Abstract
The World Health Organization estimates that there are 650,000 prevalent cases of multidrug-resistant (MDR) tuberculosis (TB) globally, and since children (<15 years of age) constitute up to 20 % of the TB caseload in high-burden settings, the number of children with drug-resistant (DR) TB is likely to be substantial. Because bacterial burden at the site of disease is often low, diagnosis involves collection of multiple specimens and a laboratory capable of performing culture, although the Xpert MTB/RIF assay has improved sensitivity over smear examination. The basic principles of treatment for children are the same as those for adults with MDR-TB; however, the treatment regimen is often empiric and based on the drug susceptibility pattern of the source case, if available, or on past history of treatment. Additional challenges arise when MDR-TB is diagnosed and managed in the context of HIV coinfection. HIV-infected children are also treated with antiretroviral therapy medications, which have the potential to interact with second-line anti-TB drugs. Lack of pediatric formulations of second-line drugs and paucity of pharmacokinetic data make dosage challenging. However, when treated appropriately, children with DR TB have good outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antituberculosis drug resistance is a major public health problem that threatens progress made in tuberculosis (TB) care and control worldwide. Globally, 3.7 % (2.1 %–5.2 %) of new cases and 20 % (13 %–26 %) of previously treated cases are estimated to have multidrug-resistant (MDR) TB [1]. The World Health Organization (WHO) estimates that there are 650,000 prevalent cases of MDR-TB globally [2], and since children (<15 years of age) constitute up to 20 % of the TB caseload in high-burden settings [3, 4], the number of children with drug-resistant (DR) TB is undoubtedly high. Data regarding this vulnerable population, however, are lacking; a recent systematic review of children with MDR-TB was able to include only eight studies from five countries [5••]. Children serve as a “sentinel” of TB transmission in the community, and drug resistance in this group mirrors the situation in the adult population in the region.
Major obstacles to understanding the epidemiology of pediatric TB in general and DR-TB in particular include the difficulty of confirming the diagnosis (needing multiple specimens other than sputum and a laboratory capable of performing culture), a higher proportion of smear- and culture-negative and extrapulmonary TB in young children, and the low priority given to this group by public health programs. However, the occurrence of DR-TB among children has been documented by several groups [6, 7•, 8••, 9–11]. In the Western Cape, repeat surveys among children, done in 1997–1998, 2001–2002, and again in 2005–2006, showed that resistance to isoniazid (INH) or rifampicin (RIF) increased from 6.9 % to 12.9 % to 15.1 % and multidrug resistance from 2.3 % to 5.6 % to 6.7 % [12, 13]. Drug resistance among children has been documented in both pulmonary and extrapulmonary disease [14].
When children have MDR-TB, it is usually “primary resistance”—that is, they are infected with strains transmitted from adults with MDR-TB—rather than secondary resistance acquired as a result of suboptimal therapy or nonadherence [13]. The concordance between the Mycobacterium tuberculosis strain infecting the child and the adult index case varies from 45 % to 80 % in different studies, suggesting that children are exposed to TB both within and outside the household [15].
Diagnosis
The diagnosis of pediatric MDR-TB is often delayed due to reliance on the adult case definition and the need for bacteriologic confirmation [16]. Systematic approaches to the diagnosis of children with suspected drug resistance and consensus case definitions have been proposed recently [17, 18]. A diagnosis of TB in children can be made on clinical and radiological grounds in the majority of cases, when bacteriological confirmation is not possible. Depending on the age of the child, site of disease, and available facilities, attempts can be made to obtain sputum, gastric aspirates, induced sputum, biologic fluid samples, nasopharyngeal aspirates, lymph node aspiration biopsy, or tissue biopsy [19–23]. With extensive sampling, the proportion of children with a confirmed diagnosis can be >50 % [24]. Invasive methods, such as bronchoalveolar lavage, bronchoscopic biopsy, or open lung biopsy may sometimes be required [25].
Diagnostic Assays
Culture can be performed using solid media, such as the egg-based Lowenstein–Jensen or the agar-based Middlebrook medium, where the cultures are examined after 3–4 weeks, instead of 4–6 weeks using the classic method. Liquid media systems such as the radioactive (Bactec) or nonradioactive (MGIT), allow detection of growth in 8–14 days. Table 1 shows the TB diagnostic tests in use recently endorsed by the WHO [26].
