Abstract
Purpose of Review
Hypertension (htn) is a polygenic disorder that effects up to one third of the US population. The endoplasmic reticulum (ER) stress response is a homeostatic pathway that regulates membrane structure, protein folding, and secretory function. Emerging evidence suggests that ER stress may induce endothelial dysfunction; however, it is unclear whether ER stress-associated endothelial dysfunction modulates htn.
Recent Findings
Exogenous and endogenous molecules activate ER stress in the endothelium, and ER stress mediates some forms of neurogenic htn, such as angiotensin II-dependent htn. Human studies suggest that ER stress induces endothelial dysfunction, though direct evidence that ER stress augments blood pressure in humans is lacking. However, animal and cellular models demonstrate direct evidence that ER stress influences htn.
Summary
ER stress is likely one of many players in a complex interplay among molecular pathways that influence the expression of htn. Targeted activation of specific ER stress pathways may provide novel therapeutic opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension (HTN) is a polygenic disorder associated with activation of the sympathetic nervous system, upregulation of the renin-angiotensin-aldosterone system (RAS), altered G-protein coupled receptors, epigenetics, and inflammation. Vascular endothelial cells line the blood vessels and serve as a barrier and signal transducer between the blood and interstitium. Endothelial dysfunction is related to disruption of normal vascular endothelial homeostasis, due in part to imbalances between vasodilators and vasoconstrictors, growth factors and inhibitors, and inflammatory and anti-inflammatory molecules. Endothelial dysfunction plays a key role in hypertension (htn), atherosclerosis, peripheral arterial disease, and thombotic conditions and is associated with reductions in nitric oxide (NO), impaired endothelium-dependent dilation (EDD), platelet aggregation, reduction in anti-oxidants, increased pro-inflammatory cytokines, leukocyte adhesion, and fibrinolysis (rev in [1]). In this review, we will predominately focus on endothelial dysfunction associated with hypertension.
Background on Endoplasmic Reticulum Stress
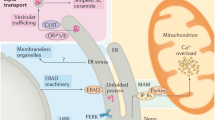
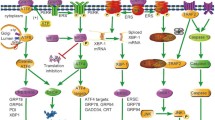
The endoplasmic reticulum (ER) folds, modifies, degrades, and transports proteins, and plays a key role in calcium storage, lipid biosynthesis, and numerous metabolic processes. Pathophysiological stress, including nutrient deprivation or excess, altered protein glycosylation, oxidative stress, reducing agents, lipids, changes in ER calcium content, microRNAs (miRNAs), infections, and TLR signaling, can interfere with normal three-dimensional protein folding in the ER, causing the accumulation of toxic misfolded proteins. Thus, cells have evolved a complex intracellular signaling pathway, the unfolded protein response (UPR) [2, 3], which represses protein synthesis and increases ER chaperone content to restore normal ER function. The UPR promotes adaptive responses to rapidly changing cellular conditions in a dynamic and coordinated manner; however, when these pathways are overwhelmed by sustained ER stress, the UPR initiates pro-apoptotic pathways (rev. in [4,5,6]) and autophagy [7]. Indeed, it has been posited that the UPR transitions to ER stress when the ER functions in an environment that lies outside of its normal physiological range [4].
The UPR plays its most vital role in secretory cells including endothelial cells, which secrete factors that regulate vascular homeostasis (rev. in [8, 9]). In mammalian cells, there are three major arms of the UPR: (1) inositol requiring protein-1α/X box binding protein-1 (IRE1α/XBP-1), (2) protein kinase RNA (PKR)-like ER kinase (PERK), and the (3) activating transcription factor-6 (ATF6) pathways. Seventy-eight kilodaltons glucose-regulated protein/immunoglobulin binding protein (GRP78/BiP) is an ER chaperone that senses and activates the UPR. In unstressed cells, GRP78/BiP binds to the ER luminal domains of IRE1α, PERK, and ATF-6 and maintains them in a dormant state. During ER stress, GRP78/BiP binds to the misfolded proteins and dissociates from and activates the transmembrane sensors (IRE1α, PERK, and ATF6). After GRP78/BiP dissociation, full activation of the UPR may require binding of unfolded proteins to the luminal domains of IRE1α, PERK, and ATF-6 [10, 11]. Once activated, the PERK pathway rapidly attenuates protein translation, whereas the ATF6 and the IRE1α/XBP-1 cascades transcriptionally upregulate ER chaperone genes to promote efficient folding and degradation of proteins, facilitating efficient ER function.
