Abstract
The hepatitis D virus (HDV), the smallest virus known to infect man, causes the most severe form of chronic viral hepatitis, hepatitis delta. It is estimated that about 15 to 20 million people are suffering from chronic HDV infection. HDV is a defective satellite virus depending on the hepatitis B surface antigen (HBsAg) for transmission. Chronic hepatitis delta is associated with a rapid progression of liver fibrosis and a high prevalence of liver cirrhosis, even in younger patients. Immunization against hepatitis B virus (HBV) protects from HDV infection, but there is no specific vaccine against HDV available for HBsAg-positive individuals. Treatment options for hepatitis delta patients are limited. So far, only interferon-alpha has shown an antiviral efficacy against HDV. Recent trials showed sustained virological response rates concerning HDV in 25 %–30 % of patients treated with pegylated interferons. HDV is dominant over HBV in the majority of cases, but HBV DNA-positive subjects should be treated with HBV polymerase inhibitors. Combination therapy of pegylated interferon-alpha and adefovir showed a more pronounced HBsAg decline, but the exact role of combination therapies in hepatitis delta requires further investigation. Alternative future treatment strategies may include prenylation inhibitors and HBV entry inhibitors, which are in early clinical development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prevalence of Hepatitis Delta Virus
An estimated 350 million people worldwide are suffering from hepatitis B virus (HBV) infection. The number of patients coinfected with HBV and the hepatitis delta virus (HDV) is believed to be between 15 and 20 million [1, 2]. HDV prevalence rates differ between the different areas. HDV has been endemic in the Mediterranean area, the Middle East, Central Africa, and the Amazonas [3]. In the Western world, the proportion of HBsAg-positive patients coinfected with HDV is much lower [4, 5]. Main risk factors for HDV infection in Europe are intravenous drug abuse and being born in a country in an HDV-endemic area [6, 7]. Similarly, a recent U.S. study revealed a high anti-HDV prevalence of around 40 % in HBsAg-positive intravenous drug users [8]. During the 1980s and 1990s, the anti-HDV prevalence in HBsAg-positive patients was more than 20 % in certain Mediterranean countries [9]. After the implementation of vaccination programs against HBV infections, the number of patients coinfected with HBV and HDV decreased substantially [10]. However, an eradication of HDV in Europe could not be achieved. Immigrant populations from endemic areas have been especially responsible for rather high prevalences in Germany and other European countries. At Hannover Medical School, the prevalence of anti-HDV-positive among HBsAg-positive individuals is around 8 %–10 % [5]. The vast majority (75 %) of these patients were not born in Germany [6].
Virology and Life Cycle of HDV
The HDV genome consists of about 1,700 nucleotides and currently is the smallest animal virus known to infect humans. HDV has only a single-stranded circular RNA [11], which encodes a single protein, the hepatitis delta antigen (HDAg). Two different forms of HDAg are known: a large form with 214 aminoacids (L-HDAg) and a smaller protein (S-HDAg) with only 195 aminoacids. The exchange of one base at position 196 (A → G, stop codon) is necessary to produce the S-HDAg. Both forms of the delta antigen are important for distinct steps within the HDV life cycle. The small form is crucial for the synthesis of different forms of the virus-specific RNA, whereas the large protein is crucial for viral assembly [11, 12]. Host polymerases are used for the replication of viral RNA [13, 14]. The complete virion consists of the envelope protein of the hepatitis B virus (HBsAg) and the ribonucleoprotein consisting of genomic RNA, as well as several small and large delta antigens.
Hepatitis D Virus Genotypes
Eight different HDV genotypes have been described so far [15, 16]. Genotype 1 can be found in nearly all areas around the world, with the highest prevalences in Europe and North America [17]. In contrast, HDV genotype 3 has been identified only in patients living in South America [18], whereas genotype 2 is endemic in Eastern Asia [19]. The remaining genotypes have been found in patients living in Africa [16]. Of note, genotype 1 seems to be associated with a more severe clinical course, as compared with HDV genotype 2 infection [20].
