Abstract
The manipulation of the gut microbiota by diet, antibiotics, or probiotics could promote, prevent, or reverse the development of specific diseases, including obesity. A link has been proposed between obesity and the growth promoters (probiotics and antibiotics) that have been used in animals for more than 40 years to induce weight gain. Several species of the Lactobacillus genus that are frequently used as probiotics for human consumption merit particular attention because they are increased in the gut microbiota under high-fat diets, are more abundant in obese humans, and are selected by growth-promoter antibiotics; moreover, the administration of these bacteria in experimental models is linked to the development of obesity. However, other species or strains of the same genus are associated with an antiobesity effect. Newborns and infants are a particularly susceptible population in which the administration of antibiotics or probiotics could be related to the development of obesity in adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the strangest phenomena that one of us (D.R.) has observed in his career, which has included the writing of over 1,500 scientific papers, is the lack of scientific communication between fields. Gut microbiota manipulation is an extreme example of this phenomenon [1, 2]: Despite the widespread manipulation of the gut microbiota in farm animals in all industrialized countries to induce weight gain in these animals (e.g., pigs, calves, and chickens) [3, 4], this practice has been neglected for a long time in the field of medicine [5]. The manipulation of the gut microbiota is achieved in farm animals not only through the use of low doses of antibiotics, but also through probiotics administration, including mainly Lactobacillus, Bifidobacterium, and Enterococcus species.
During the course of our work on Lactobacillus ingluviei [6], we serendipitously discovered that this bacterium induced significant weight gain in chickens [7]. These findings were supported by similar studies of ducks and mice [8, 9]. We believe that this discovery provided the first indications to the scientific community of the existence of a possible link between growth promoters (probiotics and antibiotics) and the current epidemic of obesity [1, 5]. This work has led to vehement reactions from researchers who are funded by the food industry [10].
On the whole, work on probiotics is currently hampered by the fact that the vast majority of probiotics investigators are subsidized or have previously been sponsored by the food industry. In contrast to research in other sectors, such as the pharmaceutical industry, studies in the food industry never inform the public about the conflicts of interest that are associated with this type of financing. Most of the works and symposia that promote the positive effect of probiotics are sponsored by the food industry [11].
The Observed Modifications of the Gut Microbiota in Obesity
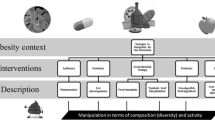
This topic has recently been extensively reviewed [12••]. Ley and Gordon first observed that different digestive microbiota were present in obese subjects than in lean subjects at both the bacterial taxonomic [13] and gene functional [14] levels and determined that the microbiota of obese subjects displayed enhanced energy-harvesting abilities. Numerous subsequent studies have associated particular bacterial genera and species either with obesity (Lactobacillus, Staphylococcus, and Faecalibacterium) or with a lean phenotype (Bacteroidetes, Methanobrevibacter, and Bifidobacterium) (see Fig. 1; Table 1) [12••]. Notably, we linked Bifidobacterium with a lean phenotype in a meta-analysis that included five studies with consistent results from four countries (Finland, Germany, Spain, and China), demonstrating that this type of obesity-associated alteration transcends geographical diversity [12••].
The role of the manipulation of the gut microbiota in obesity. Specific bacterial clades in the human gut microbiota have been linked with obesity or lean status. Consistent data report that administration of such bacteria through oral probiotics administration could lead to weight change accordingly. Antibiotics have been linked with weight gain plausibly by the perturbation of the established gut microbiota increasing obesity-associated bacteria and decreasing lean-associated bacteria
Within the Lactobacillus genus, different species are associated with an obese profile (Lactobacillus reuteri) or a lean profile (Lactobacillus gasseri and Lactobacillus plantarum) such that the microbiota composition is related to body weight and obesity at the species level, which is extremely relevant for clinical studies and for the management of obesity. Similar to our pioneering work on Lactobacillus [15], other research groups have found correlations between the body mass index (BMI) and the Lactobacillus count by culture [16•], particularly for one bacterial species, L. sakei.