PERK
PERK is an ER transmembrane protein that is activated during ER stress and phosphorylates eukaryotic translation initiation factor 2α (eIF2α) which suppresses translation of 90% of cellular mRNAs [5, 12, 13]. A subset of genes including activating transcription factor-4 (ATF4) [14] are preferentially translated by phosphorylated eIF2α (p-eIF2α). ATF4 binds to promoter/enhancer regions and transcriptionally augments expression of UPR target genes, which include C/EBP homologous protein (CHOP, GADD153) [15], GADD34 [16], vascular endothelial growth factor (VEGF) A [17], TRB3 [18], E-selectin, and genes important in amino acid metabolism [19, 20]. PERK also phosphorylates and activates NRF2 [21].
IRE1α/XBP-1 Pathway
IRE1α is a membrane-bound serine/threonine kinase with endonuclease activity [3, 22] that splices a 26 bp intron from XBP-1 during ER stress. XBP-1 splicing induces a translational frame-shift that generates a transcription factor, which transcribes genes involved in ER maintenance, expansion, and ER-associated degradation (ERAD) [23, 24]. IRE1α also activates apoptosis signal-regulating kinase (ASK1), c-Jun N-terminal kinase (JNK), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB, rev. in [25]), which are involved in apoptotic, autophagy, and inflammatory pathways [26,27,28,29,30]. XBP-1 preserves cell survival during the UPR; however, after prolonged stress, the IRE1α/XBP-1 arm of the UPR is attenuated, sensitizing the cells to apoptosis mediated by the PERK/CHOP pathway [31, 32]. XBP-1 regulates VEGFA expression [33] and VEGFA rapidly activates all three ER stress sensors (IRE1α, PERK, and ATF6) and promotes endothelial survival [34].
IRE1α also directly cleaves mRNAs in a process described as regulated IRE1-dependent decay (RIDD) [35, 36]. RIDD assists the PERK arm of the UPR in reducing ER accumulation of misfolded proteins. RIDD activity may also induce the rapid clearance of microRNAs [37] and activate the Nod-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome to promote inflammation and programmed cell death [38]. However, the relevance of RIDD activity in the endothelium is unknown.
ATF6
ATF6 is the third ER stress sensor that is bound as an inactive precursor in the ER membrane. During ER stress, ATF6 is transported to the Golgi and cleaved to release its cytoplasmic bZIP domain [39], which translocates to the nucleus and activates the transcription of target genes which include GRP78/BiP, XBP-1, GRP94, oxygen-regulated protein 150 (ORP150), ER oxidoreductin 1β (ERO1β), p58IPK, and ER degradation-enhancing α-mannosidase-like protein (EDEM) (rev. in [40]).
Central/Neurogenic Hypertension and ER Stress
Seminal studies by Young and colleagues revealed that ER stress in the brain mediates angiotensin (Ang) II-dependent htn. Intraventricular injection of ER stress inducers (thapsigargin (TG) and intracerebral tunicamycin (TM)) increases mean arterial pressure (MAP) in mice [41••, 42]. Low-dose Ang II increases expression of ER stress markers in the brain subfornical region (SFO) and distension of the ER cisternae and reduces ribosome density. This is associated with increases in reactive oxygen species (ROS) and NFκB expression. Additionally, treatment with an ER stress inhibitor (tauroursodeoxycholic acid, TUDCA) and overexpression of GRP78/BiP (an ER chaperone) in the SFO prevent Ang II-induced htn [41••]. Interestingly, Ang II-mediated ER and oxidant stress does not increase apoptotic cell death in the SFO [43]. In a similar manner in spontaneously hypertensive rats (SHR), Chao and colleagues observed increased expression of ER stress markers and evidence of autophagy in the rostral ventrolateral medulla (RVLM) prior to the development of htn. Intracisternal treatment with salubrinal (a molecule that reduces dephosphorylation of phosphorylated-eIF2α) and ROS scavengers stabilize ER stress and reduce BP [42].
