Abstract
‘Primary progressive aphasia’ (PPA) refers to core linguistic disorders caused by neurodegenerative disease. Three main PPA variants are recognized: nonfluent/agrammatic, semantic and logopenic. Correctly classifying patients during life according to the underlying histopathology will become increasingly important as cause-specific treatments become available. This article reviews clinical and histopathological studies of PPA, with particular reference to updated PPA classifications. Currently, one-to-one relationships do not exist within PPA subtypes. The semantic variant has the best correspondence between the clinical syndrome and the underlying pathological cause and the logopenic variant the worst correspondence. The use of future biomarkers should facilitate accurate clinicopathological correlation of patients during life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aphasia is defined as an impairment of language affecting the production and/or comprehension of speech caused by dysfunction of the brain. The term ‘primary progressive aphasia’ (PPA) describes aphasias that are caused by neurodegenerative disease. The first descriptions of such progressive aphasias were published over 100 years ago by Arnold Pick [1, 2] and Paul Sérieux [3]. The term ‘primary progressive aphasia’ (PPA) was coined in the 1980s [4] following Marsel Mesulam’s seminal work in 1982 describing six cases of PPA [5]. In recent years, there has been a surge of scientific research into PPA that has enhanced our understanding of the clinical, genetic and pathological aspects of PPA.
Ideally, the clinical classification and diagnosis of neurodegenerative disorders would reflect the underlying neuropathology. This concept of clinicopathological correlation is vitally important in advancing our understanding of neurodegenerative disease. At a histopathological level, an ever-increasing range of specific proteinopathies are being identified, and hopes for future disease-modifying treatments in neurodegeneration rest heavily on targeting such protein-specific processes. Some conditions, such as Alzheimer’s disease (AD), are regarded as clinicopathological entities where the clinical diagnosis of AD strongly predicts Alzheimer-type pathology. However, in PPA the situation is more complicated, and it is necessary to recognize changes in clinical classification of PPA over time in order to understand the impact this has on clinicopathological relationships in PPA. In this review, we will describe each of the currently recognized subtypes of PPA, briefly recap the historical changes in nosology and explore their relationship to the underlying pathological causes.
Historical Perspective
Since Mesulam’s description of six cases in 1982, it has been apparent that PPA is a heterogeneous disorder. Mesulam’s initial work aimed to group PPAs together despite this clinical heterogeneity, principally in order to distinguish PPA from aphasic AD [4]. Notably, Mesulam used ‘aphasic AD’ to refer to patients who present with aphasia, albeit without focal linguistic impairment, and in whom other cognitive deficits typical of AD can be appreciated. PPA subsequently came to be associated with frontotemporal lobar degeneration (FTLD) spectrum pathology. In 1998, clinical FTLD criteria were published with the aim of clinically identifying patients with underlying FTLD pathological features [6]. Consequently, the criteria included the three clinical syndromes associated with FTLD pathology: behavioural variant frontotemporal dementia (bvFTD)—which causes alterations in social behaviour/personality; progressive nonfluent aphasia—a primary linguistic disorder; and semantic dementia (SD), which affects semantic knowledge. SD can present with prominent deficits in verbal semantics and/or visual semantics, i.e. an associative agnosia [7, 8]. The visual/verbal distinction in SD is often presented in terms of asymmetry of atrophy, with visual disorders termed ‘right temporal lobe onset’ and with verbal disorders termed ‘left temporal lobe onset’. Historically, some investigators referred to this semantic group as ‘fluent’ aphasia in counterpoint to the nonfluent group. Although the 1998 criteria aimed to improve clinicopathological correlations, a proportion of patients meeting these criteria for SD and progressive nonfluent aphasia, particularly the latter, were found to have non-FTLD pathological diagnoses [9–16].
