Abstract
The neuroanatomical substrate of vascular cognitive impairment (VCI) has traditionally included the subcortex of the brain, especially sub-frontal white matter circuits, strategic areas of single infarction that may mediate cognitive impairment such as the dominant thalamus or angular gyrus, and the left hemisphere, and bilateral brain infarcts or volume-driven cortical-subcortical infarctions reaching a critical threshold of tissue loss or injury. We provide an update on the neuroanatomical substrates of VCI and emphasize the following structures or areas: (1) new concepts in relation to hippocampal involvement in VCI based on neuropathological and MRI studies of microinfarcts and the role of traditional cardiovascular risk factors in possibly mediating or potentiating cognitive impairment; (2) advances in our understanding of cerebral microbleeds; and (3) an update on white matter hyperintensities and small vessel disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent times we have observed an evolution of terminology in relation to the definition of dementia or cognitive impairment associated with stroke. Once called multi-infarct dementia (MID) and later vascular dementia (VaD), we now use the term vascular cognitive impairment (VCI) to refer to the entity of post-stroke cognitive impairment. VCI is the preferred term as it is more encompassing than the antecedent terms and provides a means to identify persons across the entire spectrum of degree of cognitive abnormality and stage of risk [1]. For research purposes, VaD, the full-blown or extreme form of VCI, has been studied and defined by different criteria [2, 3]. In clinical practice, VCI connotes a cognitive disorder associated with cerebrovascular disease ranging from mild cognitive deficits to frank dementia, and a syndrome in which there is clinical stroke or subclinical vascular brain injury, and cognitive impairment affecting at least 1 major cognitive domain [4••].
Central to our understanding of VCI is the neuroanatomical substrate of the disorder. Traditionally, stroke was believed to cause cognitive impairment, at least in part, based on volume of infarcted brain tissue, bilaterality of stroke lesions, number of stroke lesions, strategic neuroanatomical involvement, location and extent of involvement of white matter disease, or so-called leukoaraiosis, and co-existence of other pathologies such as Alzheimer disease (AD) [5, 6]. Seminal neuropathological observations by Tomlinson et al published in 1970 suggested that there was a relationship between occurrence of cognitive impairment and volume or strategic location of brain infarction [7]. When there was an estimated 50 mL of infarcted brain tissue, dementia might be present, and with over 100 mL of infarcted brain tissue, dementia was invariable. Over time a multiplicity of reports surfaced linking strategic brain infarct location (eg, dominant thalamus and angular gyrus, deep frontal areas, and left hemisphere) with cognitive impairment [6]. Furthermore and over time, the importance and predominance of subcortical forms of VCI have been established as have revelations related to the importance of associated measures of cerebral atrophy. Structures such as the hippocampus and entorhinal cortex, traditionally thought of as germane only to AD, have become of key importance in understanding cognitive impairment outside of the so-called neurodegenerative disorders. In addition, the role of advanced structural and functional brain imaging, cardiovascular risk factors, and cerebral amyloid angiopathy and cerebral microbleeds have become recognized as germane in understanding the VCI neuroanatomical mechanistic process [4••, 8–15].
In this update, we focus on key neuroanatomical substrates of VCI. We will emphasize involvement of the hippocampus, the significance of cerebral microbleeds, and involvement of cerebral white matter, and small vessel disease in conferring cognitive impairment after stroke. One will need to keep in mind that whereas pure forms of VCI exist, because stroke is common as we age as is AD, mixed pathology (ie, stroke plus AD) may be common in the aged, and it may be difficult to clinically determine whether the occurrence of cognitive impairment is solely a consequence of vascular disease or AD [4••].
The Hippocampus: More Than Just Alzheimer Disease
Traditionally, AD has been considered a neurodegenerative disease with gradual and progressive episodic memory dysfunction early on in the disorder and with many other cognitive domains being involved subsequently [16•]. Medial temporal lobe volume loss (atrophy) of such neuroanatomical structures as the hippocampus, entorhinal cortex, and amygdala may be observed on appropriate MRI brain sequences and considered a feature supporting a diagnosis of AD. Brain necropsy findings typically include diffuse extracellular beta-amyloid protein plaques, β-amyloid plaques containing degenerating neurons (ie, neuritic plaques), and intracellular hyperphosphorylated tau protein in the form of neurofibrillary tangles [17]. The pathological changes of AD have a propensity early in the disorder to appear in the entorhinal cortex and hippocampus and later become widespread in conjunction with loss of neurons and synapses.
