of a poor diet (and other environmental factors) and the immunological and physiological consequences of aging (enhanced inflammatory responses, decreased neurotransmitters, and increased oxidative stress)
The aforementioned hypothesis relating to the role of the gut microbiota in neurodegenerative disease has been largely based on observations in animal models. In one AD model, oral antibiotic treatment reduced amyloid plaque deposition [88]. Studies in the germ-free Aβ precursor protein (APP) transgenic mouse model revealed an altered gut microbiota. Preparation of germ-free animals of this genotype significantly reduced amyloid deposition in the brain; recolonization with bacterial populations from conventionally raised APP animals led to an increase in amyloid pathology, whereas recolonization with bacteria from wild-type animals had little effect [89]. Other AD models have also been shown to possess an altered microbiota which, interestingly, became most pronounced with advancing age [90, 91]. In a series of elegant experiments, Sampson and colleagues demonstrated the importance of the gut microbiota to the pathophysiology of neuro-inflammation in the development of motor deficits in the α-synuclein-overexpressing mouse. Colonization of these mice by feces from PD patients further accelerated the disease process [92••]. The G93A transgenic mouse model of amyotrophic lateral sclerosis also demonstrated an abnormal microbiota and impaired gut epithelial tight junctions, defects that were reversed by the administration of 2% butyrate; an intervention that also prolonged the animals’ lives [93]. Individually and collectively, these animal studies link a disturbed microbiota with systemic inflammation and neuro-inflammation, thereby laying the groundwork for the development of a neurodegenerative process, exacerbated, perhaps, by the aging process, genetic predisposition, and various environmental factors. One must be cautious not to over-interpret findings from animal models; none fully recapitulates the complete human phenotype and a failure to recognize differences in neurophysiology, immune response, and enteric microbiology between mouse and man may lead to expectations that cannot be realized.
The Gut Microbiota in Neurodegenerative Disorders—the Clinical Evidence
For some time, infections of various types have been linked to neurodegenerative disorders. Mention has already been made to the link between H. pylori and PD [84]; infection with Helicobacter pylori has been linked with impaired levodopa absorption [94] and disease severity and progression [95, 96]. Furthermore, though the data is somewhat limited, eradication of Helicobacter pylori has resulted in clinical improvement [73, 97]. Given the high prevalence of gastrointestinal dysfunction in PD [98, 99] and the more recent suggestion that the disease process in PD may originate in the gut [79, 80, 100], the microbiota of PD subjects has attracted considerable attention. Not surprisingly, given its high prevalence of gut dysmotility, PD has also been linked with small intestinal bacterial overgrowth [101, 102]. Utilizing molecular techniques to identify bacterial virulence or recognition factors, prior exposure to bacterial infections has also been associated with both AD [103] and PD [104].
The advent of high-throughput sequencing, metagenomics, metabolomics, and other techniques has revolutionized the study of the gut microbiota and its metabolism, and already, a number of studies have been performed among subjects with both PD and AD. Studies in PD have consistently demonstrated a microbial composition that deviates significantly from that of appropriate controls; less consistent has been the nature of that deviation. These studies have variably demonstrated a suppression of Prevotellaceae [105, 106] and anti-inflammatory genera such as Blautia, Coprococcus, Roseburia, and Fecalibacterium [107] with a blooming of pro-inflammatory Proteobacteria [107], Enterococcaceae [108], and Enterobacteriaceae [105] with the latter being correlated with postural instability and difficulty with gait.
A small study in multiple system atrophy also linked a pro-inflammatory microbiota with impaired gut barrier function and gut inflammation [109].
Similar observations have been made in AD where a suppression of anti-inflammatory taxa such as Eubacterium rectale and a profusion of pro-inflammatory taxa such as Escherichia and Shigella were associated with pro-inflammatory cytokines and amyloid deposition in the brain [110].
While these human studies, in general, support a role for the microbiota-gut-brain axis in PD and AD, one must also be mindful of their limitations. Most were small in size and the study population may or may not have been representative of the general disease population. All but one [108] relied on fecal sampling alone and all were prey to the many factors that may confound any study of the human microbiome in health or disease: diet, medications, and co-morbidity, to mention but a few [111•, 112, 113]. In one of the larger studies Hill-Burns and colleagues attempted to correct for several confounders and were able to define a microbial signature in PD independent of the observed effects of diet, age, region of residence, gender, and medication [114]. Depression is a common co-morbidity in AD and PD; given the pro-inflammatory phenotype and the altered microbiota signatures associated with depression, per se [115, 116], it may prove difficult to differentiate the relative contributions of depression and either AD or PD to a given microbial pattern. Interactions between disease processes and the microbiome may be bidirectional; one must remain open to the possibility that the disease drives changes in the microbiota and not the other way around [111•, 117,118,119].