Such findings led to speculation that pathological heterogeneity will always exist within PPA subtypes and thus PPAs should be grouped together on the basis of clinical symptoms and separated from nonlinguistic disorders, i.e. bvFTD and right temporal lobe onset SD [17•]. Together with an increasing interest in a third PPA subtype not included in the 1998 clinical FTLD criteria, this triggered a move towards updated classification of PPA focussing on clinical syndromes, without concern for attaining strict correspondence between PPA subtype and the underlying pathological diagnosis [17•]. These contemporary classifications are presented in Table 1 and include basic PPA criteria and criteria for three PPA subtypes. The basic PPA criteria comprise several inclusions and exclusions to ensure that only patients with circumscribed, progressive and sufficiently severe language impairments are included [17•]. If the basic PPA criteria are fulfilled, the next level of criteria can be applied, which includes criteria for three PPA subtypes: nonfluent/agrammatic variant PPA (nfvPPA; with similarities to progressive nonfluent aphasia), semantic variant PPA (svPPA; similar to left temporal lobe onset SD) and logopenic variant PPA (lvPPA). Within subtype criteria, there are three hierarchical levels of diagnosis: ‘clinical’, ‘imaging supported’ and ‘with definite pathology’. The latter allows evidence of any neurodegenerative disease as support for each of the three subtypes.
PPA Pathological Features
Gross pathological examination of brains in PPA demonstrates gross atrophy, typically centred on the perisylvian region in the left hemisphere. As mentioned already, most cases of PPA have a FTLD spectrum pathological change, yet some cases have been found to have other diseases, most commonly AD [10–16, 18–20].
FTLD spectrum pathology can be broadly separated into tauopathies, transactive response DNA binding protein of about 43 kDa (TDP-43) proteinopathies and fused in sarcoma (FUS). The tauopathies most commonly observed in FTLD are Pick’s disease, corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and frontotemporal dementia and parkinsonism linked to chromosome 17. Until 2011, subclassification of FTLD TDP-43 proteinopathies according to the distribution of TDP-43 was difficult as two different systems existed which both used numbers 1–4 to denote different subtypes [21, 22]. These difficulties were overcome with publication of ‘a harmonized classification system’ which uses letters A–D [23]. In PPA, the subtypes of FTLD pathological change typically observed include TDP-43 types A, B and C and the following tauopathies: Pick’s disease, CBD and PSP. FUS and frontotemporal dementia and parkinsonism linked to chromosome 17 pathology are not commonly reported in PPA. The distribution of pathological deposits in cases of PPA, whichever specific proteinopathy is seen, has been found to be asymmetrical; the left hemisphere is more severely affected [24•].
AD pathology is characterized by amyloid β (Aβ) plaques and intracellular neurofibrillary tangles (NFTs), comprising hyperphosphorylated tau [25]. The density and location of NFTs have been found to correlate better than those of Aβ plaques with the severity of disease and the areas of cognition affected [26–30]. Furthermore, grey matter volume loss correlates with NFT density in AD [31, 32]. In cases of PPA with AD pathological change, the gross pathology, Braak stage, neuritic plaque deposition, and TDP-43 immunoreactivity with NFT and Aβ plaques are the same as in typical AD clinical syndromes. However, there are some differences in PPA with AD versus typical AD: NFT density is higher in the left hemisphere than in the right hemisphere [33], particularly affecting the left temporoparietal cortex [15, 34], and there is a greater ratio of cortical to hippocampal NFT density [24•, 33, 34].
Relationship Between Subtypes of PPA and Pathology
Semantic Variant PPA
SvPPA is the most clinically and pathologically homogeneous of the PPA syndromes. The characteristic features of svPPA are marked anomia and impaired single-word comprehension. Patients are often garrulous with fluent speech, which conceals the magnitude of their disorder. When reading, patients may pronounce irregularly spelt words phonetically; such regularization errors are termed ‘surface dyslexia’. Although the patients’ main symptoms are in the language domain, additional deficits in object and face recognition, i.e. associative agnosia and prosopagnosia, are not uncommon, particularly with disease progression [35]. Patients with right temporal onset SD exhibit prominent associative agnosia, but typically also have some degree of verbal semantic impairment akin to that seen in svPPA.
In svPPA, neuroimaging studies typically report atrophy and hypometabolism of the anterior temporal and ventromedial frontal lobes predominantly in the left hemisphere [36–40]. As the name suggests, in right temporal onset SD the same regions are affected, but predominantly in the right hemisphere [7, 8].