Tuberculin skin testing, using purified protein derivative and chest radiography, is used as an adjunct to smear microscopy (and culture, if available); however, the former has poor sensitivity and specificity for active TB, and the latter is often not available at the point of primary patient care [26].
In a large, multicountry study in adults, Boehme et al. evaluated an automated tuberculosis assay (Xpert MTB/RIF) for the presence of Mycobacterium tuberculosis (MTB) and resistance to RIF. With a single test, this assay identified 98 % of patients with smear-positive and culture-positive TB (including more than 70 % of patients with smear-negative and culture-positive disease) and correctly identified 98 % of bacteria that were resistant to RIF [27•]. It has several advantages over conventional nucleic acid amplification tests, which have been licensed for nearly 20 years: simple to perform with minimal training, not prone to cross-contamination, requires minimal biosafety facilities, and has a high sensitivity in smear-negative TB (the last factor being particularly relevant in patients with HIV infection) [27•].The Xpert MTB/RIF assay has demonstrated sensitivity of 50 %–70 % in specimens like gastric aspirates and induced sputum [28••, 29].
Molecular line probe assays focused on rapid detection of RIF resistance alone or in combination with INH resistance are now widely used; examples are the INNO-LiPARif.TB kit (Innogenetics, Zwijndrecht, Belgium) [30], labeled for use on M. tuberculosis isolates grown on solid culture, and the Genotype MTBDR and Genotype MTBDRplus assays (Hain Lifescience, Germany) [31], labeled for use on isolates from solid and liquid culture, as well as directly on smear-positive pulmonary specimens. Both assays are complete, polymerase chain reaction (PCR)-based, hybridization assays simultaneously detecting M. tuberculosis complex and specific mutations in the rpoB gene conferring RIF resistance. The Genotype MTBDRplus assay also simultaneously detects specific mutations in the katG gene conferring high-level INH resistance, as well as those in the inhA conferring low-level resistance.
The molecular basis of resistance to INH and RIF (and some other drugs) is now understood (Table 2) [32]. Resistance to INH is due to mutations at one of two main sites, in either the katG or the inhA gene [33, 34]. Resistance to RIF is nearly always due to point mutations in the rpo gene in the beta subunit of DNA-dependent RNA polymerase [35]. These mutations are not directly connected, and so separate mutations are required for organisms to change from a drug-susceptible isolate to MDR-TB. However, genetic probes that detect drug resistance to RIF with >95 % accuracy is very suggestive of MDR-TB; <10 % of RIF resistance is monoresistant, and so RIF resistance is a marker for MDR-TB in >90 % of cases [36]. Whenever RIF and/or INH resistance is determined by a rapid molecular test, the results should be confirmed by phenotypic testing.
There must be recognition, however, that there will be a group of children who need treatment for MDR-TB in whom bacteriological confirmation is either pending or not possible. The category of “probable” MDR-TB will allow providers to initiate timely care within programmatic guidelines in order to decrease the morbidity and mortality of MDR-TB in children, while at the same time ensuring that any potential therapeutic “chaos” does not ensue.
Children with signs and symptoms of active TB disease who, in addition, have the following risk factors should be considered as having “probable” MDR-TB and started on MDR-TB treatment, even in the absence of bacteriological confirmation [8••]:
-
1.
Close contact with a known case of MDR-TB;
-
2.
Close contact with a person who died whilst on TB treatment;
-
3.
Close contact with a person who failed TB treatment;
-
4.
Failure of a first -line regimen;
-
5.
Previous treatment with second-line medications.
Treatment
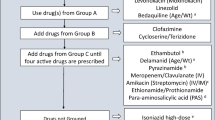
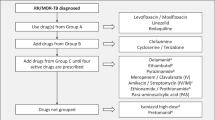
The basic principles of treatment regimen design for children are the same as for adults with MDR-TB [37]. One major difference for children is that their treatment is often empiric and based on the drug susceptibility pattern of the source case, if available, or on past history of treatment. Depending on country guidelines, the regimen used is either individually constructed or a standardized one, such as the Category IV regimen recommended by WHO [18].
The basic principles are the following:
-
Use any first-line medication to which susceptibility is documented or likely (high-dose INH could be included routinely, unless high-level INH resistance or Kat-G mutation is documented).
-
Use of at least four second-line drugs to which the strain is likely to be sensitive; one of these agents should be an injectable, one should be a fluoroquinolone, and PZA should be continued.