In pregnant women, the physiological increase in adiposity in the third quarter appears to be associated with a profound change in the digestive microbiota [17]. Santacruz et al. [18] reported that pregnant women with excessive weight gain had digestive microbiota that were enriched in Staphylococcus, Enterobacteriaceae, and Escherichia coli but depleted in Akkermansia muciniphila and Bifidobacterium. In this study, the Bifidobacterium genus was again associated with a statistically significant protective role against overweight and obese tendencies.
By contrast, Arumugam and the MetaHIT consortium [19] did not report any association between obesity and newly identified bacterial taxonomic enterotypes; however, their study was not designed to elucidate these types of correlations. Employing the same approach, Fraser et al. [20] identified three communities of interacting bacteria in the Old Order Amish sect that were similar to the three enterotypes that were described by Arumugam. Regression analyses for phenotype clusters found significantly different BMI among these enterotypes with a very significant p-value for network effect (Supplemental Table 2 of [20]).
The Effects of Diet
Diet drastically changes the diversity and composition of the digestive microbiota [21]. Ley et al. demonstrated that a low-calorie diet increased the proportion of Bacteroidetes in the gut microbiota of obese individuals to a level similar to the Bacteroidetes level of lean controls [13]. Recently, Murphy et al. [22•] found that a high-fat diet was associated with the opposite effect in mice, producing a decrease in Bacteroidetes and an increase in Firmicutes, and observed that this change corresponded more specifically to an increase in Lactobacillus and a decrease in Bacteroides. All of these results favor correlations between Bacteroidetes and lean status and between higher levels of particular Lactobacillus species and obese tendencies. The existence of apparently paradoxical significant results that indicate a correlation between a high-fat diet and decreased Lactobacillus levels [23, 24] does not contradict the role of Lactobacillus in the regulation of weight but stresses the importance of species-level analyses of the Lactobacillus in the microbiota [25, 26].
A structural resilience of the gut microbiota under high-fat dietary perturbations has been reported [27]; thus, it is unlikely that a diet that is devoid of probiotics or antibiotics can irreversibly alter the gut microbiota. However, many "natural" probiotics that are present in normal human food shape the digestive microbiota. In particular, lactobacilli are important in the production of foods that require lactic acid fermentation, such as dairy products (yogurt and cheese), fermented vegetables (olives, pickles, and sauerkraut), fermented meats (salami), and sourdough bread [28].
Gut Microbiota Transplantation
The most convincing evidence of causality between gut microbiota and obesity is the observation that obese phenotypes are transmissible by the transplantation of gut microbiota from obese donors; these transplantation experiments were first performed by Ley and Gordon [14, 17, 29]. More recently, Vijay-Kumar et al. [30••] found that the transplantation of the microbiota of obese TLR5-KO mice into axenic WT mice conferred many aspects of the donor phenotype, including hyperphagia, obesity, hyperglycemia, insulin resistance, colomegaly, and a high level of proinflammatory cytokines. This result suggests that the modification of the intestinal flora is a contributing factor in the development of obesity.