Pathological examination in svPPA reveals predominant left lateralized atrophy of the temporal lobes and FTLD TDP-43, predominantly type C histological features [24•, 41•]. Right temporal onset SD is also associated with FTLD TDP-43 pathology, which accounts for more than 70 % of the svPPA and SD cases described [9, 11, 14, 42–45] (Table 2). Some patients with svPPA and SD have been found to have other FTLD pathological features, most frequently Pick’s disease, and patients have been reported with the pathological changes of AD [9, 11, 14, 42–45]. Notably, there is a large variation in pathological specificity reported by different research groups (50–100 %; see Table 2). This suggests that differences exist in the clinical diagnosis of svPPA/SD between groups. Interestingly, research studies evaluating the updated classifications have shown a higher concordance between svPPA and TDP-43 proteinopathy than studies prior to the updated classifications, although the numbers are small in the post-2011 studies (Table 2).
SvPPA and right temporal onset SD are usually sporadic [43]. This is in contrast to bvFTD, where a positive family history may be seen in up to 40 % of cases [46].
New PPA classifications force a separation between right temporal onset SD and svPPA. Yet they share a significant number of clinical features, have neuroimaging that demonstrates asymmetrical temporal lobe atrophy and are frequently caused by the same pathological disease. The presence of behavioural changes in right temporal lobe onset SD can lead to such patients being diagnosed with bvFTD. By the same token, semantic impairments seen in patients with mutations in MAPT (microtubule-associated protein tau gene), associated with symmetrical temporal lobe atrophy [47], can lead to such patients being clinically diagnosed with SD. Therefore, the prediction of the underlying pathology in patients with progressive semantic impairment remains difficult, and current clinical criteria do not fully aid clinicians in teasing out the differences in such cases.
Nonfluent/Agrammatic Variant PPA
NfvPPA encompasses clinical syndromes characterized by problems in grammar/syntax and or problems with speech production, and is associated with various underlying pathological changes (Table 2). Distinctive features of nfvPPA include agrammatism, phonemic paraphasias, impaired articulatory planning or apraxia of speech (AOS) and dysprosody [6, 37, 48–54]. Understanding the relationship between nfvPPA and the underlying pathological diagnosis is greatly hampered by the broad range of clinical syndromes that can be subsumed under the rubric of ‘nonfluent’ aphasia. It is perhaps best to consider the pathology encountered in the nfvPPA group as a whole and then look at whether splitting the nfvPPA group up further leads to greater understanding of the pathological relationships.
Neuroimaging studies suggest patients with nfvPPA have atrophy and hypometabolism of the left posterior inferior frontal lobe [37, 49, 52, 55, 56]. When AOS is a prominent feature, the premotor and supplementary motor cortex are also affected [49].
At autopsy, atrophy of the temporal and frontal lobes with an asymmetric, left-sided emphasis is seen. Histology in the FTLD spectrum is typically observed in over 70 % of cases (Table 2) [24•, 41•, 42, 57]. Within the FTLD spectrum, over 50 % of nfvPPA patients have FTLD tau (including Pick’s disease, CBD, PSP), but a reasonable proportion of patients (around 20 %) are reported with TDP-43 proteinopathy (predominantly type A).
It therefore seems that, compared with svPPA, the overall diagnosis of nfvPPA does not predict a specific underlying disease with a great deal of accuracy. In the past, some patients now classified as having lvPPA might have been diagnosed with progressive nonfluent aphasia. This may account for the significant variation in the proportion of nfvPPA patients reported with AD over the years (Table 2). Clinicopathological correlation in nfvPPA may therefore improve further with implementation of the updated classifications and improved recognition of lvPPA. However, the misattribution of lvPPA as nfvPPA does not account for the variability of the diseases within the FTLD spectrum encountered at autopsy.
Given that nfvPPA seems to encompass at least two subtypes (agrammatic and AOS predominant), it is reasonable to explore whether such subdivision on clinical grounds leads to better specificity of aetiological diagnosis. Indeed, some studies have reported a significant association between AOS and FTLD tau [42, 49]. In a large series of PPA patients, the link between FTLD tauopathies and AOS was limited to PSP histology; the majority of patients with other FTLD tauopathies (CBD and Pick’s disease) exhibited prominent agrammatism [24•]. The suggestion from this observation is that clinicopathological correlations may be found between some specific clinical and pathological subtypes rather than between general PPA groups and overarching pathological groupings. Further complicating the understanding of the relationships between AOS, agrammatism, and specific proteinopathy in nfvPPA is a recent study reporting that AOS was the commonest feature in 11 cases of nfvPPA with FTLD, including both tau and TDP-43 (nine tau, two TDP-43 type A) [55], whereas some degree of agrammatism was present in the tau and TDP-43 groups [55]. AOS and agrammatism can be difficult to diagnose and quantify. It is possible that some of the discrepancies in the literature are due to inconsistencies between research laboratories in identifying these clinical features. Further work describing and outlining how best to identify these features is required.