-
All doses should be given using DOT (directly observed therapy) to ensure that patients adhere to treatment.
-
Treatment duration should be for 18–24 months, at least 12 months after the last positive culture/smear with minimal disease or 18 months with extensive (lung cavities or widespread parenchymal involvement) disease.
Table 3 shows the five groups of drugs recommended by WHO for use in treating DR-TB in children [5••]. The pharmacokinetics and toxicity of drugs in children differ considerably from those in adults. Almost every aspect of pharmacokinetics (absorption, distribution, metabolism, excretion) is subject to age-related change. Young children often require a higher mg/kg bodyweight dosage of a drug to achieve the same pharmacokinetic exposure as in adults. Current dosing recommendations are based on adult mg/kg doses [18].
For children, amikacin is usually given in preference to kanamycin, since it has a lower minimum inhibitory concentration and the available ampoule sizes are smaller, preventing wastage. Capreomycin is usually reserved for the treatment of extensively DR (XDR) TB. The fluoroquinolones have a central role in the management of MDR-TB in children. Resistance to early generation fluoroquinolones (ofloxacin) may not necessarily imply resistance to later generations (moxifloxacin or levofloxacin) [38]. Few studies have assessed the pharmacokinetics of fluoroquinolones in children; the available data are largely from studies in older children with cystic fibrosis [39].
The second-line drugs are rarely produced in pediatric formulations or appropriate tablet sizes, necessitating breaking, splitting, crushing, or grinding. Hence, dosing may be inaccurate, and subtherapeutic or toxic levels are possible. The taste of the medications is often unpalatable.
Adherence to treatment is a critical factor in the management of MDR-TB, and adverse events associated with second-line drugs could have a severe impact on adherence [40]. In general, children tolerate drugs better than do adults, and most side effects are mild and manageable with counseling and symptomatic drugs. The published information on treatment outcomes for children with MDR-TB suggests that when appropriately treated, outcomes are as good if not better than in adults [41•].
MDR-TB and HIV Coinfection
In settings with a high burden of TB and HIV, up to 40 % of children with MDR-TB are also HIV infected [42]. However, there are few reports of DR-TB/HIV cotreatment in pediatric patients [43–45]. The combination of MDR-TB and HIV can have serious psychological effects. Both conditions are stigmatized and are perceived to carry poor prognosis. The second- and third-line TB regimens demonstrate their own distinct cumulative toxicities with concomitant antiretroviral administration; the nephrotoxicity associated with tenofovir may be compounded by the antituberculous aminoglycosides, and the peripheral neurotoxicity induced by stavudine and didanosine and psychiatric disturbances associated with efavirenz may be exacerbated by the antituberculous agent cycloserine. Additionally, the pill burden and gastrointestinal distress associated with drug-susceptible TB regimens are even greater with MDR-TB and XDR-TB regimens [46, 47].
Studies have demonstrated that, even in a setting of high HIV prevalence, it is possible to achieve favorable outcomes among children treated for MDR-TB using early empiric treatment delivered through a comprehensive community-based program [16, 41•, 48–50]. Four pediatric XDR-TB patients with HIV coinfection were successfully cured with cotreatment in South Africa [43]. Another study in South Africa examined outcomes in 111 children with MDR-TB, including 43 children with HIV coinfection, most of whom initiated ART prior to or during MDR-TB treatment. In that report, 82 % of patients achieved favorable outcomes, and 5 of the 13 deaths occurred before confirmation of MDR-TB and initiation of appropriate treatment [45].
Supportive Care
In addition to TB drugs, guidelines recommend that children with TB should be given pyridoxine if they are HIV infected, malnourished, or breast fed or are being given terizidone, cycloserine, or high-dose INH [51, 52]. Most experts put all children being treated for DR-TB on multivitamin supplements. Nutritional and metabolic requirements should be assessed, because these children are commonly malnourished, and supplements should be provided when necessary [44, 45]. Physiotherapy and occupational therapy may be of benefit for children with respiratory and musculo-skeletal deficit. Social workers should assess home circumstances and support the caregiver to look after a child who may have complex medical needs and must take multiple medications.
New TB Drugs
There are six novel drugs in four new classes in clinical trials, including TMC207 (Bedaquiline), OPC-67683 (Delamanid), PA824, SQ109, and Oxazolidinones (PNU-100480 and AZD5847) [53].
