Abstract
Purpose of Review
In this review, we discuss the role of perivascular adipose tissue (PVAT) in the modulation of vascular contractility and arterial pressure, focusing on the role of the renin-angiotensin-aldosterone system and oxidative stress/inflammation.
Recent Findings
PVAT possesses a relevant endocrine-paracrine activity, which may be altered in several pathophysiological and clinical conditions. During the last two decades, it has been shown that PVAT may modulate vascular reactivity. It has also been previously demonstrated that inflammation in adipose tissue may be implicated in vascular dysfunction. In particular, adipocytes secrete a number of adipokines with various functions, as well as several vasoactive factors, together with components of the renin-angiotensin system which may act at local or at systemic level. It has been shown that the anti-contractile effect of PVAT is lost in obesity, probably as a consequence of the development of adipocyte hypertrophy, inflammation, and oxidative stress.
Summary
Adipose tissue dysfunction is interrelated with inflammation and oxidative stress, thus contributing to endothelial dysfunction observed in several pathological and clinical conditions such as obesity and hypertension. Decreased local adiponectin level, macrophage recruitment and infiltration, and activation of renin-angiotensin-aldosterone system could play an important role in this regard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Potential Role of PVAT in the Modulation of Arterial Pressure
The majority of blood vessels are surrounded by variable amounts of perivascular adipose tissue (PVAT). For many years, PVAT was routinely removed from arteries during in vitro studies in order to simplify the experiments on isolated blood vessels. However, due to the increasing evidence of the role of the adipose tissue as an endocrine organ [1] and of the close proximity of PVAT to a vast network of blood vessels, investigators have begun to study the effects of PVAT (Table 1).
In 1991, Soltis and Cassis were the first to test the hypothesis that PVAT might be important for vascular regulation [2••]. They demonstrated the presence of a diminished response to noradrenaline in intact arteries compared with vessels having PVAT removed in isolated aortic ring preparations from rats. They attributed this effect to the dense sympathetic innervation in PVAT [2••]. Ten years later, the interest in PVAT was renewed by Lohn et al. [3]. They demonstrated that the vasocontractile response with angiotensin II (Ang II), serotonin, and phenylephrine was 95, 80, and 30% lower, respectively, after removal of PVAT [3]. Furthermore, this effect was independent of nitric oxide (NO) formation and appeared to be mediated by a substance released from intact aortic rings surrounded with fat into the organ bath since transferral of bath solution from vessels with fat or cultured rat adipocytes to vessels without fat resulted in rapid relaxation of pre-contracted arteries. The factor involved was considered to be more likely a protein than a lipid, as it was not adsorbed by essentially fatty acid-free serum albumin and it was inactivated by heating. Therefore, the authors concluded that “adventitium-derived relaxing factor” (ADRF) was released by PVAT [3].
ADRF release was shown to depend on extracellular calcium and can be modulated by changes in calcium concentration in the plasma in vivo [4]. Furthermore, ADRF effect is regulated by intracellular tyrosine kinase, protein kinase A, and voltage-dependent smooth muscle potassium channel but not by NO, cytochrome P450 pathways, or prostaglandins [4]. We now know that the relaxing factor is released from the adipose tissue and not from the tunica adventitia [9] and therefore we can use the term PVAT-derived relaxing factor (PDRF). Subsequent studies confirmed the role of PVAT as an inhibitor of arterial contraction in internal thoracic arteries [7] and in human internal mammary arteries [5]. The mechanism of the vasodilatory capacity of PVAT varies depending on anatomical location and animal species. In rat aorta, PDRF appears to cause vasorelaxation by opening ATP-dependent K+ (KATP) channels in vascular smooth muscle cell [4], while in rat mesenteric arteries, voltage-dependent K+ channels (Kv) seem to be involved [6]. In a recent study, Gao et al. identified two different pathways for explaining the PVAT relaxation on rat aorta. They described an endothelium-dependent and endothelium-independent mechanism, working through a release of nitric oxide with calcium sensing K+ channels activation (KCa) [9] and hydrogen peroxide with soluble guanylate cyclase activation [9], respectively. Several studies both in vitro and in vivo have confirmed the anti-contractile property of PVAT, but despite several candidates being proposed, the real identity of PDRF is partly unknown.
Candidates for PDRF
Leptin
Leptin has been proposed as PDRF because of its direct vasodilator effect by release of nitric oxide with endothelium-dependent mechanism [8, 15]. However, leptin possesses also contractile effects by activation of the sympathetic nervous system and several other effects such as increase in vasoconstrictor endothelin-1 [16] and in toxic reactive oxygen species (ROS) [17]. Therefore, leptin may contribute to arterial pressure homeostasis and to the anti-contractile effect of PVAT, but its role as PDRF seems to be unlikely.