Prevention of HDV Infections
The best available option to prevent people from getting HDV infection is vaccination against HBV. HDV is a defective satellite virus lacking the envelope and is, therefore, dependent on the hepatitis B surface antigen (HBsAg) to infect hepatocytes. Therefore, vaccination against HBV also prevents hepatitis delta. Vaccinating all family members of HBV- or HDV-infected patients against hepatitis B is crucial. HDV is thought to be transmitted only parenterally, but transmission of HDV has been found to occur between family members [21], and therefore, precautions are very important in families with more than one HBsAg-positive carrier.
Clinical Course of Hepatitis Delta Infection
Simultaneous HBV/HDV infection is associated with a more severe form of acute hepatitis, leading to acute liver failure in some cases [22, 23]. However, spontaneous clearance of both infections has been reported in up to 95 % of cases. Simultaneous HBV/HDV infection has become a rare event in Western countries. Patients with fulminant hepatitis should be referred to in liver transplant centers. The remaining cases without acute severe hepatitis are generally self-limiting and do not need any specific antiviral therapy.
More common and, therefore, more important are HDV superinfections of HBsAg-positive individuals. Patients suffering from superinfections can initially present with signs of acute hepatitis, but in most cases, symptoms are very nonspecific. In contrast to simultaneous HBV/HDV infections, the large majority of patients with superinfections take a chronic course with persistence of HDV RNA. HBV patients coinfected with HDV show a much faster progression of liver fibrosis. In HBV/HDV-infected patients, liver cirrhosis can frequently be detected at a much younger age than in HBV-monoinfected individuals. HDV coinfection is also associated with earlier development of hepatic decompensation and a higher likelihood of liver transplantation or liver-related death [24–27]. The nature of the relationship between HDV infection and hepatocellular carcinoma (HCC) is controversial, and further studies are needed to answer the question of whether HDV infection increases the risk for the development of HCC [26–29]. The higher frequency of liver cirrhosis in HDV-infected patients clearly represents a risk factor for HCC, but HDV infection itself does not seem to confer an additional oncogenic hit.
The individual course of hepatitis delta can be variable. Different patterns of viral dominance have been described, and direct interactions between HDV and HBV that inhibit viral replication of the respective other virus have been suggested [30, 31]. In the majority of cases, HDV is the more dominant virus and suppresses HBV replication, which is independent of the phase of HBV infection. Thus, in both HBeAg-positive and HBeAg–negative patients, HBV-DNA is frequently found at very low levels in HDV/HBV-coinfected individuals [24]. In the long term, each virus may become dominant, and fluctuating levels of both HBV DNA and HDV RNA have been observed [32, 33]. Thus, HBV replication may significantly contribute to disease progression in HDV infection, an observation that needs to be considered when making treatment decisions.
Diagnosis of HBV/HDV Coinfection
We recommend testing every HBsAg-positive patient for anti-HDV-IgG at least once. HDV antibodies usually persist even after resolved infections; however, antibody titers may decline and become negative in some individuals. In our experience, anti-HDV antibodies remained detectable in most patients for several years after liver transplantation, even in the absence of HDV reinfection [34]. A positive anti-HDV test should lead to subsequent investigation of HDV replication, using nucleic acid testing (HDV RNA) [35]. Unfortunately, there are as yet no international standards for HDV RNA quantification. Therefore, results from different labs can differ considerably. Moreover, not all published assays cover all eight HDV genotypes; thus, false negative HDV RNA tests should be considered in anti-HDV-positive patients with significant, otherwise unexplained hepatitis. Samples from patients receiving antiviral treatment should be tested during therapy with one assay performed by the same laboratory.
HDV genotyping might be helpful in certain geographic regions where different HDV genotypes are prevalent [20]. However, HDV genotyping is not recommended as part of routine diagnostic workup in Europe and North America, where the great majority of patients are infected with HDV genotype 1. Moreover, different HDV genotypes are currently treated with the same therapeutic approach, and genotyping therefore has no direct clinical consequences [2].
Because HCV infection is a coinfection of HBV-infected individuals, serological and molecular markers of hepatitis B should also be evaluated. Quantitative HBsAg levels are of particular importance as the best clinical endpoint of antiviral therapy in hepatitis delta HBsAg seroconversion with development of anti-HBs antibodies. The decrease of HBsAg levels during therapy may be important in determining optimal treatment duration. The patient’s HBeAg status should also be determined, even though the natural history of hepatitis delta seems largely not to be influenced by the presence of absence of HBeAg [24]. HBV DNA testing is also required to determine whether a patient requires antiviral therapy with HBV polymerase inhibitors.