Routes of Communication
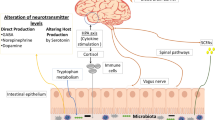
Much ignored in the discussion of the microbiota-gut-brain axis is the simple but vexing question—how could a tiny microbe in the gut communicate with the very distant galaxy that is the brain? Several possibilities have been proposed. First, and much discussed in this review, is the immune response. In this concept, an abnormal microbiota (or, perhaps an aberrant response to a normal microbiota) generates an inflammatory response that results in the release of cytokines [120•] that then results in neuro-inflammation. Mast cell activation, in contrast, may be neuroprotective [121]. Other routes of influence, including the vagus nerve (especially relevant to PD) [122] and microbial metabolites, which may include neurotransmitters and neuromodulators have also been invoked [21••, 123]. Of relevance to AD, bacteria have also been shown to produce amyloid [72•, 75]. It is safe to say that many links in that long journey that leads from the gut lumen to the brain remain to be identified and that some of the hypotheses that have been generated, such as the widely reported “leaky gut” story, represent gross oversimplifications [124].
Conclusions
Epidemiological observations and experiments in animal models support a general schema which implicates the gut microbiota through the microbiome-gut-brain axis in the pathogenesis of common neurodegenerative diseases, such as Parkinson’s and Alzheimer’s disease. Many factors may conspire with bacteria and the host response to lead to the neuropathology that characterizes these disorders. Limited clinical data tends to support this concept but there are many caveats. Leaving aside the many methodological shortcomings of extant studies, it must, first and foremost, be remembered that microbiota-gut-brain communications are bidirectional and one must always consider the possibility that any changes observed in the microbiota are secondary. Quite how bugs communicate with the brain remains to be deciphered. There are certainly no shortage of options: through neuroactive peptides synthesized by bacteria, via other bacterial metabolites that can impact on the blood-brain barrier or brain function, through inflammatory mediators released locally or in the liver that can mediate neuro-inflammation or along the vagus. Each will need to be considered as we attempt to unravel this mystery.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance ••Of major importance
Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135:25–30.
Costello ME, Robinson PC, Benham H, Brown MA. The intestinal microbiome in human disease and how it relates to arthritis and spondyloarthritis. Best Pract Res Clin Rheumatol. 2015;29:202–12.
Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;9:1087–99.
Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–96.
Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–55.
Claesson MJ, O'Toole PW. Evaluating the latest high-throughput molecular techniques for the exploration of microbial gut communities. Gut Microbes. 2010;1:277–8.
Wang WL, SY X, Ren ZG, Tao L, Jiang JW, Zheng SS. Application of metagenomics in the human gut microbiome. World J Gastroenterol. 2015;21:803–14.
Kim Y, Koh I, Rho M. Deciphering the human microbiome using next-generation sequencing data and bioinformatics approaches. Methods. 2015;79-80:52–9.
Vernocchi P, Del Chierico F, Putignani L. Gut microbiota profiling: metabolomics based approach to unravel compounds affecting human health. Front Microbiol. 2016;7:1144.
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80.
GD W, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8.
Neu J. The microbiome during pregnancy and early postnatal life. Semin Fetal Neonatal Med. 2016;21:373–9.
Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–66.
Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Brück WM, Berger B, et al. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio. 2015;6:e02419–4.
Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, et al. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS One. 2016;11:e0152751.
Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7.
Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17:553–64.
Diaz Heijtz R. Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016;21:410–7.
•• Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 167:915–32. A comprehensive and insightful review from leaders in the field.
•• Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595:489–503. A detailed and carefully argued discussion of the importance of the gut microbiome in CNS development and in the pathophysiology of CNS disorders from the individuals who have popularized the term “microbiota-gut-brain axis”.
Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15:307–10.
• Kumar M, Babaei P, Ji B, Nielsen J. Human gut microbiota and healthy aging: recent developments and future prospective. Nutr Healthy Aging. 2016;4:3–16. Given the age profile of many patients with neurodegenerative diseases, changes in the microbiome related to aging per se are important.
Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84.
Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90.
Shanahan F, van Sinderen D, O'Toole PW, Stanton C. Feeding the microbiota: transducer of nutrient signals for the host. Gut. 2017;66:1709–17.
Doré J, Blottière H. The influence of diet on the gut microbiota and its consequences for health. Curr Opin Biotechnol. 2015;32:195–9.
Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–54.
Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–21.
Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20:779–86.
McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66:1241–51.
Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–20.
Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24.
Heinritz SN, Weiss E, Eklund M, Aumiller T, Louis S, Rings A, et al. Intestinal microbiota and microbial metabolites are changed in a pig model fed a high-fat/low-fiber or a low-fat/high-fiber diet. PLoS One. 2016;11:e0154329.