Table 4 shows the overview of anti-TB drugs in the clinical pipeline [54]. These agents are anticipated to shorten and improve the treatment of drug-resistant, and possibly drug-susceptible, tuberculosis—used either separately or in novel combinations. A recent study from South Africa evaluated several novel combinations in an early bactericidal activity study, which measures decline in sputum colony counts per day among patients with sputum smear-positive pulmonary TB, and got encouraging results [55•].
Conclusions and Future Directions
MDR-TB in children is often an underrecognized and neglected problem. Although accurate prevalence or incidence data are not available, wherever surveillance has been done, the rates have been found similar to those for adults in the region. Diagnosis should be presumptive, based upon a number of clinical and epidemiologic factors, in situations where bacteriologic confirmation is not available. While principles of treatment are similar to those for adults, lack of pediatric formulations and paucity of information on pharmacokinetics of second-line drugs in children make treatment challenging. Outcomes are good when appropriate therapy is initiated, even in the presence of HIV coinfection. Research is urgently required to establish optimal dosing schedules of second-line drugs, investigate shorter, more patient-friendly, fully oral regimens for treatment and prevention, and initiate dose-finding and safety studies of newer anti-TB molecules (e.g., Bedaquiline, PA 824, and Delamanid).
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization. Global tuberculosis report 2012. WHO/HTM/TB/2012.6.
World Health Organization, Geneva, Switzerland. Global tuberculosiscontrol.WHO/HTM/TB/201116 2011. Available at:http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf.
Marais BJ, Hesseling AC, Gie RP, et al. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–63.
van Rie A, Beyers N, Gie RP, et al. Childhood tuberculosis in anurban population in South Africa: burden and risk factor. Arch Dis Child. 1999;80:433–7.
•• Seddon JA, Furin JJ, Gale M, Del Castillo Barrientos H, Hurtado RM, Amanullah F, et al. Caring for children with drug-resistant tuberculosis. Am J Respir Crit Care Med. 2012;186(10):953–64. doi:10.1164/rccm.201206-1001CI. The review covers treatment initiation, regimen design and treatment duration, management of comorbid conditions, treatment monitoring, adverse events, adherence promotion, and infection control, all within a multidisciplinary environment.
Debré R et al. Infection of children by strains of tubercle bacilli initiallyresistant to streptomycin or isoniazid. Int J Tubercul Lung Dis. 1959;80:326–39.
• Stephen M. Graham, Tahmeed Ahmed, FarhanaAmanullah, Renee Browning et al. Evaluation of tuberculosis diagnostics inchildren: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an Expert Panel. Journal of Infectious Diseases Advance Access published March 23, 2012. The definitions presented in this article are intended for use in clinical research to evaluate diagnostic assays and not for individual patient diagnosis or treatment decisions.
•• Seddon JA, Perez-Velez CM, Simon Schaaf H, Furin JJ, Marais BJ, et al. Consensus statement on research definitions for drug-resistant tuberculosis in children. J Ped Infect Dis. 2013. doi:10.1093/jpids/pit012. first published online. The study suggests consistent terminology, as well as definitions for measures of exposure, drug resistance testing, previous episodes and treatment, certainty of diagnosis, site and severity of disease, adverse events, and treatment outcome.
TB India 2012. RNTCP Annual Status report. Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, NirmanBhawan, New Delhi - 110108. Available from: http://www.tbcindia.nic.in. March 2012.
World Health Organization (WHO). Guidelines for the programmatic management of drug-resistant tuberculosis – 2010 Available at URL: http://www.tbrieder.org/publications/books_english/who_treatment_mdr.pdf.
Shah I, Rahangdale A. Partial extensively drug resistance (XDR) tuberculosis in children. Indian Pediatr. 2011;48:977–8.
Swaminathan S, Datta M, Radhamani MP, Mathew S, Reetha AM, et al. A profile of bacteriologically confirmed pulmonary tuberculosis in children. Indian Pediatr. 2008;45:743–7.
Schaaf HS, Marais BJ, Hesseling AC, Brittle W, Donald PR. Surveillance of antituberculosis drug resistance among children from the Western Cape Province of South Africa–an upward trend. Am J Public Health. 2009;99(8):1486–90.