Adiponectin
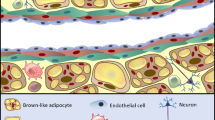
The PVAT has been shown to secrete other vasodilator products like hydrogen peroxide [18] and adiponectin [19, 20]. In particular, adiponectin is a protein secreted exclusively by adipose tissue [21] and has been reported to possess several protective properties against hypertension [22,23,24], atherosclerosis [25], and diabetes [26]. Several studies have demonstrated that adiponectin acts as a vasodilator by activation of endothelial NO synthase (eNOS) and consequent increase of NO production [26]. It has the ability to inhibit macrophage activation, to reduce proliferation of vascular smooth muscle cells, to improve insulin signaling pathways, and to reduce ROS levels [27, 28]. Hypoadiponectinemia has been correlated to endothelial dysfunction [29], obesity [30], hypertension [23], atherosclerosis [24], type 2 diabetes [25], and myocardial infarction. In particular, it has been shown that in patients with coronary artery disease, epicardial tissue expresses lower adiponectin levels [31]. Recent studies have demonstrated adiponectin-mediated anti-contractile properties of PVAT in human small arteries, which represent the main regulators of peripheral resistance and hence of blood pressure [11••]. In particular, in that study, it has been demonstrated that the anti-contractile function of PVAT disappears in healthy human arteries after blockade of adiponectin receptor 1 [11••]. Considering its beneficial and protective effects, adiponectin could be an important therapeutic target. Therefore, adiponectin might represent a reliable candidate for the role of PDRF. Aside from vasodilator products, PVAT has also been reported to secrete vasoconstrictor peptides like endothelin and Ang II [32] (Fig. 1).
Hormones and other substances secreted by adipose tissue. Abbreviations: ASP, acylation stimulating protein; IL-6, interleukin 6; PAI-1, plasminogen activator inhibitor type 1; TF, tissue factor; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α. Diagram modified from [33]
Inflammatory Cytokines
The adipokines group also includes proinflammatory cytokines (such as interleukin 6 (IL-6) and tumor necrosis factor (TNF-α)), chemokines (like interleukin 8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1)), growth factors (transforming growth factor-β (TGF-β)), and pro-thrombotic factors (e.g., plasminogen activator inhibitor type 1 (PAI-1)).
There is a compelling evidence for an increased level of adipokines in inflammatory state, such as in obesity, and of a decreased cardiometabolic protective role of adiponectin. These obesity-related changes in adipose tissue are probably linked to the development of insulin resistance, type 2 diabetes mellitus, hypertension and, thus, metabolic syndrome [34,35,36,37].
In addition to this emergent role of PVAT as modulator of arterial tone, in a recent study, it has been suggested that PVAT may act also as a regulator of vein function. In particular, PVAT is able to reduce contractile responses of the inferior vena cava to various agonists mediated by angiotensin (1–7) through activation of Kv channels [14].
Therefore, the PVAT may play a role as dual modulator of vascular tone.
It is clear that adipose tissue and in particular PVAT is a secretory and endocrine organ which is involved in the regulation of vascular tone. PVAT might play an important role in the modulation of blood pressure, in the hemodynamic homeostasis, and in other conditions associated to vascular dysfunction such as atherosclerosis, obesity, and type 2 diabetes mellitus. This suggests that a balance between adipose tissue-derived vasodilator and vasoconstrictor mediators might be extremely important for the maintenance of an appropriate vascular tone.
The Adipose Tissue and the Renin-Angiotensin Axis
The renin-angiotensin system (RAS) is involved in systemic blood pressure regulation and in renal electrolyte homeostasis. Several studies have demonstrated the presence of local RAS activity in both white and brown perivascular adipose tissues [38•].
A number of animal studies have demonstrated the expression of most of the RAS system components in adipose tissue [39]; however, data from human tissues are relatively few [40] (Fig. 2).
Perivascular adipose tissue and local renin-angiotensin system [41•]
Angiotensinogen (AGT) expression in adipose tissue was first identified in periaortic brown adipose tissue in 1987 [42]. Subsequently, AGT secretion and AGT-mRNA were detected in rat and human adipose tissue depots, in adipocytes isolated from rat arterial vessels walls and mesentery [43], in primary cultured human adipocytes [12], and in differentiating human pre-adipocytes [44]. Thus, the AGT expression and secretion is considered a late marker of adipocyte differentiation [45]. Various studies have demonstrated that AGT expression is higher in visceral compared with subcutaneous adipose tissue in rats [46] and humans [47]. In addition, white adipose tissue is considered to be one of the major sources of angiotensinogen outside the liver [48]. The AGT activation by free fatty acids and overfeeding provides a possible link between adipose-tissue RAS, obesity/insulin resistance, and obesity/hypertension.