Antibiotics
Antibiotics have been used for decades as growth promoters [3, 4, 31] and are used in humans for the treatment of malnutrition [32]. In the 1950s, pioneering studies showed that administration of tetracyclines in premature infants [33] and young recruits of the U.S. Navy [34] was associated with weight gain. In patients with cystic fibrosis, long-term prescriptions of minocycline [35] or azithromycin [36–38] have been associated with weight gain. Other antibiotics have been associated with weight gain in humans, such as erythromycin [39], cotrimoxazole [40], or clarithromycin [41]; the last of these drugs is associated with acquired obesity in the context of eradicating Helicobacter pylori. Recently, Cho et al. [42] demonstrated that minor doses of antibiotics may produce the same type of growth-promoting phenomena in mice. Penicillin and vancomycin have both been tested in this context; these antibiotics cause mice to experience a change in their gut microbiota that is associated with weight and adiposity gains. Interestingly, the number of Lactobacillaceae sequences tripled in the group that received antibiotics. The same research group found that antibiotics-receiving infants are larger than controls [43••]. We reported that weight gain was observed in patients who were receiving vancomycin [44]; this molecule is similar to avoparcin, which is heavily used as a growth promoter in the farm industry. These data, in combination with the fact that the microbiota of obese individuals may be enriched in lactobacilli [15] (which are among the gram-positive bacteria that are resistant to vancomycin), strongly suggest that antibiotics may impact the metabolic phenotype through the pervasive perturbation of gut microbiota [45]; in particular, growth-promoter antibiotics appear to increase the prevalence of specific bacterial species that have been associated with obesity and to decrease bacterial species linked to a lean status.
Probiotics
A number of probiotics have been used for more than 40 years, with a European regulation on their use, to fatten animals, particularly young pigs. The existing literature regarding probiotics has recently been subjected to a meta-analysis that indicates that certain microbes are likely to cause weight gain in humans and animals, such as Lactobacillus acidophilus, Lactobacillus fermentum, L. ingluviei, and perhaps L. reuteri [25, 26]. In animals, studies have revealed that L. reuteri can increase weight gain in situations in which growth depression is caused by a lack of dietary protein and not by contagious disease [46]. This observation raises the possibility that L. reuteri improves the intestines’ ability to absorb and process nutrients and increase food conversion. Moreover, Nahashon et al. [47] reported that feeding laying Leghorns with Lactobacillus significantly improved the retention of fat and produced increased cellularity of the Peyer’s patches of the ileum. In an experimental model, we demonstrated that young mice fed with two doses of L. ingluviei experienced not only a weight gain, but also an increase in fat and the presentation of a metabolic syndrome profile [9]. In humans, bottle-fed infants receiving an L. acidophilus strain exhibited significant weight gain, as compared with controls [48].
By contrast, certain strains or species, such as L. gasseri and L. plantarum, appear to have a protective effect against obesity [26]. In a randomized controlled trial, Kadooka found that the oral administration of L. gasseri in adults with obese tendencies resulted in weight loss [49••], whereas in animal models involving a high-fat diet, L. plantarum resulted in weight loss through the secretion of conjugated linoleic acid [50].
It is notably difficult for allochthonous microbes introduced into a stable ecosystem to establish themselves, particularly if members of the same bacterial genus are previously established. This phenomenon is referred to as “competitive exclusion” and is the reason that a probiotic strain is frequently detectable in the gut only while the continued consumption of a probiotic product is occurring [28]. Conversely, Tannock reported that L. reuteri persist at constant population levels throughout life in the guts of formerly Lactobacillus-free mice that were inoculated by mouth with a pure culture on a single occasion [28]. In humans, Abrahamsson et al. [51•] demonstrated that the administration of L. reuteri to the mother and newborn during the first year of life was associated with a significant increase in the gut microbiota concentration of L. reuteri at 24 months, which was 1 year after the end of the administration period. Unfortunately, the weight of these subjects was not evaluated. These data support a dramatic and pervasive modification of the gut microbiota by probiotics administered in the first months of life. Long-term safety studies must certainly be conducted to assess whether Lactobacillus probiotics in newborns may favor obesity in adulthood.
Conclusion
Since 2006, when the first scientific studies on the metagenomic analysis of the human microbiota found a gut microbiota alteration that was associated with obesity [13, 14], a link has been proposed between the growth promoters that have been used for more than 40 years to induce weight gain in farm animals and the occurrence of continuous weight gain in humans. It appears necessary for the scientific community to analyze humans via prospective epidemiological studies. The role of products used in the food industry to fatten animals must be clarified to determine whether these products cause the same effects in humans. Several Lactobacillus species that are frequently used as probiotics for human consumption, typically in certain yogurts and juices [52], deserve special attention because they are increased in the gut microbiota of mice with high-fat-diet-induced obesity, they are found more frequently and abundantly in obese humans, they are selected by antibiotics inducing weight gain in animals and humans, and their administration in experimental models is linked to weight gain. These effects vary greatly for different species and strains of Lactobacillus, and the species- and strain-dependent nature of these effects will be of importance in future studies (see Fig. 1; Table 1). Finally, newborns and infants appear to be a particularly susceptible population in which the manipulation of the developing gut microbiota by probiotics and antibiotics could plausibly be linked with acquired obesity.