Recent advances in our knowledge of risk factors for AD, have established a central role for stroke and cardiovascular risk factors which were once not thought to be risks for AD [4••]. This knowledge has led to advanced imaging studies to begin to better elucidate the relationship between vascular risk factors and AD. A number of provocative studies have been published recently including those focusing on the hippocampus.
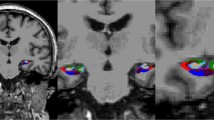
To better understand the relationship between the hippocampus and stroke or cardiovascular risk factors, one must first be aware of the vascular supply to the medial temporal lobe. Szabo et al recently characterized hippocampal stroke and distinguishable phenotypic lesions. They described the following hippocampal vascular supply accordingly to the Stephens and Stilwell atlas source reference: mainly from the posterior cerebral artery (PCA) and to a lesser degree from the anterior choroidal artery (AChA) with the occipital two-thirds being supplied by PCA branches from the P2 segment’s anterior, middle and posterior hippocampal arteries, and the rostral third by branches from the AChA which provide variable supply to the head of the hippocampus [18, 19]. The middle and posterior hippocampal branches supply the body and tail of the hippocampus and the anterior branch supplies the head of the hippocampus and the uncus. Szabo et al showed that there was verbal episodic long-term memory deficit in left and nonverbal episodic long-term memory deficit in right hippocampal infarction in a number of patients after formal neuropsychological examination [18]. A hallmark in the study was that infarcts were not limited to the hippocampus but were more widespread.
Wu et al carried out a MRI brain study of high-resolution functional maps of the hippocampal formation in 240 community-based nondemented persons who had a mean age of 79.7 years, of whom 25 % had type 2 diabetes, and 74 had MRI evidence of brain infarcts [20]. The authors concluded that the hippocampal subregion (dentate gyrus) linked to diabetes was associated with blood glucose level as the pathogenic mechanism and provided a causal pathway for age-related memory decline. Furthermore, the hippocampal region linked to infarction (CA1 region) was associated with transient hypoperfusion as the proposed causal mechanism. The hippocampus has a high concentration of insulin receptors [21]. The Wu et al findings provide a possible neuroanatomical target for testing interventions related to glucose metabolism that might prevent or slow cognitive impairment or decline [20].
The same core group of investigators carried out another study to determine whether brain infarcts and diminished hippocampal volume were independently associated with poorer memory function to suggest that both the integrity of the hippocampus and more diffuse brain networks involved with the hippocampus in memory function were essential [22]. Furthermore, the investigators studied brain infarcts and hippocampal volume in relation to a possible unique profile of cognitive deficits. They showed the following: (1) brain infarcts and hippocampal volume were associated with specific aspects of memory function; (2) brain infarcts were associated with smaller hippocampal volume; and (3) both hippocampal size and presence of infarcts were associated with global memory function [22]. The data support the contention that infarcts may be associated with poor memory function and not just nonmemory performance domains, as was traditionally believed in VCI. Since the hippocampus is part of a network with other areas of the brain involved in memory such as the thalamus, basal forebrain, and amygdala, ischemic injury to these latter neuroanatomical structures could result in abnormal memory processing or performance [22]. In addition, and based on neuropsychological performance profiles, the investigators observed that hippocampal volume was associated with performance on long-term recall, delayed recognition, and delayed free recall; subcortical infarcts with performance on learning, long-term recall and delayed recall; and cortical infarcts with performance on delayed recognition of the selective reminding test. Finally, main results from a brain necropsy study by Arvanitakis et al lends further support to the belief that brain infarcts exert a negative effect on memory function independent of traditional AD pathology such as hippocampal atrophy [23].
In the Honolulu Asia Aging Autopsy study, Launer et al showed that microinfarcts of the brain were substantially associated with cognitive function and mediated by brain weight but not neurofibrillary tangles or neuritic plaques in those without dementia. In those with dementia, however, neurofibrillary tangles were strongly associated with brain weight and cognitive functions, while microinfarcts were only modestly associated with cognitive function in the latter group [24]. Therefore, microinfarcts as shown by brain pathology study seem to significantly be associated with brain atrophy and cognitive impairment, especially before dementia is clinically manifest. As stroke is preventable, these findings provide additional evidence of the possibility to intervene or prevent cognitive impairment or decline through vascular risk factor management. Validated biomarkers might be useful to help guide the timing of these interventions [24].