Although renin activity has been detected in brown adipose tissue [49], Gàlvez-Prieto et al. showed the absence of renin expression in mesenteric and aortic PVAT from male Wistar Kyoto rats [39]. Nevertheless, they confirmed the presence of the (pro)renin receptor which may act with a circulating enzyme to increase local synthesis of angiotensin I from AGT. The peroxisome proliferator-activated receptors (PPARs), of which two isoforms (α and γ) have been identified in PVAT, have both been shown to positively regulate renin transcription [50]. This receptor has been detected in subcutaneous and visceral human adipose tissues [51].
Ang II is the major component of RAS with a variety of physiological actions including vasoconstriction, stimulation of aldosterone release from the adrenal gland, renal sodium and water reabsorption, increase of blood pressure, cell growth, inflammation process, vascular and cardiac remodeling, activation of the sympathetic nervous system, and ROS production [52]. In the adipose tissue, Ang II may play a role in adipocyte growth and differentiation, stimulating lipogenesis, pre-adipocytes recruitment, and their differentiation in mature adipocytes [41•].
The exact role of Ang II in the differentiation and proliferation of human adipocytes remains controversial; it has been shown that pre-adipocytes from human adipose tissue express a functional RAS [53]. Sharma hypothesized that Ang II may be able to inhibit the adipogenic differentiation of primary cultured pre-adipocytes in humans [54]. Ang II levels in mesenteric adipose tissue are higher than in periaortic adipose tissue [39], demonstrating a regional different expression of the RAS.
The effects of Ang II are mediated by two main membrane receptors: the angiotensin type 1 receptor (AT1R) and the angiotensin type 2 receptor (AT2R). Matsushita et al. demonstrated that Ang II stimulated differentiation of adipocytes through AT1R and inhibited it through AT2R [55]. The AT1R is responsible for most biological effects of Ang II, such as pressure, trophic, and pro-inflammatory effects, whereas the AT2R antagonizes several AT1R-mediated effects. It has been demonstrated that Ang II through AT1R stimulates transcription factor expression, which may promote adipocyte differentiation and increase of triglycerides content in human adipocyte culture and in 3T3-L1 cells, thus leading to adipocyte hypertrophy [56]. The crucial role of AT1R is confirmed by the demonstration that, in essential hypertensives patients, in adipocytes in culture and in a rat model of type 2 diabetes, AT1R antagonist is able to increase adiponectin concentrations and improve insulin sensitivity [57, 58]. In addition, it has been demonstrated that telmisartan, a selective AT1R blocker, increases adiponectin level and reduces myocardial damage in infarcted Zucker diabetic fatty rat models [59]. It has already been shown that plasma adiponectin levels were significantly increased in patients with hypertension after treatment with telmisartan [60]. A previous study had also shown that treatment with the AT1R antagonist, olmesartan, in Ang II-infused rats resulted in an increase of adiponectin level [61].
Conversely, the binding between Ang II and its AT2R may determine tissue regeneration [30] and upregulation of adiponectin production in neonatal rat ventricular myocytes [62].
Although the role of Ang II on differentiation and proliferation of adipocytes is still controversial, Ang II-related effects on adipose tissue, with its trophic and pro-inflammatory effects, could play an important role for vascular function and structure and could be implicated in hypertension-related obesity.
Changes of the RAS Axis in Obesity and Hypertension: Contribution of the Tissue RAS of PVAT
Abdominal obesity is an important risk factor for the development of arterial hypertension, insulin resistance, and type 2 diabetes. Abdominal obesity is also associated with metabolic and morphological abnormalities including sodium retention, endothelial dysfunction, left ventricular hypertrophy, dyslipidemia, hyperinsulinemia, microalbuminuria, elevated markers of inflammation, and oxidative stress [63].
Increasing evidence suggests a link between renin-angiotensin-aldosterone system (RAAS) and other components of the metabolic syndrome, in particular abdominal obesity. A Japanese study has shown that oxidative stress, an important mechanism of metabolic dysregulation in obesity, is able to reduce mRNA-AGT levels in adipose tissue of obese and in hypertrophic murine adipocytes [64]. Oxidative stress is closely correlated to RAAS; Ang II is a potent inductor of ROS, and during obesity, systemic and related adipose tissue Ang II levels are increased.
In addition to Ang II, aldosterone is also an important mediator of RAAS effects. In obesity-related hypertension, aldosterone levels are higher and are associated with endothelial dysfunction [65] and inflammation [66]. In a study of 2009, it has been shown that the blockade of mineralcorticoid receptors improves adipocyte dysfunction and insulin resistance in a murine model of obesity [67]. A recent review discusses the known interaction between adiponectin and aldosterone, demonstrating an inverse relationship [68]. Several effects of aldosterone are mediated through pathways similar to those induced by Ang II. Furthermore, recent data demonstrate the presence of cross-talk between aldosterone and Ang II which modulates and stimulates the signaling transduction such as the Ang II effects on PVAT [69].