Finally, patients with hepatitis delta should be screened for HCV and/or HIV coinfections. One out of three anti-HDV-positive patients seen in Germany also tested positive for anti-HCV [6]. In HIV-infected patients, coinfection with HCV is not uncommon, although there is a considerable difference in rates of coinfection found between different European regions [36].
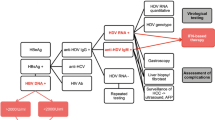
As in any other patient with chronic liver disease, the clinical workup of a HCV-infected patient should include abdominal imaging and a liver biopsy to determine fibrosis stage and inflammatory activity [37]. Endoscopy should be performed in patients with liver cirrhosis in order to evaluate for the presence and severity of esophageal varicoses. Additionally, a form of regular HCC screening is recommended for every cirrhotic patient [38, 39•] (Fig. 1).
Current Treatment Options for Chronic Hepatitis Delta
HDV exclusively uses enzymes provided by the host’s hepatocytes for viral replication. As such, HDV lacks specific viral enzymes that could be targeted therapeutically in order to inhibit replication. In the last decades, several studies have been performed to investigate potential antiviral effects of various compounds. To date, interferon-alpha appears to be the only available drug with appreciable antiviral activity against HDV. Conventional, as well as pegylated, interferon alpha has been able to suppress HDV replication [40–45] (Table 1). Different studies are often difficult to compare directly because different forms, doses, and durations of interferon-alpha therapy were investigated. Moreover, sensitivities of the assays used to test for HDV RNA varied considerably. Even taking into consideration these variabilities of the studies, overall, between 25 % and 40 % of patients showed a sustained virological response concerning HDV RNA after 1–2 years of therapy.
The HIDIT I trial, carried out by the German Network of Competence for Viral Hepatitis (Hep-Net) in collaboration with Turkish and Greek centers, has so far been the largest multicenter study in hepatitis delta. Pegylated interferon-alpha was tested in combination with adefovir dipivoxil versus either drug alone in 91 patients with hepatitis delta [42••]. Overall, 28 % of patients receiving 180 μg of pegylated Interferon-alpha-2a once weekly for 48 weeks were cured of HDV infection [42••]. Moreover, combination therapy with adefovir showed a more pronounced decline of HBsAg levels, leading to HBsAg clearance in 2/30 patients. Thus, the combination therapy may have some advantages, since HBsAg clearance represents the ultimate cure of hepatitis delta.
A key question for any antiviral therapy is to what extent suppression of viral replication translates to a more beneficial clinical long-term outcome and whether liver-related clinical endpoints can be prevented. Importantly, Farci and colleagues showed that patients treated with high doses of interferon-alpha had a significantly better long-term survival than did patients treated with low-dose Interferon [46].
Several questions related to interferon-alpha-based therapy for HDV infection remain unanswered. For example, the optimal treatment duration needs to be defined for individual patients. Longer therapies appear to be associated with higher response rates [47], but it is not yet clear which patients may safely stop treatment after 1 year and which patients should continue. In the HIDIT II trial of the German Hep-Net (NCT00932971), 120 patients are currently being treated for 2 years with 180 μg pegylated Interferon-alpha-2a once weekly plus placebo or 245 mg Tenofovir daily for 96 weeks. The results of this trial will be reported during the year 2013. Single-case reports have also been published using duration of therapy of up to 12 years, waiting until the patient’s HBsAg has been cleared [48]. Other unanswered issues include establishing universal stopping rules (i.e., defining when therapy can be discontinued in patients with poor chances to clear HDV). At this time, it seems reasonable to monitor HBsAg and HDV RNA levels during therapy and to consider discontinuing treatment after 6–12 months if both parameters do not show any significant declines.