In contrast, in a murine model of deoxycorticosterone acetate (DOCA)-salt-induced htn, intra-ventricular administration of TUDCA and adenoviral expression of GRP78/BiP does not reduce BP [44] but attenuates an 80 beat per minute reduction in heart rate and reduces saline intake (and urine output) [45]. DOCA-salt intake also increases CHOP (an ER stress-induced transcription factor) expression and induces ultrastructural ER changes in the SFO and supraoptic nuclei. In this study, DOCA-treated CHOP-deficient mice had modest reductions in saline intake compared with their wild-type controls, suggesting that CHOP may modulate saline intake. Again, there were no differences in BPs, suggesting that DOCA-salt causes ER stress in the brain and it is “mechanistically linked” with changes in saline intake, but not hypertension [42, 45]. These studies suggest that ER stress likely contributes to the pathogenesis of some forms of neurogenic htn, likely independently of endothelial dysfunction.
Human Studies
Many ER stress studies utilize ER chaperones such as TUDCA and 4-phenylbutyrate (PBA) to abrogate ER stress. In a clinical study, Walsh and colleagues demonstrated that oral TUDCA mitigates post-prandial hyperglycemia-induced endothelial dysfunction (brachial artery flow-mediated dilatation, FMD) independently of changes in blood glucose. However, the investigators did not confirm that TUDCA reduces endothelial expression of ER stress markers [46••]. Additionally, Kaplon and colleagues used fluorescent microscopy and showed enhanced UPR activation in human endothelial cells obtained from non-diabetic obese patients compared with controls [47]. Admittedly, confirmatory Western blotting or mRNA evaluation would have strengthened their observations. In endothelial cells and peripheral blood mononuclear cells (PBMC) derived from healthy individuals, Intralipid® infusions (IV dietary fat emulsions) activate the UPR [48]. Thus in humans, obesity, hyperglycemia and hyperlipidemia can activate the endothelial UPR.
Studies have shown that in scleroderma patients (systemic sclerosis), presence of the HLA-B35 allele confers a higher risk of pulmonary htn. Lenna and colleagues demonstrated in cultured endothelial cells that HLA-B35 upregulates endothelin-1 (ET-1) and reduces eNOS expression, and this is associated with activation of the UPR [49•, 50]. Additionally, in PBMCs isolated from patients with limited cutaneous systemic sclerosis, presence of the HLA-B35 allele correlates with elevated GRP78/BiP and DNAJ homolog subfamily B member 1 (ER stress markers), inflammation (IL-6), and proliferation [51]. Interestingly, dasatinib, an oral tyrosine kinase inhibitor used to treat chronic myelogenous leukemia, can cause pulmonary htn. Parallel human and rat studies suggest that pulmonary htn associated with dasatinib is related to activation of ER stress in the pulmonary endothelium [52]. As a whole, these studies provide very weak evidence that activation of the UPR induces endothelial dysfunction; however, direct evidence that ER stress augments blood pressure in humans is lacking.
Animal Models
Rodent models have provided more convincing evidence regarding the pathophysiological connections between ER stress and hypertension. In a murine model of Ang II-induced Htn (2 week infusion in mice), Ang II increases expression of ER stress markers (ATF4, CHOP mRNA, and p-eIF2α) and this is associated with reduced phosphorylation of eNOS and EDD in the aorta and mesenteric resistance arteries (MRA). ER chaperones (PBA and TUDCA) significantly reduce systolic blood pressures (SBP, by >30 mmHg) and augment eNOS phosphorylation and EDD [53••, 54, 55]. Kassan’s studies suggest that ER stress impairs macrovascular endothelial function in a TGFβ1-dependent manner and microvascular endothelial function via an oxidative stress-dependent mechanism [53••].