Gemmell et al investigated the impact of cellular pathology on cognitive function in stroke and neurodegenerative diseases associated with medial temporal atrophy and dementia [25]. Specifically, hippocampal pyramidal neuron density and soma volume were studied in relation to cognitive function in stroke patients who developed delayed post stroke dementia (PSD) or did not have dementia. The investigators showed that there was reduced neuronal volume (ie, neuronal atrophy) associated with cognitive impairment in delayed PSD and other age-related cognitively impairing disorders. Pyramidal neuronal volumes in the CA1 and CA2 hippocampal subfields were 10 %–20 % smaller in the delayed PSD group and all of the other dementia groups compared with elderly controls. Furthermore, the findings might be selective for PSD as there was negligible AD-type lesion burden. Finally, Gemmell et al raised the possibility of the development of therapeutic strategies to maintain or restore functional morphology of existent neurons to prevent further cognitive decline in PSD and other dementias [25].
Several other studies with implications for the hippocampal neuroanatomical substrate and function have shown the following: (1) in the Framingham Offspring Cohort study, hypertension was associated with accelerated white matter hyperintensity volume (WMHV) and worsening executive function; midlife diabetes and smoking with more rapid increase in temporal horn volume (a surrogate of accelerated hippocampal atrophy); midlife smoking predicted a more marked reduction in total brain volume and an increase in extensive WMVH; and waste-to-hip ratio with marked decline in total brain volume (TBV) [14]; (2) in a Mayo Clinic study of older adults without dementia, posterior cingulum tract fractional anisotropy (FA) on diffusion tensor imaging (DTI) study was associated with the cognitive domains of memory, language, attention/executive function, and visual-spatial processing [26]; and (3) in the Atherosclerosis Risk in Communities study, diabetes at baseline was associated with incident infarcts and worsening sulcal widening on MRI, and hypertension was associated with incident infarcts [27].
Significance of Cerebral Microbleeds
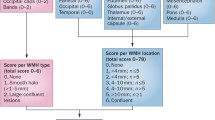
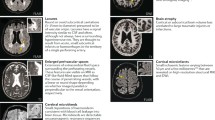
Cerebral microbleeds (CMBs) are characterized as small (<5 mm) hypointense foci that are detected on T2*-weighted gradient-recalled echo (GRE) MRI sequences [28]. The hypointense signal on the GRE sequence represents hemosiderin, a product of red blood cell hemolysis, which is cloistered indefinitely by perivascular macrophages [29]. In an update of the Rotterdam Scan study, Poels et al reported that the prevalence of CMBs increases with age. In the cohort of 3979 subjects, the prevalence of CMBs was 35.7 % in subjects 80 years of age and older, whereas the prevalence was 6.5 % in subjects 45 to 50 years of age [30]. Similar to previous findings, risk factors associated with CMBs differed based on location. Subcortical CMBs are associated with classic cardiovascular risk factors such as uncontrolled hypertension and smoking, but lobar CMBs are associated with cerebral amyloid angiopathy (CAA) [15, 30, 31]. A small 5-year cohort follow-up of 21 subjects with ischemic stroke or transient ischemic attack showed that the greatest predictors of new CMB formation were baseline CMBs (OR 1.2; 95 % CI, 1.02–141.34; P = 0.048) and mean systolic blood pressure (OR 1.28 per unit increase; 95 % CI, 1.23–1.33; P < 0.001) [32]. A study of 742 healthy subjects without classic cardiovascular risk factors suggests a high prevalence of hippocampal atrophy in subjects with CMBs vs. subjects without CMBs (P < 0.0005); however, the number of subjects with CMBs in this study was small (n = 17) [33].
CBMs are associated with VCI, and appear to effect specific cognitive domains [34, 35]. In elderly patients with moderate to severe CAA, there was an association with impaired perceptual speed and episodic memory, but spared semantic and working memory [34]. A small longitudinal cohort of stroke patients showed an association of CMBs with frontal executive dysfunction after a median follow-up of 5.7 years [35]. Interestingly, Tang et al found that an absence of CMBs was an independent factor for reversion from cognitive impairment, no dementia to normal cognition at 15-month follow-up after an acute ischemic stroke (OR 4.3, P = 0.027) [36].