The tissue RAS of PVAT may operate in combination or independently of circulating RAS. The precise function of RAS in perivascular adipose tissue is still controversial but may contribute to vascular tone, structural and functional alterations, especially in pathological conditions such as hypertension and obesity, amplifying the effect of systemic RAS. It has been hypothesized that cross-talk between smooth muscle cells and endothelial cells which could explain at least in part the implication of tissue RAS in the perivascular adipose tissue on structural alterations of small resistance arteries.
In view of the relevant role of the RAS system and PVAT on vascular function, it is interesting to understand the possible relationship between them.
In 2006, Gàlvez and colleagues observed several changes in PVAT function and mass in spontaneously hypertensive rats (SHR) which could contribute to increased vascular resistance [8]. In the SHR model, PVAT has a reduced anti-contractile effect, being the mass of mesenteric PVAT less than in control mice with smaller adipocytes and lower leptin content [8]. Subsequently, Lee demonstrated that the changes on PVAT structure and function were observed also in Ang II-induced hypertension in adult male Wistar normotensive rats [12]. In addition, it has also been shown that Ang II is able to mediate PVAT-associated increase of contractile response to perivascular neuronal excitation by EFS, possibly through superoxide production [70]. Recently, the same author confirmed the reduced anti-contractile function of PVAT in SHR [71] and this functional impairment could involve angiotensin (1–7) [71]. In fact, angiotensin (1–7) blockade is able to inhibit the anti-contractile effect of PVAT [13]. Ang 1–7 has also been implicated on anti-contractile function showed by PVAT on inferior vena cava [14].
In a study of 2011, in a mouse model, we demonstrated that PVAT in small mesenteric arteries loses its anti-contractile effect, after stimulation with two physiological stimuli of inflammation: aldosterone and hypoxia [72••].
In that study, we demonstrated that the presence and activation of macrophages in adipose tissue was a key modulator of the increased in contractility in arteries with PVAT following induction of inflammation. Among multiple factors that may be involved in determining the vascular consequences of obesity and hypertension, we have shown a favorable effect of eplerenone in inducing a reduction of the inflammatory effects of both aldosterone and hypoxia [72••]. This property of mineralcorticoid receptor blockers may be of potential therapeutic interest. In a recent study, we demonstrated that an ACE inhibitor and an AT1R blocker also have similar effects on anti-contractile properties of the perivascular adipose tissue following hypoxia [73•]. In particular, we have shown that hypoxia induced a loss of this anti-contractile effect which could be completely prevented with captopril or telmisartan. Therefore, our in vitro induction of a hypoxic environment can simulate the loss of anti-contractile perivascular adipose tissue function seen in vivo in obese patients, and this can be prevented using inhibitors of the renin-angiotensin cascade; RAS blockade may protect the vasculature and may reverse the effect of perivascular and systemic RAS.
The Role of Oxidative Stress and Inflammation in PVAT Function
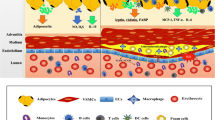
It is well known that vascular dysfunction is mediated by adipose tissue inflammation which could be contrasted by anti-inflammatory agents such as adiponectin. Recently, a review from Huang Cao et al. described the important role of PVAT on regulation of vascular tone underlining the importance of the functional integrity of PVAT, rather than the amount of PVAT itself, because it is essential for the control of blood pressure and it is protective against the development of hypertension and cardiovascular disease [74•]. In particular, in lean conditions, the PVAT is able to secrete several adipokines which act on endothelial cells through a nitric oxide-dependent mechanism and directly on small muscle cells to induce vasorelaxation of the vessel wall. Instead, during obesity, the secretory profile of PVAT change with a reduction of expression of vasorelaxing factors and an increase in vasoconstricting factors with cell infiltration leading to inflammatory state [74•]. The cause of the onset of inflammation still remains unknown although a recent hypothesis is related to hypoxia; in obesity, adipocytes become hypertrophic leading to inadequate perfusion and consequent local hypoxia. Hypoxia-inducible factor (HIF-1 α) alpha is a key mediator of hypoxia. Its levels are higher in adipose tissue of obese subjects, and they decrease after weight loss [75]. HIF-1 α is a main hypoxia-inducible transcription factor responsible for increased inflammatory mediator production, like TNF-α and IL-6, and for reduced adiponectin level [76]. Greenstein et al. have shown that the application of TNF-α and IL-6 to PVAT around healthy blood vessels decreased the dilator effect of PVAT. Similarly, it has been shown that the induction of experimental hypoxia for 2.5 h causes inflammation and loss of anti-contractile function of PVAT. Both conditions could be reversed and the anti-contractile property rescued by catalase and superoxide dismutase or cytokine antagonists [11••]. From these observations, it is clear that PVAT loses its dilating effect after the increase of oxidative stress.