Ideally, patients should be identified prior to therapy and prioritized on the basis of who appears to need treatment most urgently and where treatment can be performed with a reasonable risk–benefit ratio. Interferon-alpha therapy is contraindicated in patients with decompensated liver cirrhosis. For example, low platelet counts are a marker of advanced liver disease, and we should be very cautious to initiate therapy in subjects with platelet counts below 90,000/μl. On the other hand, patients with very mild disease may not need immediate antiviral therapy. We have suggested a baseline-event-anticipation score for HDV infection, which is easy to use and which may distinguish individuals with a very mild course of disease from subjects with a higher risk to develop liver-related complications (www.hepatitis-delta.org).
The Role of Nucleoside and Nucleotide Analogues As Well As Other Antiviral Compounds in the Treatment of Chronic HDV Infections
Since the late 1970s, several compounds with antiviral effects on different viruses have been tested for their efficacy in hepatitis delta. In particular, nucleoside and nucleotide analogues (NUCs), including lamivudine, adefovir, and entecavir, have been investigated. Because these agents are able to suppress HBV replication, it was speculated that their secondary effects on HDV replication may have been due to potential decreases in HBsAg levels. However, none of the known NUCs achieved significant reductions of HBsAg levels, and none of the NUCs tested so far have had a significant short-term antiviral effect on HDV [42••, 47, 49–53]. However, longer treatment with NUCs for several years may induce HBsAg declines in some patients [54]. Indeed, HIV-infected patients with hepatitis delta who have been exposed to tenofovir for several years as part of the antiretroviral HIV therapy showed significant HBsAg declines that were parallel to HDV RNA declines and led to HDV RNA negativity in individual patients [55]. In a recent study investigating HIV–HBV–HDV coinfected patients on NUC treatments, a clearance rate of 2.6/100 patients/year was reported. With a total of 7 patients becoming HBsAg negative, 2 of them were HDV coinfected, and these patients did not seroconvert to anti-HBs. For this reason, antiviral therapy was continued [56].
In cases of HDV infection with a high HBV viral load, NUC therapy is recommended [33, 57, 58]. Because chronic HBV infection may well contribute to the progression of liver disease, suppression of HBV DNA replication is clearly associated with a reduced risk of developing liver-related morbidity and mortality [59–61]. Importantly, compounds with low resistance barrier should be used carefully, since resistances located in the HBV polymerase gene could also induce changes in the HBsAg, due to overlapping reading frames. These mutations could potentially influence HDV formation and release [62], with yet unknown clinical consequences.
Liver Transplantation in Patients With End Stage Liver Disease Due to Chronic HDV Infection
For patients suffering from end stage liver disease who cannot be treated with interferon-alpha-based therapies, liver transplantation is the only remaining treatment option. Fortunately, the overall outcome for patients transplanted for hepatitis delta is excellent. With the use of hepatitis B immunoglobulins and potent NUCs, HBV reinfection of the transplanted liver with HBV and, subsequently, also with HDV can be inhibited [63]. HDV RNA becomes negative within the first days after transplantation, and HDV RNA values parallel HBsAg levels [34]. However, HDV antigen can be detected in the transplanted liver in hepatocytes for several months after transplantation, suggesting HDV latency in the absence of HBV replication. We therefore would recommend continuing lifelong efforts to prevent HBV reinfection, since reappearance of HBsAg may lead to HDV reactivation and reinfection.
Possible Future Treatment Options for Chronic Hepatitis Delta
HDV is the smallest known animal virus and, unlike HIV, HCV, or HBV, does not encode for its own enzymes, which could be targeted therapeutically. However, virus assembly and posttranslational modification are crucial steps in the life cycle of HDV. One important modification is prenylation of the HDAg. In vitro models and certain studies with different types of animals showed antiviral effects of prenylation inhibitors against HDV replication [64]. Prenylation inhibitors have already been developed as anticancer therapies, and first studies in humans with hepatitis delta have already started (www.clinicaltrials.gov).
Other strategies for the treatment of HDV infection could be to target other steps in the HBV life cycle with the end point of HBsAg clearance. An entry inhibitor for hepatitis B called Myrcludex B consists of HBVpreS lipopeptides and has been shown to inhibit HBV infection in vitro and also in vivo in a humanized mouse model [65]. Myrcludex B also inhibits the entry of hepatitis virions into hepatocytes. Subsequently, the drug had beneficial effects in a mouse model of HDV infection [66]. Myrcludex B is currently in early phase I/IIa clinical development, and first trials in hepatitis delta patients are expected to start during the next 18 months.