In normotensive Sprague-Dawley rats, TM (10 μg/kg/day, subcutaneous osmotic pump for 28 days) increases SBP by over 30 mmHg and this is associated with increased aortic vascular smooth muscle fibrosis and apoptosis [54]. TM (10 pg/g × 7 days) also increases SBP and DBP in C57BL/6 mice [55]. In SHR, suppression of ER stress reduces BPs and endothelium-dependent contractions (EDC) in aortae and this is associated with reduced endothelial expression of cyclooxygenase-1 (COX-1), H2O2, cytosolic phospholipase A2 (cPLA2) activity, and pro-apoptotic marker expression [56•, 57]. Additionally, in rat-isolated aortic rings, TM causes insulin-stimulated vasoconstriction (impaired vasorelaxation) and this is likely related to ER stress-associated expression of ET-1 [58]. TUDCA treatment also reduces endothelial dysfunction in diabetic mice (db/db) [59]. Indeed, studies suggest that in endothelial cells, CHOP directly inhibits transcriptional activation of the eNOS promoter [60]. Thus, these studies clearly show in rodents that activation of ER stress by exogenous agents induces endothelial dysfunction and hypertension.
However, in mice, ER stress induction with intra-peritoneal TM (1 mg/kg of two injections/week × 2 weeks) increases aortic and MRA expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) activity in the absence of significant changes in SBP, suggesting that a threshold level of ER stress must be overcome to increase BP [53••, 61]. Notably, a single dose of TM (1 μg/g bw) induces apoptosis of renal tubular cells and acute tubular necrosis, and it is well-known that renal dysfunction impacts BP [62, 63]. Therefore, renal pathophysiological changes should be thoroughly evaluated when investigating ER stress and its links with endothelial dysfunction and htn.
The Western diet is high in saturated fats and has been associated with insulin resistance, vascular dysfunction, and inflammation [64]. Cheang and colleagues demonstrated that high fat diet (HFD)-induced endothelial dysfunction and aortic ER stress is reversed by TUDCA [65•]. HFD feeding of rats causes endothelial apoptosis, swollen mitochondria, and extended ERs in the thoracic aorta [66]. Similarly, fenofibrate (a PPARα agonist) reduces impaired EDD and ER stress in HFD-fed rats. However, it is not clear whether fenofibrate’s effects are related to lipid-lowering or activation of PPARα. In db/db mice and HFD-fed mice, exercise (4 weeks running) reduces ER (and oxidative) stress and restores aortic EDD and insulin-induced dilation of mesenteric arteries. These findings are attenuated in PPARδ-deficient mice, suggesting that PPARδ inhibits ER stress and plays a key role in exercise-induced improvements in diabetic endothelial dysfunction [67•].
Additionally, acetylcholine (ACH)-mediated vasodilation is impaired in chronically HFD-fed rats [68]. In rat aortae, TUDCA and an AMPK activator (aminoimidazole-4-carboxamide ribonucleotide, AICAR) restore impaired palmitate-induced EDD and eNOS phosphorylation [69]. Kim and colleagues also demonstrated that insulin-stimulated vasodilatation of mesenteric arterioles is impaired in HFD-fed mice and this effect is dependent on TLR4 [70]. Thus, studies in HFD-fed rodents have revealed clear links between ER stress and endothelial dysfunction and identified key players including PPARδ and TLR4.
Cellular Models of ER Stress and Endothelial Dysfunction
At the cellular level, palmitate, the most abundant circulating FFA, induces endothelial dysfunction. Physiological concentrations of palmitate activate ER stress in rodent endothelial cells and increase oxidative stress, inflammation (increased interleukin (IL)-1β, IL-6, and vascular cell adhesion protein (VCAM)) and impair EDD (decrease eNOS phosphorylation and NO). ER stress inhibitors (TUDCA, PBA) and AMPK activators (AICAR and salicylate) attenuate these effects [66, 68, 69, 71]. In human aortic endothelial cells (HAEC), palmitate increases expression of E-selectin, tumor necrosis factor-α (TNF-α), IL-1β, IL-6, spliced XBP-1, and phosphorylated eIF2α in a TLR4-dependent manner [70].