The location of the CMBs play a role in the type of cognitive domains effected, although there is some inconsistency among the various studies even after adjustment for other vascular risk factors [37, 38]. These incongruences mostly appear related to the differences between study population demographics and sample sizes. The Radbound University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) study of 500 subjects without dementia showed that CMBs located in the frontal and temporal lobes as well as isolated subcortical CMBs were correlated with impaired cognitive function as measured by the Mini Mental State Examination (MMSE) and Cognitive Index [37]. A substudy of 439 subjects in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER), showed that CMBs with an infratentorial location were associated with impaired cognitive function specifically for memory as measured by the Immediate Picture-Word Learning test and Delayed Picture-Word Learning test, and activities of daily living as measured by the Instrumental Activities of Daily Living [38].
The AGES-Reykjavik study is an evaluation of memory, executive function, and processing in 3906 older subjects with CMBs and retinal microvascular damage. Multiple CMBs located in the subcortex or infratentorial region were associated with lower cognitive scores on all of the tested batteries, and multiple CMBs were also associated with increased odds ratio of vascular dementia although this was not location specific (OR 2.32, 95 % CI 1.02–5.25). A finding of both multiple CMBs and retinopathy were correlated with impaired executive function and processing speed, but not memory. There was also increased odds ratio of vascular dementia with concurrent retinopathy and multiple CMBs with a lobar location (OR 3.11, 95 % CI 1.11–8.62) [39]. The Rotterdam Scan study is another large study evaluating CMBs and specific cognitive domains as measured by the MMSE and neuropsychological batteries in 3979 subjects without dementia. The presence of 5 or more microbleeds was correlated with impaired cognitive function except for memory when located in purely lobar regions. Cognitive impairment was not seen for deep or infratentorial CMBs after adjusting for other risk factors [40]. An ongoing study that may further clarify some discrepancies regarding CMB location and VCI is the Lothian Birth Cohort 1936. The 1901 healthy subjects underwent intelligence testing at age 11 and are currently undergoing extensive testing which includes cognitive batteries and T2*GRE MRI sequences to assess CMBs after subjects reach 70 years of age [41].
White Matter Lesions and Small Vessel Disease Neuroanatomical Substrates
White matter lesions (WMLs) or leukoaraiosis are radiographic manifestations of ischemia from cerebral small vessel disease (SVD). Lacunar infarcts are often correlated with WMLs on MRI, and also represent ischemia from SVD [8]. The relationship between SVD and VCI has been examined in multiple studies to date. A small cohort of 70 elderly subjects in the Oxford Project to Investigate memory and Ageing (OPTIMA) who did not meet criteria for AD at autopsy and had cognitive assessment at their last clinical appointment showed a negative association of severity of SVD and MMSE score (P < 0.008) [42]. A Japanese study of 350 elderly “community-dwelling” subjects without dementia assessed for VCI as measured by the MMSE and modified Stroop test (MST). Cognitive impairment was found in 15.7 % of subjects and was associated with increased WMLs and significant cerebral atrophy. Frontal executive dysfunction was found in 14.9 % of subjects and associated with the number of silent lacunar infarcts, significant cerebral atrophy, and increased WMLs [43].
The Leukoaraiosis and Disability (LADIS) study started in 2001 to evaluate the association between WMLs and disability in subjects ranging in age from 65 to 84 years. Substudies from LADIS have evaluated the association between WMLs and VCI [44]. Benistry et al evaluated the significance of lacunar infarct location on cognition. Lacunar infarcts located in the thalamus were associated with lower global cognitive function as measured by the MMSE and decreased composite scores for executive function and speed and motor control. Lacunar infarcts in the putamen had a negative association with memory function, but this association was not found with lacunar infarcts in the caudate, internal capsule, or lobar white matter [45].
Jokinen et al evaluated the combined effect of WMLs and lacunar infarcts on cognitive function over a 3 year period on independently functional subjects. Moderate to severe WMLs and lacunar infarcts were associated with cognitive decline specifically in the domains of psychomotor speed, executive function and global cognitive function. The authors found that WMLs and lacunar infarcts were also correlated with a 3-fold increase for developing dementia [46]. In an additional LADIS study, Verdelho et al evaluated 639 elderly, but independently functional subjects for cognitive impairment. After 3 years of follow-up, 14 % of the subjects were diagnosed with dementia and 23 % were diagnosed with cognitive impairment without dementia, which was independently related to WML severity after correcting for other risk factors except for diabetes. Factors predictive of VCI included WML severity, history of stroke and medial temporal atrophy; however, only medial temporal atrophy alone was associated with AD [47].