A third possible approach to optimizing the current hepatitis delta treatment is the development of alternative immunotherapies, including alternative interferons, toll like receptor (TLR) agonists, or therapeutic vaccination. Today, most of the interferons used in the field of hepatology are type I interferons, with receptors on many different cells throughout the body causing various side effects. Interferon-lambda may have advantages, since receptors are less widely expressed. Potent activities with a better tolerability have been shown in early trials using this agent for treatment of hepatitis C [67]. It will be interesting to see whether interferon-lambda may be an alternative treatment option also for patients infected with HBV, including individuals with hepatitis delta.
Although the hepatitis delta virus was discovered more than 30 years ago, treatment options for hepatitis delta are still limited. However, there is some hope on the horizon, and the development of drugs acting directly against HDV seems to be feasible. In addition, the awareness of this so-called rare disease has increased in recent years. Hepatitis delta can be cured only if patients are identified, and efforts should only be made to find new drugs, but also to educate physicians about this most severe form of chronic viral hepatitis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011.
Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31–40.
Arboleda M, Castilho MC, Fonseca JC, Albuquerque BC, Saboia RC, et al. Epidemiological aspects of hepatitis B and D virus infection in the northern region of Amazonas, Brazil. Trans R Soc Trop Med Hyg. 1995;89:481–3.
Gaeta GB, Stroffolini T, Smedile A, Niro G, Mele A. Hepatitis delta in Europe: vanishing or refreshing? Hepatology. 2007;46:1312–3.
Wedemeyer H, Heidrich B, Manns MP. Hepatitis D virus infection–not a vanishing disease in Europe! Hepatology. 2007;45:1331–2. author reply 1332–1333.
Heidrich B, Deterding K, Tillmann HL, Raupach R, Manns MP, et al. Virological and clinical characteristics of delta hepatitis in Central Europe. J Viral Hepat. 2009;16:883–94.
Cross TJ, Rizzi P, Horner M, Jolly A, Hussain MJ, et al. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J Med Virol. 2008;80:277–82.
Kucirka LM, Farzadegan H, Feld JJ, Mehta SH, Winters M, et al. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis. 2010;202:845–52.
Farci P. Delta hepatitis: an update. J Hepatol. 2003;39 Suppl 1:S212–9.
Gaeta GB, Stroffolini T, Chiaramonte M, Ascione T, Stornaiuolo G, et al. Chronic hepatitis D: a vanishing Disease? An Italian multicenter study. Hepatology. 2000;32:824–7.
Taylor JM. Hepatitis delta virus. Virology. 2006;344:71–6.
Weiner AJ, Choo QL, Wang KS, Govindarajan S, Redeker AG, et al. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988;62:594–9.
Lai MM. RNA replication without RNA-dependent RNA polymerase: surprises from hepatitis delta virus. J Virol. 2005;79:7951–8.
Greco-Stewart VS, Schissel E, Pelchat M. The hepatitis delta virus RNA genome interacts with the human RNA polymerases I and III. Virology. 2009;386:12–5.
Radjef N, Gordien E, Ivaniushina V, Gault E, Anais P, et al. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol. 2004;78:2537–44.
Makuwa M, Caron M, Souquiere S, Malonga-Mouelet G, Mahe A, et al. Prevalence and genetic diversity of hepatitis B and delta viruses in pregnant women in Gabon: molecular evidence that hepatitis delta virus clade 8 originates from and is endemic in central Africa. J Clin Microbiol. 2008;46:754–6.
Shakil AO, Hadziyannis S, Hoofnagle JH, Di Bisceglie AM, Gerin JL, et al. Geographic distribution and genetic variability of hepatitis delta virus genotype I. Virology. 1997;234:160–7.
Parana R, Kay A, Molinet F, Viana S, Silva LK, et al. HDV genotypes in the Western Brazilian Amazon region: a preliminary report. AmJTrop Med Hyg. 2006;75:475–9.