It has been argued that the classification of primary progressive AOS should be separate from PPA classifications [58]. Owing to the overlap between primary progressive AOS and nfvPPA with prominent AOS, the separation of these disorders may be difficult and would likely cause a degree of diagnostic confusion. However, as we gain a better understanding of the clinicopathological relationships in nfvPPA and AOS, further subdivision of nfvPPA may prove useful.
Special attention ought to be brought to those rare instances of familial nfvPPA. Most cases of nfvPPA are sporadic, but some familial forms of nfvPPA are associated with progranulin (GRN) mutations, which are also found in some familial cases of bvFTD [59–66]. Interestingly, members of the same family with known GRN mutations have been found to present with different clinical syndromes [61, 64]. In addition, a handful of nfvPPA patients have been described with the C9ORF72 GGGGCC hexanucleotide repeat expansion [67, 68]. The presence of such mutations is helpful in predicting the underlying disease as patients with GRN and C9ORF72 mutations typically have FTLD TDP-43.
Logopenic Variant PPA
LvPPA is a heterogeneous clinical and pathological entity. ‘Logopenia’, the characteristic feature of lvPPA, is defined by slowed speech rate due to marked word-finding difficulties. This leads to fluent speech interspersed with nonfluent periods. Cases of PPA described with this feature date back to Mesulam’s initial descriptions of PPA in 1982 [5], but lvPPA was only presented as a discrete clinical syndrome in 2004 [37]. Single-word comprehension and syntax are typically not affected in lvPPA.
Atrophy and hypometabolism of the left posterior temporal lobe and inferior parietal atrophy is typically reported in neuroimaging studies of lvPPA [37, 69–73].
LvPPA is a relatively new entity, and therefore there are only a few published studies of clinicopathological case series which include lvPPA. Gross inspection has shown atrophy of the temporal and parietal regions with a left-sided preponderance [24•]. Most patients with lvPPA have AD (around 60 %), but a significant minority of patients have other diseases (Table 2) [24•, 41•]. Non-AD proteinopathies in patients with lvPPA are varied and include FTLD TDP-43 type A, dementia with Lewy bodies, cerebrovascular disease and Creutzfeldt–Jakob disease [41•]. Owing to the association between lvPPA and AD, several studies have used biomarkers to detect AD pathology during life. Similarly to pathology studies, around 60 % of lvPPA patients have been found to have CSF biomarkers of AD (i.e. elevated tau levels and reduced Aβ 42 levels) [74, 75]. In contrast, studies using positron emission tomography with the ligand Pittsburgh compound B to detect Aβ have found a much higher proportion (between 90 and 100 %) of lvPPA patients to be positive for Pittsburgh compound B, suggestive of underlying AD pathological change [76–78].
There are no genetic mutations known to cause lvPPA. The presence of an apolipoprotein E (APOE) ε4 allele is a risk factor for AD [79, 80]. Findings have been mixed as to whether the same is true for lvPPA, with some authors finding a similarly high frequency of APOE ε4 carriers in lvPPA versus typical AD [34, 37] and other authors reporting a lower frequency [24•, 81, 82].
Unclassifiable PPA
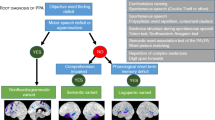
Recent studies evaluating the 2011 PPA classifications have found that a PPA subtype cannot be ascribed to all patients with PPA [41•, 83–85], and some patients meet the criteria for more than one PPA subtype [83–85]. In unclassifiable and mixed PPA cases, predicting the underlying disease is especially challenging.
A variety of underlying diseases have been reported in unclassifiable PPA, including FTLD tau, FTLD TDP-43 and AD (Table 2) [24•, 41•, 49]. In the clinicopathological study from our centre, two ‘unclassified’ PPA patients had GRN mutations [41•]. Interestingly, two other PPA patients in the study also had GRN mutations, but a PPA subtype could be ascribed to them (one nfvPPA patient and one lvPPA patient). The most salient feature of PPA with GRN mutations is typically reported to be anomia; agrammatism may be present, in which case patients may fall into the nfvPPA category [61–63, 86, 87]. However, if agrammatism is not present, it is conceivable that these patients would fulfil the lvPPA criteria. Indeed, one study has reported a high proportion GRN mutations in patients who met the criteria for lvPPA [88]. These findings suggest that patients may present with a syndrome akin to lvPPA owing to their marked anomia. The characterization of the language disorder associated with GRN mutations may require further study. Future criteria could include separate PPA–GRN criteria or include characteristic features of PPA–GRN within the nfvPPA criteria.