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22:971–82.
Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–5.
Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20:769–78.
Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100.
Bonder MJ, Tigchelaar EF, Cai X, Trynka G, Cenit MC, Hrdlickova B, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8:45.
• Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124:4212–8. Highlights another important confounder in human studies.
Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–5.
Freedberg DE, Toussaint NC, Chen SP, Ratner AJ, Whittier S, Wang TC, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology. 2015;149:883–5.
Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56.
Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–6.
Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36.
Surana NK, Kasper DL. Deciphering the tête-à-tête between the microbiota and the immune system. J Clin Invest. 2014;124:4197–203.
Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–93.
Dey N, Wagner VE, Blanton LV, Cheng J, Fontana L, Haque R, et al. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107.
Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, et al. The gut microbiota keeps enteric glial cells on the move; prospective roles of the gut epithelium and immune system. Gut Microbes. 2015;6:398–403.
Savidge TC. Epigenetic regulation of enteric neurotransmission by gut bacteria. Front Cell Neurosci. 2016;9:503.
Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–8.
Carmody RN, Turnbaugh PJ. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest. 2014;124:4173–81.
Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–66.
Yarandi SS, Peterson DA, Treisman GJ, Moran TH, Pasricha PJ. Modulatory effects of gut microbiota on the central nervous system: how the gut could play a role in neuropsychiatric health and disease. J Neurogastroenterol Motil. 2016;22:201–12.
Phillips GB, Schwartz R, Gabuzda GJ Jr, Davidson CS. The syndrome of impending hepatic coma in patients with cirrhosis of the liver given certain nitrogenous substances. N Engl J Med. 1952;247:239–46.
Martini GA, Phear EA, Ruebner B, Sherlock S. The bacterial content of the small intestine in normal and cirrhotic subjects: relation to methionine toxicity. Clin Sci. 1957;16:35–51.
Phear EA, Ruebner B, Sherlock S, Summerskill WH. Methionine toxicity in liver disease and its prevention by chlortetracycline. Clin Sci. 1956;15:93–117.
Quigley EMM. Gastrointestinal dysfunction in liver disease—gut-liver interactions revisited. Dig Dis Sci. 1996;41:557–61.
Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation and variceal bleeding in cirrhosis. Gut. 2005;54:556–63.
Quigley EM, Abu-Shanab A, Murphy EF, Stanton C, Monsour HP Jr. The metabolic role of the microbiome: implications for NAFLD and the metabolic syndrome. Semin Liver Dis. 2016;36:312–6.
Stärkel P, Schnabl B. Bidirectional communication between liver and gut during alcoholic liver disease. Semin Liver Dis. 2016;36:331–9.
•• Obata Y, Pachnis V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology. 2016;151:836–44. Emphasizes the role of microbiota-host interactions.
Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–38.
Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–64.
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–52.
Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–8.
• Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. 2015;78:e7–9. Nice evidence for the role of the microbiota in neurogenesis.
Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurol. 2016;44:2654–66.
Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotrophic factor and behavior in mice. Gastroenterology. 2011;141:599–609.
Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6.
Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21.
• Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev. 2016;74:624–34. Summarizes the current status of the microbiota-gut-brain axis in Alzheimer’s disease.
Dobbs SM, Dobbs RJ, Weller C, Charlett A, Augustin A, Taylor D, et al. Peripheral aetiopathogenic drivers and mediators of Parkinson’s disease and co-morbidities: role of gastrointestinal microbiota. J Neuro-Oncol. 2016;22:22–32.
Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3.
Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and alzheimer’s disease. J Alzheimers Dis. 2017;58:1–15.
Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017.
Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer's disease. J Alzheimers Dis. 2015;43:725–38. https://doi.org/10.3233/JAD-160152.
Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, et al. Microbes and Alzheimer’s disease. J Alzheimers Dis. 2016;51:979–84.
Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21:10609–20.
Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM. Microbiota-gut-brain signalling in Parkinson’s disease: implications for non-motor symptoms. Parkinsonism Relat Disord. 2016;27:1–8.
Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep. 2016;6:34477. https://doi.org/10.1038/srep34477.
Noble JM, Scarmeas N, Celenti RS, Elkind MS, Wright CB, Schupf N, et al. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One. 2014;9:e114959.
Pereira PAB, Aho VTE, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:61–7.
Shen X, Yang H, Wu Y, Zhang D, Jiang H. Association of Helicobacter pylori infection with Parkinson’s diseases: a meta-analysis. Helicobacter. 2017;22:e12398.
Scheperjans F, Pekkonen E, Kaakkola S, Auvinen P. Linking smoking, coffee, urate, and Parkinson’s disease—a role for gut microbiota? J Parkinsons Dis. 2015;5:255–62.