Rekha B, Swaminathan S. Childhood tuberculosis–global epidemiology and the impact of HIV. Ped Respir Rev. 2007;8:99–106.
Whalen CC, Zalwango S, Chiunda A, Malone L, Eisenach K, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLoS ONE. 2011;6(2):e16137.
Mukherjee JS, Joseph JK, Rich ML, et al. Clinical and programmatic considerations in the treatment of MDR-TB in children: a series of 16 patients from Lima, Peru. Int J Tuberc Lung Dis. 2003;7:637–44.
Bayona J et al. Contact investigations as a means of detection and timelytreatment of persons with infectious multidrug-resistant tuberculosis. Int J Tubercul Lung Dis. 2003;7(12):S501–9.
Boston, USA: The Sentinel Project for Pediatric Drug-Resistant Tuberculosis. Management of drug-resistant tuberculosis in children: a field guide. November 2012.
Nicol MP, Zar HJ. New specimens and laboratory diagnostics forchildhood pulmonary TB: progress and prospects. Paediatr Respir Rev. 2011;12:16–21.
Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputumversus gastric lavage for microbiological confirmation of pulmonarytuberculosis in infants and young children: a prospective study. Lancet. 2005;365:130–4.
Wright CA, Hesseling AC, Bamford C, Burgess SM, Warren R, Marais BJ. Fine-needle aspiration biopsy: a first-line diagnostic procedure inpaediatric tuberculosis suspects with peripheral lymphadenopathy? Int J Tuberc Lung Dis. 2009;13:1373–9.
Oberhelman RA, Soto-Castellares G, Gilman RH, Caviedes L, Castillo ME, Kolevic L, et al. Diagnostic approaches for paediatric tuberculosis by use ofdifferent specimen types, culture methods, and PCR: a prospectivecase-control study. Lancet Infect Dis. 2010;10:612–20.
Zar HJ, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Rapid molecular diagnosis ofpulmonary tuberculosis in children using nasopharyngeal specimens. Clin Infect Dis. 2012;55:1088–95.
Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracictuberculosis. Clin Infect Dis. 2006;42:e69–71.
Goussard P, Gie RP, Kling S, Nel ED, Louw M, Schubert PT, et al. The diagnostic value and safety oftransbronchial needle aspiration biopsy in children with mediastinallymphadenopathy. Pediatr Pulmonol. 2010;45:1173–9.
New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin Infect Dis. 2010;50 (Supplement 3):S173–7 doi:10.1086/651488.
• Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. The MTB/RIF test provides sensitive detection of tuberculosis and rifampin resistance directly from untreated sputum in less than 2 h, with minimal hands-on time.
•• Bates M, O'Grady J, Maeurer M, Tembo J, Chilukutu L, Chabala C, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect Dis. 2013;13(1):36–42. Analysis of GLA samples with the Xpert MTB/RIF assay is a sensitive and specific method for rapid diagnosis of pulmonary tuberculosis in children who cannot produce sputum.
Nicol MP, Workman L, Isaacs W, Munro J, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis. 2011;11(11):819–24. doi:10.1016/S1473-3099(11)70167-0.
Innogenetics. INNO-LiPARif. TB products insert. 25956 v3. 2007-03-21. Innogenetics NV, Gent, Belgium.
HainLifescience. Genotype® MTBDRplus product insert, Ver 1.0. HainLifescience GmbH, Nehren,Germany. Also available at http://www.hain-lifescience.com/pdf/304xx_pbl.pdf.
Ormerod LP. Multidrug-resistant tuberculosis (MDR-TB): epidemiology, prevention and treatment. Br Med Bull. 2005;73–74(1):17–24.
Zhang Y, Heym B, Allen B, et al. The catalase-peroxidase gene and isoniazid resistance in M. tuberculosis. Nature. 1992;358:591–3.
Piatek AS, Telenti A, Murray MR, et al. Genetotypic analysis of Mycobacterium tuberculosis in two distinct populations using molecular beacons: implications for rapidsusceptibility testing. Antimicob Agents Chemother. 2000;44:103–10.
Telenti A, Imboden P, Marchesi F, et al. Detection of rifampicin resistancemutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–50.
Drobniewski FA, Pozniak AL. Molecular diagnosis, detection of drug resistance and epidemiology of tuberculosis. Br J Hosp Med. 1996;56:204–8.