During ER stress, higher ROS production is related to reductions in anti-oxidants and increased protein folding in the ER. In coronary endothelial cells, ER stress upregulates NADPH oxidase (Nox2 and Nox4 mRNA) and p38 mitogen-activated protein kinase and this is associated with a reduction in eNOS activity and impaired vascular function [61] (rev in [72]). Thioredoxin-interacting protein (TXNIP) binds to and negatively regulates thioredoxin’s anti-oxidant function (scavenges ROS). During ER stress, the IRE1α and PERK pathways activate the NLRP3 inflammasome via induction of TXNIP [38, 73, 74]. In rat aortic endothelial cells (RAEC), palmitate activates ER stress, augments TXNIP expression, and activates the inflammasome. AICAR attenuates TXNIP induction, NLRP3 inflammasome activation, and subsequent endothelial expression of IL-1β, IL-6, and VCAM, again suggesting that AMPK plays a key role in inhibition of ER stress. In this study, the authors hypothesized that AMPK phosphorylates dynamin-related protein-1 (Drp-1) and inhibits mitochondrial fission thereby inhibiting ROS expression and ER stress [69]. In human umbilical vein endothelial cells (HUVEC) and murine endothelial cells sirtuin, a class III histone deacetylase prevents ER stress and microRNA-204 expression and regulates caveolin 1 (Cav1) function and endothelial vasorelaxation [75]. Oxidized phospholipids also induce ER stress in aortic endothelial cells, and this is associated with inflammatory gene expression in an ATF4- and XBP-1-dependent manner. Indeed, in human atherosclerotic lesions, there is evidence of UPR activation in the regions containing oxidized phospholipids [76•]. Thus, at the cellular level, activation of ER stress by endogenous and exogenous mediators induces a plethora of inflammatory mediators, which negatively modulate endothelial function and likely impact blood pressure.
CKD
Chronic Kidney Disease (CKD) is associated with endothelial dysfunction and htn. In primary HAEC, incubation with urea (20 mM for 48 h) increases ER stress markers, ROS and NOX activity, and these changes are abrogated by over-expression of uncoupling protein 1 (UCP-1) and reductions in mitochondrial ROS. However, the investigators did not use ER stress inhibitors to demonstrate direct links between induction of ER stress and their endothelial model of CKD [77]. In HUVECs, incubation of uremic sera induces ER stress, impairs proliferation, increases expression of monocyte chemotactic protein-1 (MCP-1), VEGF, and NFκB [78], and reduces insulin-stimulated NO release and eNOS phosphorylation [79]. Pretreatment with ER stress inhibitors (PBA or TUDCA), anti-oxidants, and overexpression of GRP78/BiP inhibit uremic serum-induced ER stress and insulin resistance [78, 79]. In a similar manner, in aortae isolated from rats with 5/6 nephrectomy, CKD is associated with activation of ER stress and insulin-resistance [79]. In Dahl salt-sensitive (SS) hypertensive rats, PBA reduces salt-induced htn, albuminuria, and preserves the glomerular filtration barrier [80]. Interestingly, reduction of BP with anti-hypertensive agents does not improve renal pathology suggesting that ER stress inhibition limits the progression of CKD in a hypertensive rat model, independent of its effects on BP. Accordingly, in a model of diabetes and htn, Wang and colleagues demonstrated that activation of ER stress likely explains the pathophysiological synergy between htn and hyperglycemia promoting renal injury [81•]. Moreover, in ischemia-reperfusion injury, ischemic preconditioning of the renal arteries may be protective by modulating ER stress and augmenting NO release in the kidney [82]. These studies suggest that ER stress is activated in the renal endothelium and likely plays a key role in endothelial dysfunction associated with CKD and potentially systemic htn.
Hyperhomocysteinemia
Homocysteine (HC) is an amino acid derived from the metabolism of methionine, and hyperhomocysteinemia is associated with endothelial dysfunction and higher risks of coronary artery, cerebrovascular, peripheral arterial disease, and venous thrombosis. HC may activate ER stress by disrupting disulfide bond formation and increasing the unfolded protein load [83]. HC impairs EDD (rev. in [84]) by activation of ER and oxidative stress via the PERK and IRE-1 pathways [85,86,87] (rev. in [84]). In human vascular endothelial cells and HUVECs, HC induces endothelial apoptosis by activation of activating transcription factor 3 (ATF3) and JNK in a CHOP- and IRE-1α-dependent manner [86,87,88,89]. HC-induced ER stress also causes endothelial detachment-mediated apoptosis associated with expression of T cell death-associated gene 51 (TDAG51) [90]. Additionally in porcine coronary endothelial cells, HC impairs endothelial vasorelaxation by reducing currents and cell surface expression of Ca2+-activated K+ channels that regulate vascular relaxation. These effects of HC are restored by ER stress inhibition [91]. Thus, HC activates ER stress in endothelial cells and negatively regulates endothelial viability and vasorelaxation.