In addition to hypoxia, there are also different HIF activators such as growth factors, cytokines, and hormones. In particular, it has been demonstrated that in vascular smooth muscle cells, Ang II is a potent activator of HIF-1 α by mechanisms even different from hypoxia, involving also mitochondrial reactive oxygen species (mtROS) [77, 78]. As previously mentioned, it has been shown that hypoxia and aldosterone can reproduce the effect of an inflammatory phenotype in adipose tissue, with loss of anti-contractile property [72••]. The macrophage activation is responsible for both conditions because free radical scavengers are able to rescue the anti-contractile function of adipose tissue [72••]. In conclusion, inflammation and oxidative stress are interrelated and associated with adipose tissue dysfunction, and they could contribute to arterial stiffness and hypertension [79•, 80], maybe through endothelial dysfunction (Fig. 3). Macrophages in adipose tissue represent a key modulator of oxidative stress and systemic inflammation through the production of IL-6 and ROS [81]. The important role of IL-6 and MCP-1 in the recruitment of inflammatory cells, in particular, monocytes and macrophage, and in the pathology of vascular disease during obesity is described in a recent review [74•]. Decreased adiponectin level is involved in the process of macrophage infiltration [82] and is a consequence of inflammation and oxidative stress [81] (Fig. 3).
Therefore, it is clear that adiponectin is one of the key mediators of PVAT function which is altered in obesity. It was demonstrated that the effect of adiponectin on vascular tone is mediated by activation of potassium channel calcium dependent (BKCa channels) on vascular smooth muscle cells and adipocytes and by endothelial mechanisms [83•]: adiponectin hyperpolarizes wild-type arteries but not vessels from BKCa knockout mice. Furthermore, it seems that stimulated release of a hyperpolarizing factor (maybe adiponectin) from mesenteric artery PVAT induces electrical changes that involve myocyte BKCa channels [84]. Thereafter, the intracellular downstream mechanisms which involve cGMP-dependent protein kinase (PKG) as a regulator of normal PVAT function was described [85•]. Recently, the involvement of BKCa channels in anti-contractile function of perivascular adipose tissue was confirmed [86]. In particular, it has been shown that the anti-contractile effect of PVAT completely disappeared after pre-incubation with iberiotoxin, a scorpion toxin that inhibits BKCa channels, in ob/ob mice, an animal model of obesity (B6. V-Lepob/OlaHsd) [86]. In that study also, the effects of melatonin, an endogenous hormone with anti-oxidant and vasculoprotective properties, on anti-contractile properties of PVAT were addressed.
The Role of Melatonin in Vascular Dysfunction
Melatonin is an endogenous hormone that exerts anti-oxidant, anti-inflammatory, anti-hyperlipidemic, and anti-hypertensive actions, and it also modulates insulin secretion and action [87].
It has been demonstrated that melatonin is able to improve mesenteric small resistance artery structure and endothelial function in spontaneously hypertensive rats [88]. It has been shown that melatonin therapy is able to improve blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome [89]. Furthermore, chronic administration of melatonin in rats fed a normal or high-fat diet, resulted in significant reduction in body weight, in circulating insulin, glucose, and triglyceride mean levels and was able to modulate the normal circadian pattern of plasma adiponectin [90]. It has also been proposed that melatonin-induced hypertrophy and functional activation of brown adipose tissue may provide a potential target for treatment of obesity in humans [91•]. Therefore, in obesity, melatonin might be able to reduce pro-inflammatory cytokines and to increase adiponectin level with consequent modulation of PVAT function. Recently, it has been demonstrated that prolonged administration of melatonin is able to reduce hyperglycemia associated with obesity in hyperphagic mouse and to ameliorate the inflammation in the perivascular environment [86]. In particular, ob/ob mice treated with melatonin showed a marked reduction in the expression of endothelin-1 (ET-1), IL-6, and metalloproteases 2 and 9; in addition, we observed that the increased expression of both TNF-α and CD68 in visceral fat sections from ob/ob mice was significantly reduced after melatonin treatment. Anti-contractile function of PVAT, partially lost in a model of animal obesity, may be restored by melatonin treatment but only in the presence of an intact PVAT, indicating the importance of PVAT oxidative stress in vascular dysfunction observed in obese animals [86].