Schaaf HS, Marais BJ. Management of multidrug-resistant tuberculosis in children: a survival guide for paediatricians. Paediatr Respir Rev. 2011;12(1):31–8. Epub 2010 Oct 14.
Kam KM, Yip CW, Cheung TL, et al. Stepwise decrease in moxifloxacin susceptibility amongst clinical isolates of multidrug-resistant Mycobacterium tuberculosis: correlation with ofloxacin susceptibility. Microb Drug Resist. 2006;12:7–11.
Schaefer HG, Stass H, Wedgwood J, et al. Pharmacokinetics of ciprofloxacin in pediatric cystic fibrosis patients. Antimicrob Agents Chemother. 1996;40:29–34.
World Health Organization. In: Gupta R, Arnadottir T, editors. Guidelines for establishing DOTSplus pilot projects for the management of MDR-TB. Geneva: WHO; 2000. WHO/CDS/TB/2000.279.
• Ettehad D, Schaaf HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):449–56. The treatment outcomes of pediatric MDR-TB are at least as good as those reported for adults.
Schaaf HS, Seddon JA, Willemse M, Hesseling AC, Donald PR. Results from the field. MDR-TB in children: clinical features and outcome of culture-confirmed cases. Berlin, Germany: Abstract International Union of Tuberculosis and Lung Disease Conference; 11–15 November 2010.
Thomas TA, Shenoi SV, Heysell SK, Eksteen FJ, Sunkari VB, et al. Extensively drug-resistant tuberculosis in children with human immunodeficiency virus in rural South Africa. Int J Tuberc Lung Dis. 2010;14:1244–51.
Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi SA. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC Infect Dis. 2011;11:28. doi:10.1186/1471-2334-11-28.
Seddon J, Hesseling A, Willemse M, Donald P, Schaaf H. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clin Infect Dis. 2012;157–166.
Shenoi S, Heysell S, Moll A, Friedland G. Multidrug-resistant and extensively drug-resistant tuberculosis: consequences for the global HIV community. Curr Opin Infect Dis. 2009;22(1):11–7.
Migliori GB, Dheda K, Dheda K. Review of multidrug-resistant and extensively drug-resistant TB: global perspectives with a focus on sub-Saharan Africa. Trop Med Int Health. 2010;15(9):1052–66.
Drobac PC, Mukherjee JS, Joseph JK, Mitnick C, Furin JJ, et al. Community-based therapy for children with multidrug-resistant tuberculosis. Pediatrics. 2006;117:2022–9. doi:10.1542/peds.2005-2235.
Feja K, McNelley E, Tran CS, Burzynski J, Saiman L. Management of pediatric multidrug-resistant tuberculosis and latent tuberculosis infections in New York City from 1995 to 2003. Pediatr Infect Dis J. 2008;27:907–12. doi:10.1097/INF.0b013e3181783aca.
Schaaf HS, Gie RP, Kennedy M, Beyers N, Hesseling PB, et al. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics. 2002;109:765–71. doi:10.1542/peds.109.5.765.
World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. WHO/ HTM/TB/2006.371, WHO/FCH/CAH/2006.7. Geneva: WHO; 2006.
Cilliers K, Labadarios D, Schaaf HS, Willemse M, Maritz JS, Werely CJ, et al. Pyridoxal-5-phosphate plasma concentrations in children receiving tuberculosis chemotherapy including isoniazid. Acta Paediatr. 2010;99:705–10.
Swindells S. New drugs to treat tuberculosis. F1000 Med Rep. 2012;4:12. doi:10.3410/M4-12. Epub 2012 Jun 1.
Van den Boogaard J, Kibiki GS, Kisanga ER, Boeree MJ, Aarnoutse RE. New drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother. 2009;53(3):849–62.
• Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet. 2012;380(9846):986–93. doi:10.1016/S0140-6736(12)61080-0. Epub 2012 Jul 23. PA-824-moxifloxacin-pyrazinamide combination is potentially suitable for treating drug-sensitive and MDR-TB.
Compliance with Ethics Guidelines
Conflict of Interest
Navaneetha Pandian Poorana Ganga Devi and, Soumya Swaminathan declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poorana Ganga Devi, N.P., Swaminathan, S. Drug-Resistant Tuberculosis: Pediatric Guidelines. Curr Infect Dis Rep 15, 356–363 (2013). https://doi.org/10.1007/s11908-013-0363-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-013-0363-z