Yuan T, Ma H, Liu W, Niesen DB, Shah N, Crews R, et al. Pomegranate’s neuroprotective effects against alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem Neurosci. 2016;7:26–33.
Wang D, Ho L, Faith J, Ono K, Janle EM, Lachcik PJ, et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res. 2015;59:1025–40.
Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 2016;6:30028.
Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802.
Shen L, Liu L, Ji HF. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis. 2017;56:385–90.
Brandscheid C, Schuck F, Reinhardt S, Schäfer KH, Pietrzik CU, Grimm M, et al. Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. J Alzheimers Dis. 2017;56:775–88.
•• Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–80. Though largely based on animal models, the study provides a compelling argument for a critical role for the microbiota in PD.
Zhang YG, Wu S, Yi J, Xia Y, Jin D, Zhou J, et al. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther. 2017;39:322–36.
Pierantozzi M, Pietroiusti A, Sancesario G, Lunardi G, Fedele E, Giacomini P, et al. Reduced L-dopa absorption and increased clinical fluctuations in Helicobacter pylori-infected Parkinson’s disease patients. Neurol Sci. 2001;22:89–91.
Dobbs SM, Dobbs RJ, Weller C, Charlett A, Bjarnason IT, Lawson AJ, et al. Differential effect of Helicobacter pylori eradication on time-trends in brady/hypokinesia and rigidity in idiopathic parkinsonism. Helicobacter. 2010;15:279–94.
Tan AH, Mahadeva S, Marras C, Thalha AM, Kiew CK, Yeat CM, et al. Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:221–5.
Hashim H, Azmin S, Razlan H, Yahya NW, Tan HJ, Manaf MR, et al. Eradication of Helicobacter pylori infection improves levodopa action, clinical symptoms and quality of life in patients with Parkinson’s disease. PLoS One. 2014;9:e112330.
Quigley EM. Gastrointestinal dysfunction in Parkinson’s disease. Semin Neurol. 1996;16:245–50.
Mukherjee A, Biswas A, Das SK. Gut dysfunction in Parkinson’s disease. World J Gastroenterol. 2016;22:5742–52.
Perez-Pardo P, Kliest T, Dodiya HB, Broersen LM, Garssen J, Keshavarzian A, Kraneveld AD. The gut-brain axis in Parkinson's disease: possibilities for food-based therapies. Eur J Pharmacol. 2017.
Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E, et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2013;28:1241–9.
Cassani E, Barichella M, Cancello R, Cavanna F, Iorio L, Cereda E, et al. Increased urinary indoxyl sulfate (indican): new insights into gut dysbiosis in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:389–93.
Andreadou E, Pantazaki AA, Daniilidou M, Tsolaki M. Rhamnolipids, microbial virulence factors, in Alzheimer’s disease. J Alzheimers Dis. 2017;59:209–22.
Goldman SM, Kamel F, Ross GW, Jewell SA, Marras C, Hoppin JA, et al. Peptidoglycan recognition protein genes and risk of Parkinson’s disease. Mov Disord. 2014;29:1171–80.
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–8.
Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9:39.
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–60.
Hopfner F, Künstner A, Müller SH, Künzel S, Zeuner KE, Margraf NG, et al. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–5.
Engen PA, Dodiya HB, Naqib A, Forsyth CB, Green SJ, Voigt RM, et al. The potential role of gut-derived inflammation in multiple system atrophy. J Parkinsons Dis. 2017;7:331–46.
Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–8.
• Quigley EMM. Gut microbiome as a clinical tool in gastrointestinal disease management: are we there yet? Nat Rev Gastroenterol Hepatol. 2017;14:315–20. Highlights the limitations of microbiome analysis in human disease.
Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65:63–72.
Devkota S. Prescription drugs obscure microbiome analyses. Science. 2016;351:452–3.
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739–49.
Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs. 2016;30:1019–41.
Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, et al. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal. 2017;138:231–9.
Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–57.
Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163:1428–43.
Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805.
• Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–55. A very up-to-date review.
Girolamo F, Coppola C, Ribatti D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav Immun. 2017;65:68–89.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5.
Forsythe P, Kunze W, Bienenstock J. Moody microbes or fecal phrenology: what do we know about the microbiota-gut-brain axis? BMC Med. 2016;14:58.
Quigley EM. Leaky gut—concept or clinical entity. Curr Opin Gastroenterol. 2016;32:74–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Eamonn M. M. Quigley declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr Neurol Neurosci Rep 17, 94 (2017). https://doi.org/10.1007/s11910-017-0802-6
Published:
DOI: https://doi.org/10.1007/s11910-017-0802-6