The Honolulu-Asia Aging study examined the effect of WMLs on cognitive decline over 5 years. In total, 267 Japanese-American men ranging from 74 to 95 years of age underwent baseline cognitive screening and 5-year follow-up of global intellectual functioning as measured by the Cognitive Abilities Screening (CASI) score. Cognitive decline over 5 years was twice as prominent in subjects with WMLs as subjects without WMLs (OR = 1.97, 95 % CI; 1.08–3.61, P = 0.03) [48]. A study from the Women’s Health Initiative evaluated the association between retinopathy and cognitive function as measured by the modified Mini-Mental State Examination (3MSE) in 511 women 65 years of age and older over 10 years. Retinopathy was associated with cognitive impairment in addition to increased ischemic cerebral volumes. This finding suggests that retinopathy is a marker for SVD, which may lead to cerebral ischemia and subsequent cognitive impairment [49].
Conclusion
The term vascular cognitive impairment (VCI) encompasses the spectrum of cognitive dysfunction associated with cerebrovascular disease. Cerebral ischemic volume was previously thought to primarily influence the degree of cognitive impairment; however, more recent studies suggest that the specific location of the involved neuroanatomical substrate plays an influential role in VCI. Medial temporal atrophy is a known feature in AD, but hippocampal volume loss is also associated with cerebral infarcts and cognitive impairment. CMBs are associated with VCI, and the subcortical or lobar location appears to effect cognitive domains differently. WMLs and lacunar infarcts are correlated with VCI both independently and in combination. Our understanding of the neuroanatomical substrate of VCI continues to expand with advances in neuroimaging, and further bridges the gap to identify potential early markers of VCI such as retinopathy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hachinski V. Vascular dementia: a radical redefinition. Dementia. 1994;5:130–2.
Wetterling T, Kanitz RD, Borgis KJ. The ICD-10 criteria of vascular dementia. Dementia. 1994;5:185–8.
Erkinjuntti T. Clinical criteria for vascular dementia: the NINDS-AIREN Criteria. Dementia. 1994;5:189–92.
•• Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. It is a guidance paper on vascular contributions to cognitive impairment.
O’Brien MD. How does cerebrovascular disease cause dementia? Dementia. 1994;5:133–6.
Gorelick PB. Status of risk factors for dementia associated with stroke. Stroke. 1997;28:459–63.
Tomlinson BE, Blessed G, Roth M. Observations of the brains of demented old people. J Neurol Sci. 1970;11:205–42.
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Mori E. Functional brain imaging. In: Erkinjuntti T, Gauthier S, editors. Vascular cognitive impairment. London: Martin Dunitz Ltd; 2002. p. 417–31.
DeCarli C, Scheltens P. Structural brain imaging. In: Erkinjuntti T, Gauthier S, editors. Vascular cognitive impairment. London: Martin Dunitz Ltd; 2002. p. 433–57.
Stebbins GT, Nyenhuis DL, Wang C, et al. Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke. 2008;39:785–93.
Gorelick PB, Bowler JV. Advances in vascular cognitive impairment 2007. Stroke. 2008;39:279–82.
Gorelick PB, Bowler JV. Advances in vascular cognitive impairment. Stroke. 2010;41:e93–8.
Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–8.
Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871–80.
• Ballard C, Gauthier S, Corbett A, et al. Alzheimer’s disease. Lancet. 2011;377:1019–31. This is instrumental in revising the definition of VAD.
Mayeux R. Early Alzheimer’s disease. N Engl J Med. 2010;362:2194–201.
Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke. Clinical and MRI findings. Stroke. 2009;40:2042–5.
Stephens RG, Stilwell KL. Arteries and veins of the human brain. Springfield: Charles C. Thomas; 1969.
Wu W, Brickman AM, Luchsinger J, et al. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64:698–706.
Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia. Arch Neurol. 2009;66:300–5.
Blum S, Luchsinger JA, Manly JJ, et al. Memory after silent stroke. Hippocampus and infarcts both matter. Neurology. 2012;78:38–46.