Patients with GRN mutations can account for some but not all cases of unclassified PPA. It was postulated in the 2011 classifications publication that patients with early stages of PPA may not fulfil the criteria [17•]. However, in a study from our research group, although a PPA subtype could be ascribed to some ‘unclassified’ PPA patients with disease progression, this was not invariably the case [41•]. Since unclassifiable PPA cannot simply be accounted for by mild/early disease stage, this suggests that more than three PPA syndromes exist.
In a recent study, unclassified PPAs were grouped together, and the investigators found group-level patterns of atrophy predominantly involving the posterior temporal and parietal lobes on the left side, i.e. a pattern similar to that reported for lvPPA [89]. The suggestion from the authors was that such unclassifiable patients probably had AD. However, the study from our centre found that unclassifiable PPA patients have a range of clinical syndromes and underlying pathological diagnoses when evaluated individually [41•]. Therefore, simply assigning patients who do not fit the current PPA criteria to a single group may not help tease out clinicopathological correlations. Looking for trends within unclassifiable patients in order to subdivide these patients, such as those with GRN mutations, is more likely to improve our understanding of the difficult relationship between clinical symptoms and the underlying pathological diagnosis.
Since different PPA subtypes are predominantly associated with different underlying proteinopathies (Table 2), understanding clinicopathological correlations in patients who fulfil the criteria for more than one PPA subtype is problematic. Recent studies evaluating the updated criteria have found that some patients meet the criteria for both lvPPA and another PPA subtype [83–85], and some patients who initially meet the criteria for lvPPA subsequently fulfil the criteria for nfvPPA with disease progression [24•]. Indeed, the core features of lvPPA are nonspecific (Table 1) and can occur in nfvPPA and svPPA [85]. It seems increasingly obvious that the lvPPA classification recommendations are not specific enough to describe a discrete clinical syndrome, and therefore there is little chance of predicting a specific pathological diagnosis. It has been recommended that lvPPA should be defined by word-finding difficulties in the absence of agrammatism or impairments in single-word comprehension [90]. Whether this will improve accurate classification of lvPPA is yet to be determined; however, relying on the absence of core features of the other PPA subtypes to define lvPPA does not seem ideal. Future work characterizing the language disorder seen in patients with PPA with underlying AD is therefore required.
PPA with Significant Behavioural, Cognitive and/or Neurological Features
The PPA classifications are specific to linguistic features and thereby no features outside the language domain are referenced (excepting AOS). Although this narrows down the features to assess and anticipate, other features, which may help pinpoint a specific aetiology, are neglected.
Behavioural features are not uncommon in PPA, and the presence of behavioural features is much commoner in PPA with FTLD than in PPA without FTLD [41•, 91]. Conversely, the presence of certain linguistic features, such as semantic impairment, in a progressive behavioural disorder will increase the certainty that the underlying disease belongs to the FTLD spectrum.
NfvPPA has been linked with disorders of vertical gaze associated with PSP syndrome [49, 92] and symmetric apraxia associated with corticobasal syndrome. If patients with PPA exhibit prominent extrapyramidal motor disorders FTLD tau is more likely than FTLD TDP-43 [55]. Similarly, examining patients for signs of motor neurone disease (MND)/amyotrophic lateral sclerosis (ALS) can be useful since some patients with mixed nfvPPA and MND/ALS [93–95] and svPPA and MND/ALS [96–98] have been reported, and these patients are likely to have FTLD TDP-43. Therefore, monitoring neurological signs and possibly including some of these features in the clinical criteria may be a helpful way of trying to predict pathological diagnosis.