Wu JC, Chiang TY, Sheen IJ. Characterization and phylogenetic analysis of a novel hepatitis D virus strain discovered by restriction fragment length polymorphism analysis. J Gen Virol. 1998;79(Pt 5):1105–13.
Su CW, Huang YH, Huo TI, Shih HH, Sheen IJ, et al. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology. 2006;130:1625–35.
Niro GA, Casey JL, Gravinese E, Garrubba M, Conoscitore P, et al. Intrafamilial transmission of hepatitis delta virus: molecular evidence. J Hepatol. 1999;30:564–9.
Manock SR, Kelley PM, Hyams KC, Douce R, Smalligan RD, et al. An outbreak of fulminant hepatitis delta in the Waorani, an indigenous people of the Amazon basin of Ecuador. AmJTrop Med Hyg. 2000;63:209–13.
Flodgren E, Bengtsson S, Knutsson M, Strebkova EA, Kidd AH, et al. Recent high incidence of fulminant hepatitis in Samara, Russia: molecular analysis of prevailing hepatitis B and D virus strains. J Clin Microbiol. 2000;38:3311–6.
Heidrich B, Beatriz SC, Idilman R, Kabacam G, Bremer B, et al. HBeAg-positive hepatitis delta: virological patterns and clinical long-term outcome. Liver Int. 2012;32:1415–25.
Niro GA, Smedile A, Ippolito AM, Ciancio A, Fontana R, et al. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol. 2010;53:834–40.
Romeo R, Del Ninno E, Rumi M, Russo A, Sangiovanni A, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136:1629–38.
Buti M, Homs M, Rodriguez-Frias F, Funalleras G, Jardi R, et al. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat. 2011;18:434–42.
Calle-Serrano B, Grosshennig A, Heidrich B, Jaroszewicz J, Kirschner J, et al. Comparing the long-term outcome of hepatitis delta and HBV monoinfection: Is HDV-infection really worse? J Hepatol. 2011;54:144.
Ji J, Sundquist K, Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:790–2.
Williams V, Brichler S, Radjef N, Lebon P, Goffard A, et al. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J Gen Virol. 2009;90:2759–67.
Pollicino T, Raffa G, Santantonio T, Gaeta GB, Iannello G, et al. Replicative and transcriptional activities of hepatitis B virus in patients coinfected with hepatitis B and hepatitis delta viruses. J Virol. 2011;85:432–9.
Schaper M, Rodriguez-Frias F, Jardi R, Tabernero D, Homs M, et al. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol. 52: 658–64.
Wedemeyer H. Re-emerging interest in hepatitis delta: new insights into the dynamic interplay between HBV and HDV. J Hepatol. 2010;52:627–9.
Mederacke I, Filmann N, Yurdaydin C, Bremer B, Puls F, et al. Rapid early HDV RNA decline in the peripheral blood but prolonged intrahepatic hepatitis Delta antigen persistence after liver transplantation. J Hepatol. 2011.
Mederacke I, Bremer B, Heidrich B, Kirschner J, Deterding K, et al. Establishment of a novel quantitative hepatitis D virus (HDV) RNA assay using the Cobas TaqMan platform to study HDV RNA kinetics. J Clin Microbiol. 2010;48:2022–9.
Soriano V, Puoti M, Bonacini M, Brook G, Cargnel A, et al. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV International Panel. AIDS. 2005;19:221–40.
Zachou K, Yurdaydin C, Drebber U, Dalekos GN, Erhardt A, et al. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver Int. 2010;30:430–7.
Cornberg M, Protzer U, Dollinger MM, Petersen J, Wedemeyer H, et al. Prophylaxis, Diagnosis and Therapy of Hepatitis-B-Virus-(HBV-)Infection: upgrade of the guideline, AWMF-Register 021/011. Z Gastroenterol. 2007;45:525–74.
• EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009; 50: 227–42. The guideline clearly describes the clinical management of patients infected with HBV as well as patients coinfected with HBV and HDV.
Farci P, Mandas A, Coiana A, Lai ME, Desmet V, et al. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med. 1994;330:88–94.
Rosina F, Saracco G, Lattore V, Quartarone V, Rizzetto M, et al. Alpha 2 recombinant interferon in the treatment of chronic hepatitis delta virus (HDV) hepatitis. Prog Clin Biol Res. 1987;234:299–303.