Arvanitakis Z, Leurgans S, Barnes LL, et al. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7.
Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging study Autopsy study. Ann Neurol. 2011;70:774–80.
Gemmell E, Bosomworth H, Allan L, et al. Hippocampal neuronal atrophy and cognitive function in delayed poststroke and aging-related dementias. Stroke. 2012;43:808–14.
Kantarci K, Senjem ML, Avula R, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34.
Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI. The ARIC study. Neurology. 2011;76:1879–85.
Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74.
Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. Am J Neuroradiol. 1999;20:637–42.
Poels MF, Meike VW, Ikram A, et al. Prevalence and risk factors of cerebral microbleeds. An update of the Rotterdam Scan study. Stroke. 2010;41:S103–6.
Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam scan study. Neurology. 2008;70(14):1208–14.
Gregoire SM, Brown MM, Kallis C, et al. MRI detection of new microbleeds in patients with ischemic stroke: five-year cohort follow-up study. Stroke. 2010;41:184–6.
Chodhury MH, Nagai A, Bokura H, et al. Age-related changes in white matter lesions, hippocampal atrophy, and cerebral microbleeds in healthy subjects without major cerebrovascular risk factors. J Stroke Cerebrovasc Dis. 2011;20(4):203–309.
Arvanitakis Z, Leurgans SE, Wang Z, et al. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69:320–7.
Gregoire SM, Smith K, Jager HR, et al. Cerebral microbleeds and long-term cognitive outcome: longitudinal cohort study of stroke clinic patients. Cerebrovasc Dis. 2012;33:430–5.
Tang WK, Chen Y, Lu J, et al. Absence of cerebral microbleeds predicts reversion of vascular ‘cognitive impairment no dementia’ in stroke. Int J Stroke. 2011;6:498–505.
van Norden AGW, van den Berg HAC, de Laat KF, et al. Frontal and temporal microbleeds are related to cognitive function. The Radboud University Nijemegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) study. Stroke. 2011;42:3382–6.
van Es ACGM, van der Grond J, de Craen AJM, et al. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology. 2011;77:1446–52.
Qui C, Corch MF, Sigurdsson S, et al. Cerebral microbleeds, retinopathy, and dementia. The AGES-Reykjavik study. Neurology. 2010;75:2221–8.
Poels MMF, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function. The Rotterdam Scan study. Neurology. 2012;78:326–33.
Wardlaw JM, Bastin ME, Hernandez V, et al. Brain aging, cognition in youth and old age and vascular disease in the Lothian Birth Cohort 1936: rationale, design and methodology of the imaging protocol. Int J Stroke. 2011;6:547–59.
Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol. 2012;38:337–43.
Koga H, Takashima Y, Murakawa R, et al. Cognitive consequences of multiple lacunes and leukoaraiosis as vascular cognitive impairment in community-dwelling elderly individuals. J Stroke Cerebrovasc Dis. 2009;18:32–7.
Pantoni L. 2001-2011: a decade of the LADIS (Leukoaraiosis and Disability) study: what have we learned about white matter changes and small-vessel disease? A LADIS Study Goup. Cerebrovasc Dis. 2011;32:577–88.
Benistry S, Gouw AA, Prcher R, et al. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age related white-matter changes: the LADIS study. J Neurol Neuosurg Psychiatry. 2009;80:478–83.
Jokinen H, Kalska H, Ylikoski R, et al. Longitudinal cognitive decline in subcortical ischemia vascular disease. The LADIS study. Cerebrovasc Dis. 2009;27:384–91.
Verdelho A, Madureira S, Moleiro C, et al. White matter changes and diabetes predict cognitive decline in the elderly. The LADIS study. 2010;75:160–7.
Inaba M, White L, Bell C, et al. White matter lesions on brain magnetic resonance imaging scan and 5-year cognitive decline: the Honolulu-Asia Aging study. J Am Ceriatr Soc. 2011;59:1484–9.
Haan M, Espeland MA, Klein BE, et al. Cognitive function and retinal and ischemic brain changes. The women’s health initiative. Neurology. 2012;78:942–9.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grysiewicz, R., Gorelick, P.B. Key Neuroanatomical Structures for Post-Stroke Cognitive Impairment. Curr Neurol Neurosci Rep 12, 703–708 (2012). https://doi.org/10.1007/s11910-012-0315-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-012-0315-2