Conclusions
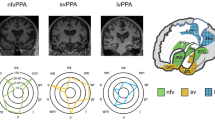
It is likely that as and when potential disease-modifying treatments for PPA become available they will be targeted at specific proteins, and thus accurate clinicopathological diagnosis will be essential. Therefore, the aim of the clinical PPA criteria should be to achieve accurate differentiation of FTLD PPA from non-FTLD PPA, and ideally to distinguish between different pathological diseases in the FTLD spectrum during life. This is a seemingly impossible task, and current PPA classifications do not provide one-to-one correspondence between pathological aetiology and PPA subtypes. However, some patterns between the clinical PPA syndrome and the underlying proteinopathies are recognized. NfvPPA and svPPA are predominantly linked with FTLD, nfvPPA more commonly with FTLD tau and svPPA with FTLD TDP-43 type C (Fig. 1). Although most lvPPA patients have AD, this group is in fact the most pathologically heterogeneous. In part this seems to be due to the nonspecific features included in current classifications, and a better understanding of language features in typical and focal presentations of AD would be useful. PPA is typically sporadic, but some genetic associations are recognized; PPA patients with GRN or C9ORF72 mutations indicate underlying FTLD TDP-43.
So far, improvements in clinicopathological correlation have been largely dependent on cognitive and postmortem neuropathology research. In life, clues to an underlying proteinopathy can be gleaned through the use of biomarkers. Structural MRI can be helpful and may disclose characteristic patterns of atrophy, such as the left anterior temporal volume loss seen in svPPA. In other PPA syndromes, left-sided perisylvian atrophy is often seen but may not help differentiate one underlying disease from another. CSF measurements of Aβ and tau, together with PET ligands that bind amyloid are now becoming increasingly used to identify the pathological changes of AD in vivo. There is hope that in due course biomarkers will become available for TDP-43 and FTLD tauopathies. The combined approach of careful clinical characterization and selective use of biomarker technology will hopefully make accurate clinicopathological diagnosis a reality in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Med Wochenschr. 1892;17:165–7.
Pick A. Zur Symptomatologie der linksseitigen Schlafenlappenatrophie. Monatsschr Psychiatr Neurol. 1904;16:378–88.
Sérieux P. Sur un cas de surdite verbale pure. Rev Med Paris. 1893;13:733–50.
Mesulam M. Primary progressive aphasia—differentiation from Alzheimer's disease. Ann Neurol. 1987;22(4):533–4.
Mesulam M. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11(6):592–8.
Neary D, Snowden J, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54.
Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196–203.
Snowden J, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127(4):860–72.
Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63(6):709–19.
Greene JD, Patterson K, Xuereb J, et al. Alzheimer disease and nonfluent progressive aphasia. Arch Neurol. 1996;53(10):1072–8.
Knibb JA, Xuereb JH, Patterson K, et al. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59(1):156–65.
Rogalski E, Mesulam M. An update on primary progressive aphasia. Curr Neurol Neurosci Rep. 2007;7(5):388–92.
Josephs KA, Whitwell JL, Duffy JR, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70(1):25–34.
Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130(10):2636–45.
Galton CJ, Patterson K, Xuereb JH, et al. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(3):484–98.
Kertesz A, McMonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(9):1996–2005.
Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11)):1006–14. This article describes the most up to date classification system for PPA and is of particular significance because no assumptions are made regarding the relationship between aphasia subtype and pathological disease.
Mesulam M. Primary progressive aphasia — a language-based dementia. N Engl J Med. 2003;349(16):1535–42.
Mesulam M. Primary progressive aphasia pathology. Ann Neurol. 2008;63(1):124–5.
Kirshner HS, Tanridag O, Thurman L, et al. Progressive aphasia without dementia: two cases with focal spongiform degeneration. Ann Neurol. 1987;22(4):527–32.
Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112(5):539–49.
Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169(4):1343–52.
Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122(1):111–3.
Mesulam MM, Weintraub S, Rogalski EJ, et al. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137(4):1176–92. This article closely examines pathological postmortem diagnosis and the topographical distribution of pathological changes in a series of PPA patients.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59.
Neary D, Snowden J, Mann DM, et al. Alzheimer's disease: a correlative study. J Neurol Neurosurg Psychiatry. 1986;49(3):229–37.
Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42(9):1681–8.
Hof PR, Vogt BA, Bouras C, et al. Atypical form of Alzheimer's disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vis Res. 1997;37(24):3609–25.
Johnson JK, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56(10):1233–9.
Guillozet AL, Weintraub S, Mash DC, et al. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60(5):729–36.
Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71(10):743–9.