Aging represents another vascular dysfunction condition which is associated with a progressive decrease of the nighttime peak of melatonin concentrations. During aging, structural and functional changes have been observed. In particular, the effect of aging on vascular endothelium and small muscle cells (SMC) has been widely investigated, while less is known about the changes of PVAT. A senescence-accelerated prone mouse (SAMP8), a model of age-related vascular dysfunction with associated increase in blood pressure and cognitive decline, has been recently investigated [92, 93]. It has been demonstrated that in SAMP8 mice, there was an overexpression of ET-1, inducible nitric oxide synthase (iNOS), and cyclooxygenase 2 (COX-2), all markers of oxidative stress together with a reduced level of endothelial nitric oxide synthase (eNOS) and cyclooxygenase 1 (COX-1) [94]. In addition, in SAMP8 mice, PVAT had lost its protective anti-contractile effect. Also, long-term treatment with melatonin in SAMP8 was able to increase some vasculoprotective markers, to decrease oxidative stress and inflammation and to restore the anti-contractile effect of perivascular adipose tissue [94]. Decreased expression of adiponectin and adiponectin receptor 1 was also observed in visceral fat of untreated aging mice, whereas a significant increase was observed after melatonin treatment [94]. The increased production/activity of adiponectin might contribute to explain the improvement of the anti-contractile action of perivascular fat observed in the mesenteric small resistance arteries of SAMP8 mice after chronic treatment with melatonin [94].
Conclusions
In conclusion, several evidences suggest a key role of PVAT as regulator of vascular tone through different mechanisms. It is clear that the RAS activity in adipose tissue plays an important role on PVAT function and the correction of the imbalance between the different RAS components is crucial for the maintenance of vascular tone. Besides the known anti-hypertensive effects of ACE inhibitors and AT1R blockers and their effect on reduction of the incidence of new-onset diabetes [95,96,97], they are able to increase circulating levels of adiponectin. In particular, it has been demonstrated that the levels of myocardial adiponectin and its type 1 receptor are decreased in type 2 diabetic rats and that telmisartan treatment may correct these alterations [98]. In addition, melatonin represents a potential therapeutic biological target in clinical conditions such as obesity and for delaying physiological and pathological alteration related to early vascular aging.
Further studies are needed to fully understand the potential implications of perivascular adipose tissue properties on vascular function in humans.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 201025;316(2):129–39.
•• Soltis EE, Cassis LA. Influence of adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13(2):277–96. First demonstration that PVAT might be important for vascular regulation.
Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16(9):1057–63.
Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2003;286(3):H1107–13.
Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, et al. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK (Ca) channels. Circulation. 2003;107(5):769–76.
Verlohren S, Dubrovska G, Tsang S-Y, EEssin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries [comment]. Hypertension. 2004;44(3):271–6.
Gao Y-J, Zeng Z-H, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovas Surg. 2005;130(4):1130–6.
Gálvez B, de Castro J, Herold D, Dubrovska G, Arribas S, González MC, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26(6):1297–302.
Gao YJ, Lu C, Su LY, Sharma AM, Lee RMKW. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. [comment]. Br J Pharmacol. 2007;151(3):323–31.
Malinowski M, Deja MA, Gołba KS, Roleder T, Biernat J, Woś S. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothorac Surg. 2008;33(2):225–31.
•• Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119(12):1661–70. This is the first demonstration of anticontractile effect of perivascular adipose tissue in obese patients.
Lee RM, Ding L, Lu C, Su LY, Gao YJ. Alteration of perivascular adipose tissue function in angiotensin II-induced hypertension. Can J Physiol Pharmacol. 2009;87(11):944–53.
Lee RMKW, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27(4):782–90.
Lu C, Zhao AX, Gao YJ, Lee RM. Modulation of vein function by perivascular adipose tissue. Eur J Pharmacol. 2011;657(1–3):111–6.
Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1.
Quehenberger P, Exner M, Sunder-Plassmann R, Ruzicka K, Bieglmayer C, Endler G, et al. Leptin induces endothelin-1 in endothelial cells in vitro. Circ Res. 2002;90(6):711–8.
Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol. 2008;295(4):H1514–21.
Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57BI/6 mice. Circ J. 2010;74(7):1479–87.
Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54(6):789–96.
Trujillo ME, Scherer PE. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257(2):167–75.
Chow W-S, Cheung BMY, Tso AWK, Xu A, Wat NMS, Fong CHY, et al. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. [comment]. Hypertension. 2007;49(6):1455–61.
Li H-Y, Chiu Y-F, Hwu C-M, Sheu WH-H, Hung Y-J, Fujimoto W, et al. The negative correlation between plasma adiponectin and blood pressure depends on obesity: a family-based association study in SAPPHIRe. [comment]. Am J Hypertens. 2008;21(4):471–6.
• Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43(6):1318–23. This is one of the first study that describes the several protective properties of adiponectin against hypertension.
Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278(4):2461–8.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. [comment]. Nat Med. 2001;7(8):941–6.
Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS pathway. Int J Obes. 2010;34:165–71.
Luo N, Liu J, Chung BH, Yang Q, Klein RL, Garvey WT, et al. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes. 2010;59(4):791–9.
Li FY, Cheng KK, Lam KS, Vanhoutte PM, Xu A. Cross-talk between adipose tissue and vasculature: role of adiponectin. Acta Physiol (Oxf). 2011;203(1):167–80.