•• Wedemeyer H, Yurdaydin C, Dalekos GN, Erhardt A, Cakaloglu Y, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322–31. This the so far largest clinical trial treating patients chronically infected with HDV with pegylated Interferon. Overall, the SVR rate was 25–30 %.
Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology. 2006;44:728–35.
Lamers MH, Kirgiz OO, Heidrich B, Wedemeyer H, Drenth JP. Interferon-alpha for patients with chronic hepatitis delta: a systematic review of randomized clinical trials. Antivir Ther. 2012;17:1029–37.
Degertekin H, Yalcin K, Yakut M, Yurdaydin C. Seropositivity for delta hepatitis in patients with chronic hepatitis B and liver cirrhosis in Turkey: a meta-analysis. Liver Int. 2008;28:494–8.
Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, et al. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004;126:1740–9.
Gunsar F, Akarca US, Ersoz G, Kobak AC, Karasu Z, et al. Two-year interferon therapy with or without ribavirin in chronic delta hepatitis. Antivir Ther. 2005;10:721–6.
Lau DT, Kleiner DE, Park Y, Di Bisceglie AM, Hoofnagle JH. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology. 1999;117:1229–33.
Niro GA, Ciancio A, Tillman HL, Lagget M, Olivero A, et al. Lamivudine therapy in chronic delta hepatitis: a multicentre randomized-controlled pilot study. Aliment Pharmacol Ther. 2005;22:227–32.
Yurdaydin C, Bozkaya H, Gurel S, Tillmann HL, Aslan N, et al. Famciclovir treatment of chronic delta hepatitis. J Hepatol. 2002;37:266–71.
Yurdaydin C, Bozkaya H, Onder FO, Senturk H, Karaaslan H, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat. 2008;15:314–21.
Niro GA, Ciancio A, Gaeta GB, Smedile A, Marrone A, et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology. 2006;44:713–20.
Kabacam G, Onder FO, Yakut M, Seven G, Karatayli SC, et al. Entecavir treatment of chronic hepatitis D. Clin Infect Dis. 2012;55:645–50.
Jaroszewicz J, Ho H, Markova A, Deterding K, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) decrease and serum interferon-inducible protein-10 levels as predictive markers for HBsAg loss during treatment with nucleoside/nucleotide analogues. Antivir Ther. 2011;16:915–24.
Sheldon J, Ramos B, Toro C, Rios P, Martinez-Alarcon J, et al. Does treatment of hepatitis B virus (HBV) infection reduce hepatitis delta virus (HDV) replication in HIV-HBV-HDV-coinfected patients? Antivir Ther. 2008;13:97–102.
Martin-Carbonero L, Teixeira T, Poveda E, Plaza Z, Vispo E, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25:73–9.
Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73–85.
Serrano BC, Manns MP, Wedemeyer H. Hepatitis delta and HIV infection. Semin Liver Dis. 2012;32:120–9.
Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31.
Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348–56.
Zoutendijk R, Reijnders JG, Zoulim F, Brown A, Mutimer DJ, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2012.
Vietheer PT, Netter HJ, Sozzi T, Bartholomeusz A. Failure of the lamivudine-resistant rtM204I hepatitis B virus mutants to efficiently support hepatitis delta virus secretion. J Virol. 2005;79:6570–3.
Samuel D, Zignego AL, Reynes M, Feray C, Arulnaden JL, et al. Long-term clinical and virological outcome after liver transplantation for cirrhosis caused by chronic delta hepatitis. Hepatology. 1995;21:333–9.
Bordier BB, Ohkanda J, Liu P, Lee SY, Salazar FH, et al. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J Clin Invest. 2003;112:407–14.
Petersen J, Dandri M, Mier W, Lutgehetmann M, Volz T, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–41.
Lutgehetmann M, Mancke LV, Volz T, Helbig M, Allweiss L, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology. 2012;55:685–94.
Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidrich, B., Manns, M.P. & Wedemeyer, H. Treatment Options for Hepatitis Delta Virus Infection. Curr Infect Dis Rep 15, 31–38 (2013). https://doi.org/10.1007/s11908-012-0307-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-012-0307-z