Whitwell JL, Dickson DW, Murray ME, et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer's disease: a case-control study. Lancet Neurol. 2012;11(10):868–77.
Gefen T, Gasho K, Rademaker A, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135(5):1554–65.
Josephs KA, Dickson DW, Murray ME, et al. Quantitative neurofibrillary tangle density and brain volumetric MRI analyses in Alzheimer's disease presenting as logopenic progressive aphasia. Brain Lang. 2013;127(2):127–34.
Adlam AL, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129(11):3066–80.
Mummery CJ, Patterson K, Price CJ, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47(1):36–45.
Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–46.
Rosen HJ, Kramer JH, Gorno-Tempini ML, et al. Patterns of cerebral atrophy in primary progressive aphasia. Am J Geriatr Psychiatry. 2002;10(1):89–97.
Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001;57(2):216–25.
Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49(4):433–42.
Harris JM, Gall C, Thompson JC, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81(21)):1832–9. This article examines the relationship between pathological postmortem diagnosis and clinical classification of progressive aphasia using the most recent classification recommendations.
Rohrer JD, Lashley T, Schott JM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134(9):2565–81.
Hodges JR, Mitchell J, Dawson K, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133(1):300–6.
Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88–97.
Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56(3):399–406.
Stevens M, van Duijn CM, Kamphorst W, et al. Familial aggregation in frontotemporal dementia. Neurology. 1998;50(6):1541–5.
Josephs KA, Hodges JR, Snowden JS, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122(2):137–53.
Tyrrell PJ, Kartsounis LD, Frackowiak RS, et al. Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. J Neurol Neurosurg Psychiatry. 1991;54(4):351–7.
Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(6):1385–98.
Snowden J, Thompson JC, Stopford CL, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134(9):2478–92.
Snowden J, Neary D, Mann DM, et al. Progressive language disorder due to lobar atrophy. Ann Neurol. 1992;31(2):174–83.
Grossman M, Mickanin J, Onishi K, et al. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer's disease. J Cogn Neurosci. 1996;8(2):135–54.
Caselli RJ, Jack Jr CR. Asymmetric cortical degeneration syndromes. A proposed clinical classification. Arch Neurol. 1992;49(7):770–80.
Delecluse F, Andersen AR, Waldemar G, et al. Cerebral blood flow in progressive aphasia without dementia. Case report, using 133xenon inhalation, technetium 99 m hexamethylpropyleneamine oxime and single photon emission computerized tomography. Brain. 1990;113(5):1395–404.
Caso F, Mandelli ML, Henry M, et al. In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology. 2014;82(3):239–47.
Nestor PJ, Graham NL, Fryer TD, et al. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(11):2406–18.
Kobylecki C, Thompson JC, Jones M, et al. Sporadic Creutzfeldt-Jakob disease presenting as progressive nonfluent aphasia with speech apraxia. Alzheimer Dis Assoc Disord. 2013;27(4):384–6.
Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(5):1522–36.
Mesulam M, Johnson N, Krefft TA, et al. Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Arch Neurol. 2007;64(1):43–7.
Leverenz JB, Yu CE, Montine TJ, et al. A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain. 2007;130(5):1360–74.
Snowden J, Pickering-Brown SM, Mackenzie IR, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006;129(11):3091–102.
Snowden JS, Pickering-Brown SM, Du Plessis D, et al. Progressive anomia revisited: focal degeneration associated with progranulin gene mutation. Neurocase. 2007;13(5):366–77.
Beck J, Rohrer JD, Campbell T, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008;131(3):706–20.
Le Ber I, Camuzat A, Hannequin D, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131(3):732–46.
Rohrer JD, Warren JD, Barnes J, et al. Mapping the progression of progranulin-associated frontotemporal lobar degeneration. Nat Clin Pract Neurol. 2008;4(8):455–60.
Kelley BJ, Haidar W, Boeve BF, et al. Prominent phenotypic variability associated with mutations in progranulin. Neurobiol Aging. 2009;30(5):739–51.
Mahoney CJ, Beck J, Rohrer JD, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135(3):736–50.
Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135(3):693–708.
Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2(6):511–24.
Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–34.
Mesulam M, Wieneke C, Rogalski E, et al. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66(12):1545–51.
Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Curr Opin Neurol. 2010;23(6):633–7.
Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24(5):375–98.