Cao Y, Tao L, Yuan YX, Jiao XY, Lau WB, Wang YJ, et al. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46(3):413–9.
Sowers JR. Endocrine functions of adipose tissue: focus on adiponectin. Clin Cornerstone. 2008;9:32–38, discussion 39–40.
Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29(6):251–5.
An SJ, Boyd R, Wang Y, Qiu X, Wang HD. Endothelin-1 expression in vascular adventitial fibroblasts. Am J Physiol Heart Circ Physiol. 2006;290:H700–8.
Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends in Endocrinology & Metabolism. 2000;11(9):237–2.
Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49.
Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9.
Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7.
Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–5.
• Engeli S, Gorzelniak K, Kreutz R, Runkel N, Distler A, Sharma AM. Co-expression of renin-angiotensin system genes in human adipose tissue. Journal of Hypertension. 1999;17:555–60. The study describes the role of the renin-angiotensin system in human adipose tissue.
Gàlvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, et al. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197:55–64.
Goossens GH, Jocken JW, Blaak EE, Schiffers PM, Saris WH, van Baak MA. Endocrine role of the renin-angiotensin system in human adipose tissue and muscle: effect of beta-adrenergic stimulation. Hypertension. 2007;49(3):542–7.
• Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10(2):93–8. This study is a review of the important role of adipose tissue renin-angiotensin system.
Campbell DJ, Habener JF. Cellular localization of AGT gene expression in brown adipose tissue and mesentery: quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology. 1987;121:1616–26.
Cassis LA, Lynch KR, Peach MJ. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988;62:1259–62.
Shiling P, Mallow H, Trindl A, Loffler G. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. Internal Journal Obes Relat Metab Disord. 1999;23:336–41.
Saye J, Linch KR, Peach MJ. Changes in angiotensinogen messenger RNA in differentiating 3T3-F442A adipocytes. Hypertension. 1990;15:867–71.
Hainault I, Nebout G, Ardouin BB, Quignard-Boulangé A. Developmental changes in AGT expression and its secretion in the Zucker rat: adipose tissue-specific effect of FA genotype. Int J Obes Relat Metab Disord. 1998;22(suppl 3):S103. Abstract
Giacchetti G, Faloia E, Sardu C, Mariniello B, Garrapa GGM, Gatti C, et al. Different gene expression of the RAS in human subcutaneous and visceral adipose tissue. Int J Obes Relat Metab Disord. 1999;23(suppl 5):S71. Dent Abstr
Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept. 1993;43:1–20.
Shenoy U, Cassis L. Characterization of renin activity in brown adipose tissue. Am J Phys. 1997;272:C989–99.
Kupiers I, van der Harst P, Navis G, et al. Nuclear hormone receptors as regulators of the renin-angiotensin-aldosterone system. Hypertension. 2008;51:1442–8.
Archard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M. Renin receptor expression in human adipose tissue. Am J Physiol Reg Int Comp Physiol. 2007;292:274–82.
Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72.
Schling P, Mallow H, Trindl A, Löffler G. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. Int J Obes Relat Metab Disord. 1999;23(4):336–41.
Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–11.
Matsushita K, Wu Y, Okamoto Y, Pratt RE, Dzau VJ. Local renin-angiotensin expression regulates human mesenchymal stem cell differentiation to adipocytes. Hypertension. 2006;48:1095–102.
Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology. 1997;138(4):1512–9.
Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, et al. Blockade of the renin–angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81.
Lee MH, Song HK, Ko GJ, Kang YS, Han SY, Han KH, et al. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int. 2008;74:890–900.
Rinaldi B, Di Filippo C, Capuano A, Donniacuo M, Sodano L, Ferraraccio F, et al. Adiponectin elevation by telmisartan ameliorates ischaemic myocardium in Zucker diabetic fatty rats with metabolic syndrome. Diabetes Obes Metab. 2012;14(4):320–8.
Nakamura T, Kawachi K, Saito Y, Saito T, Morishita K, Hoshino J, et al. Effects of ARB or ACE-inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J. 2009;50:501–12.
Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, et al. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism. 2006;55:478–88.
Guo B, Li Y, Han R, Zhou H, Wang M. Angiotensin II upregulation of cardiomyocyte adiponectin production is nitric oxide/cyclic GMP dependent. Am J Med Sci. 2011;341(5):350–5.
Onat A. Metabolic syndrome: nature, therapeutic solutions and options. Expert Opin Pharmacother. 2011;12(12):1887–900.
Okada S, Kozuka C, Masuzaki H, Yasue S, Ishii-Yonemoto T, Tanaka T, et al. Adipose tissue-specific dysregulation of angiotensinogen by oxidative stress in obesity. Metabolism Journal. 2010;59(9):1241–51.
Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond). 2002;103(4):425–31.
Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161(5):1773–81.
Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, et al. Blockade of mineralcorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res. 2009;84:164–72.
Flynn C, Bakris GL. Interaction between adiponectin and aldosterone. Cardiorenal Med. 2011;1(2):96–101.
Lemarié CA, Paradis P, Schiffrin EL. New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J Mol Med. 2008;86(6):673–8.
Lu C, Su LY, Lee RM, Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II. Eur J Pharmacol. 2010;634(1–3):107–12.
Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011;656(1–3):68–73.
•• Withers SB, Agabiti Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA, et al. Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Artherioscler Thromb, Vascular, Biol. 2011;31(4):908–13. This study demonstrates the involvement of macrophage activation in loss of anticontractile function of perivascular adipose tissue.
• Rosei CA, Withers SB, Belcaid L, De Ciuceis C, Rizzoni D, Heagerty AM. Blockade of the renin-angiotensin system in small arteries and anticontractile function of perivascular adipose tissue. J Hypertens. 2015;33(5):1039–45. https://doi.org/10.1097/HJH. This is the first demonstration of restore anticontractile effect of perivascular fat with blockade of the renin-angiotensin system in small arteries
• Huang Cao ZF, Stoffel E, Cohen P. Role of perivascular adipose tissue in vascular physiology and pathology. Hypertension. 2017;69:770–7. This is a recent overview of the role of perivascular adipose tissue in vascular structure and reactivity.
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86.
Chen B, Lam KSL, Wang Y, Wu D, Larn MC, Shen J, et al. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341(2):549–56.
Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell. 2010;21(18):3247–57.
Pagé EL, Robitaille GA, Pouysségur J, Richard DE. Induction of hypoxia-inducible factor-1α by transcriptional and translational mechanisms. J Biol Chem. 2002;277(50):48403–9.
• Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51(6):1651–7. This study shows that inflammation and oxidative stress are interrelated and associated with adipose tissue dysfunction and they could contribute to arterial stiffness and hypertension.
Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38(3):399–403.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61.
Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314(2):415–9.
• FM L, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, et al. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;(6):304, H786–H395. This study is the first demonstration that the mechanism underlies the anticontractile effect of perivascular adipose tissue involves the activation of BK(Ca) channels.
Weston AH, Egner I, Dong Y, Porter EL, Heagerty AM, Edwards G. Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: involvement of myocyte BKCa channels and adiponectin. Br J Pharmacol. 2013;169(7):1500–9.
• Withers SB, Simpson L, Fattah S, Werner ME, Heagerty AM. cGMP-dependent protein kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc Res. 2014;101(1):130–7. This study shows the role of cGMP-dependent protein kinase (PKG) as a principal mediators of perivascular adipose tissue activity.
Agabiti-Rosei C, De Ciuceis C, Rossini C, Porteri E, Rodella LF, Withers SB, et al. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J Hypertens. 2014;32(6):1264–74.
Bonnefont-Rousselot D, Collin F, Jore D, Gardès-Albert M. Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J Pineal Res. 2011;50(3):328–35.
Rezzani R, Porteri E, De Ciuceis C, Bonomini F, Rodella LF, Paiardi S, et al. Effects of melatonin and pycnogenol on small artery structure and function in spontaneously hypertensive rats. Hypertension. 2010;55(6):1373–80.
Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50(3):261–6.
Ríos-Lugo MJ, Cano P, Jiménez-Ortega V, Fernández-Mateos MP, Scacchi PA, Cardinali DP, et al. Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J Pineal Res. 2010;49(4):342–8.
• Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. 2011;12(3):167–88. This study describes melatonin function in brown adipose tissue metabolism suggesting a potential target for treatment of obesity in humans.
Takeda T. Senescence-accelerated mouse (SAM): a biogerontological resource in aging research. Neurobiol Aging. 1999;20(2):105–10.
Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp Gerontol. 2005;40(10):774–83.
Agabiti-Rosei C, Favero G, De Ciuceis C, Rossini C, Porteri E, Rodella LF, et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens Res. 2017;40(1):41–50.
Narkiewicz K. Diagnosis and management of hypertension in obesity. Obes Rev. 2006;7(2):155–62.
Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23(11):1170–8.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–536.
Guo Z, Zheng C, Qin Z, Wei P. Effect of telmisartan on the expression of cardiac adiponectin and its receptor 1 in type 2 diabetic rats. J Pharm Pharmacol. 2011;63(1):87–94.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Agabiti-Rosei, C., Paini, A., De Ciuceis, C. et al. Modulation of Vascular Reactivity by Perivascular Adipose Tissue (PVAT). Curr Hypertens Rep 20, 44 (2018). https://doi.org/10.1007/s11906-018-0835-5
Published:
DOI: https://doi.org/10.1007/s11906-018-0835-5