References
Papers of particular interest, published recently, have been highlighted as:• Of importance •• Of major importance
Raoult D. Human microbiome: take-home lesson on growth promoters? Nature. 2008;454:690–1.
Raoult D. The globalization of intestinal microbiota. Eur J Clin Microbiol Infect Dis. 2010;29:1049–50.
Pérez Guerra N, Pastrana Castro L. Probiotics: production, evaluation and uses in animal feed. Biotechnol Appl Biochem, Kerala, India.2009.ISBN: 978-81-308-0323-4.
Tannock GW. Probiotics a critical review. Wymondham: Horizon Scientific Press; 1999. ISBN 1-898486-15-8.
Raoult D. Probiotics and obesity: a link? Nat Rev Microbiol. 2009;7:616.
Merhej V, Armougom F, Robert C, Raoult D. Genome sequence of Lactobacillus ingluviei, bacterium associated with weight gain in animals. J Bacteriol. 2012;194:5697.
Khan M, Raoult D, Richet H, et al. Growth-promoting effects of single-dose intragastrically administered probiotics in chickens. Br Poult Sci. 2007;48:732–5.
Angelakis E, Raoult D. The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS One. 2010;5:e10463.
Angelakis E, Bastelica D. Ben Amara A et al. An evaluation of the effects of Lactobacillus ingluviei on body weight, the intestinal microbiome and metabolism in mice. Microb Pathog. 2012;52:61–8.
Ehrlich SD. Probiotics - little evidence for a link to obesity. Nat Rev Microbiol. 2009;7:901.
Intestinal Networks in Health and Disease. Nature. 2012;474:297–336.
•• Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91–109. A review linking gut microbiota and obesity with a meta-analysis reporting a consistent statistical anti-obesity effect of bifidobacteria.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Armougom F, Henry M, Vialettes B, et al. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:e7125.
• Stsepetova J, Sepp E, Kolk H, et al. Diversity and metabolic impact of intestinal Lactobacillus species in healthy adults and the elderly. Br J Nutr. 2011;105:1235–44. Another team reporting a link between gut microbiota Lactobacillus and human weight.
Koren O, Goodrich JK, Cullender TC, et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell. 2012;150:470–80.
Santacruz A, Collado MC, Garcia-Valdes L, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92.
Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80.
Zupancic ML, Cantarel BL, Liu Z, et al. Analysis of the gut microbiota in the old order amish and its relation to the metabolic syndrome. PLoS One. 2012;7:e43052.
Ravussin Y, Koren O, Spor A, et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring). 2012;20:738–47.
• Murphy EF, Cotter PD, Hogan A, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2012 Aug 9. epub ahead of print. High-fat diet induces a significant increase in Firmicutes and particularly Lactobacillus in mice.
Lam YY, Ha CW, Campbell CR, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233.
Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81.
Million M, Maraninchi M, Henry M, et al. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes (Lond). 2012;36:817–25.
Million M, Angelakis E, Paul M, et al. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100–8.
Zhang C, Zhang M, Pang X, et al. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012 Apr 12. Epub ahead of print.
Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004;70:3189–94.
Turnbaugh PJ, Backhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23.
•• Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. This study confirmed that gut transplantation led to transmission of obese traits (adiposity) suggesting that modification of the intestinal flora is a contributing factor in the development of obesity.
Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–94.