Teichmann M, Kas A, Boutet C, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain. 2013;136(11):3474–88.
Hu WT, McMillan C, Libon D, et al. Multimodal predictors for Alzheimer disease in nonfluent primary progressive aphasia. Neurology. 2010;75(7):595–602.
Leyton CE, Villemagne VL, Savage S, et al. Subtypes of progressive aphasia: application of the international consensus criteria and validation using β-amyloid imaging. Brain. 2011;134(10):3030–43.
Rabinovici GD, Jagust WJ, Furst AJ, et al. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64(4):388–401.
Rabinovici GD, Rosen HJ, Alkalay A, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77(23):2034–42.
Itabashi S, Arai H, Higuchi S, et al. APOE epsilon 4 allele in Alzheimer's and non-Alzheimer's dementias. Lancet. 1996;348(9032):960–1.
Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43(8):1467–72.
Rogalski E, Cobia D, Harrison TM, et al. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76(21):1804–10.
Rogalski EJ, Rademaker A, Harrison TM, et al. ApoE E4 is a susceptibility factor in amnestic but not aphasic dementias. Alzheimer Dis Assoc Disord. 2011;25(2):159–63.
Mesulam M, Wieneke C, Thompson C, et al. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135(5):1537–53.
Wicklund MR, Duffy JR, Strand EA, et al. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014;82(13):1119–26.
Sajjadi SA, Patterson K, Arnold RJ, et al. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78(21):1670–7.
Rohrer JD, Crutch SJ, Warrington EK, et al. Progranulin-associated primary progressive aphasia: a distinct phenotype? Neuropsychologia. 2010;48(1):288–97.
Pickering-Brown SM, Rollinson S, Du Plessis D, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131(3):721–31.
Josephs KA, Duffy JR, Strand EA, et al. Progranulin-associated PiB-negative logopenic primary progressive aphasia. J Neurol. 2014;261(3):604–14.
Sajjadi SA, Patterson K, Nestor PJ. Logopenic, mixed, or Alzheimer-related aphasia? Neurology. 2014;82(13):1127–31.
Mesulam MM, Weintraub S. Is it time to revisit the classification guidelines for primary progressive aphasia? Neurology. 2014;82(13):1108–9.
Xiong L, Xuereb JH, Spillantini MG, et al. Clinical comparison of progressive aphasia associated with Alzheimer versus FTD-spectrum pathology. J Neurol Neurosurg Psychiatry. 2011;82(3):254–60.
Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74(1):42–9.
Doran M, Xuereb J, Hodges JR. Rapidly progressive aphasia with bulbar motor neurone disease: a clinical and neuropsychological study. Behav Neurol. 1995;8:169–80.
Caselli RJ, Windebank AJ, Petersen RC, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol. 1993;33(2):200–7.
Tsuchiya K, Ozawa E, Fukushima J, et al. Rapidly progressive aphasia and motor neuron disease: a clinical, radiological, and pathological study of an autopsy case with circumscribed lobar atrophy. Acta Neuropathol. 2000;99(1):81–7.
Davies RR, Hodges JR, Kril JJ, et al. The pathological basis of semantic dementia. Brain. 2005;128(9):1984–95.
Kim SH, Seo SW, Go SM, et al. Semantic dementia combined with motor neuron disease. J Clin Neurosci. 2009;16(12):1683–5.
Yokota O, Tsuchiya K, Itoh Y, et al. Frontotemporal lobar degeneration with ubiquitin pathology: an autopsy case presenting with semantic dementia and upper motor neuron signs with a clinical course of 19 years. Acta Neuropathol. 2006;112(6):739–49.
Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66(1):41–8.
Yokota O, Tsuchiya K, Arai T, et al. Clinicopathological characterization of Pick's disease versus frontotemporal lobar degeneration with ubiquitin/TDP-43-positive inclusions. Acta Neuropathol. 2009;117(4):429–44.
Compliance with Ethics Guidelines
Conflict of Interest
Jennifer M. Harris and Matthew Jones declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Behavior
Rights and permissions
About this article
Cite this article
Harris, J.M., Jones, M. Pathology in Primary Progressive Aphasia Syndromes. Curr Neurol Neurosci Rep 14, 466 (2014). https://doi.org/10.1007/s11910-014-0466-4
Published:
DOI: https://doi.org/10.1007/s11910-014-0466-4