Dubray C, Ibrahim SA, Abdelmutalib M, et al. Treatment of severe malnutrition with 2-day intramuscular ceftriaxone vs 5-day amoxicillin. Ann Trop Paediatr. 2008;28:13–22.
Robinson P. Controlled trial of aureomycin in premature twins and triplets. Lancet. 1952;259:52.
Haight TH, Pierce WE. Effect of prolonged antibiotic administration of the weight of healthy young males. J Nutr. 1955;56:151–61.
Patterson PR. Minocycline in the antibiotic regimen of cystic fibrosis patients: weight gain and clinical improvement. Clin Pediatr [Phila]. 1977;16:60–3.
Pirzada OM, McGaw J, Taylor CJ, Everard ML. Improved lung function and body mass index associated with long-term use of Macrolide antibiotics. J Cyst Fibros. 2003;2:69–71.
Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2011;12:CD002203.
Saiman L, Mayer-Hamblett N, Anstead M, et al. Open-label, follow-on study of azithromycin in pediatric patients with CF uninfected with Pseudomonas aeruginosa. Pediatr Pulmonol. 2012;47:641–8.
Mansi Y, Abdelaziz N, Ezzeldin Z, Ibrahim R. Randomized controlled trial of a high dose of oral erythromycin for the treatment of feeding intolerance in preterm infants. Neonatology. 2011;100:290–4.
Garly ML, Bale C, Martins CL, et al. Prophylactic antibiotics to prevent pneumonia and other complications after measles: community based randomised double blind placebo controlled trial in Guinea-Bissau. BMJ. 2006;333:1245.
Lane JA, Murray LJ, Harvey IM, et al. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:922–9.
Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6.
•• Trasande L, Blustein J, Liu M, et al. Infant antibiotic exposures and early-life body mass. Int J Obes [Lond]. 2012 Aug 21. Epub ahead of print. A study that confirm the hypothesized link between antibiotic in early infancy and overweight in childhood.
Thuny F, Richet H, Casalta JP, et al. Vancomycin treatment of infective endocarditis is linked with recently acquired obesity. PLoS One. 2010;5:e9074.
Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280.
Dunham H, Casas I, Edens F, et al. Avian growth depression in chickens induced by environmental, microbiological, or nutritional stress is moderated by probiotic administrations of Lactobacillus reuteri. Biosci Microflora. 1998;17:133–9.
Nahashon SN, Nakaue HS, Snyder SP, Mirosh LW. Performance of single comb White Leghorn layers fed corn-soybean meal and barley-corn-soybean meal diets supplemented with a direct-fed microbial. Poult Sci. 1994;73:1712–23.
Robinson EL, Thompson WL. Effect on weight gain of the addition of Lactobacillus acidophilus to the formula of newborn infants. J Pediatr. 1952;41:395–8.
•• Kadooka Y, Sato M, Imaizumi K, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–43. The first randomized control trial linking Lactobacillus administration and a significant anti-obesity effect in humans.
Lee K, Paek K, Lee HY, et al. Antiobesity effect of trans-10, cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol. 2007;103:1140–6.
• Abrahamsson TR, Sinkiewicz G, Jakobsson T, et al. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J Pediatr Gastroenterol Nutr. 2009;49:349–54. Study establishing that probiotic administration during infancy lead to protracted alteration of the gut microbiota in humans.
Nagpal R, Kumar A, Kumar M, et al. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012 May 9. Epub ahead of print.
Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes. 2010;1:279–84.
Patrone V, Ferrari S, Lizier M, et al. Short-term modifications in the distal gut microbiota of weaning mice induced by a high-fat diet. Microbiology. 2012;158:983–92.
Klare I, Konstabel C, Werner G, et al. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J Antimicrob Chemother. 2007;59:900–12.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Million, M., Raoult, D. The Role of the Manipulation of the Gut Microbiota in Obesity. Curr Infect Dis Rep 15, 25–30 (2013). https://doi.org/10.1007/s11908-012-0301-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-012-0301-5