Novel Modulators of ER Stress
Recent studies have identified pharmacological agents that inhibit ER stress and endothelial dysfunction and could potentially be used as anti-hypertensive agents. These novel molecules are depicted in Table 1 and include vitamin D, which in humans weakly modulates hypertension [92]. Mice with diet-induced vitamin D deficiency have elevated BPs, high plasma renin, and reduced urinary sodium excretion [93]. Activated vitamin D (1, 25-(OH)2 vitamin D) reduces TM and high glucose-induced ER stress in HUVECs. However, in this study, the investigators did not determine whether vitamin D directly alters endothelial function [94]. A number of compounds including Salidroside (herb used to treat high altitude-sickness), Piceatannol (an analog of reservatrol), l-serine and glycine, and black tea extracts improve HC-induced ER stress, apoptosis, ROS generation, and EDD in endothelial cells [95,96,97,98]. Additionally, berberine, a component of Chinese herbal remedies, reduces endothelial-dependent contractions in carotid arteries of SHR, in a COX-2-dependent manner. Berberine also increases AMPK phosphorylation and inhibits ER stress in carotid arteries [99]. Another component of Chinese herbal remedies Ilexgenin A, attenuates ER stress, ROS, and NLRP3 inflammasome activation, increases LKB1 phosphorylation, eNOS phosphorylation, and improves EDD in HFD-fed rats [71]. A number of other compounds including mangiferin, curcumin, hydrogen sulfide, paeonol, and angiotensin 1–7 also abrogate endothelial ER stress [100,101,102,103,104]. In HUVECs, induction of heme oxygenase-1 (HO-1) by cobalt (III) protoporphyrin IX chloride (CoPP) prevents ER stress and reduces high-glucose induced oxidative stress, inflammation, and apoptosis, and improves high-glucose induced NO release, angiogenic capacity, and VEGFA expression [105]. In a similar manner, in HUVECs, carbon monoxide (CO) induces Nrf2-dependent HO-1 expression in a PERK-dependent manner and prevents apoptosis triggered by ER stress (via CHOP suppression) [106]. However, the direct effects of CO on endothelial function were not evaluated. Thus, a number of pharmacologically active compounds can alter endothelial ER stress, positively modulate endothelial function, and potentially serve as novel anti-hypertensive agents.
Common Pitfalls in Evaluation of ER Stress
As a whole, these studies support the concept that ER stress induces endothelial dysfunction and htn, and they are based on the assumption that ER stress is maladaptive. However, ER stress facilitates cellular adaptation to aberrant cellular conditions; thus, it ultimately benefits organismal survival. It remains unclear and should be an active area of research to determine whether all three arms of the UPR are simultaneously or sequentially activated, and whether certain physiological stimuli preferentially activate specific arms of the ER stress response. There are a few reports of selective activation of ER stress pathways in endothelial cells; CO increases phosphorylation of PERK and eIF2α without activating IRE1α or ATF6 [106]. Similarly, in HAEC, palmitate activates XBP-1 and phosphorylated eIF2α without upregulation of GRP78/BiP and CHOP [70]. However, XBP-1 splicing and eIF2α phosphorylation are early UPR activation events, and it is possible that GRP78/BiP and CHOP may be augmented at later time points (unpublished observations). Additionally, many of the experimental observations in endothelial cells have been made in a static manner, neglecting natural oscillations and temporal patterns. It will be important to develop more precise tools, such as fluorescent markers to facilitate investigation of these natural fluctuations.
Organismal aging is associated with the accumulation of misfolded proteins in the ER [107, 108], and htn manifests as humans age. None of these studies have accounted for the ramifications of cellular aging in the endothelium, and alternative ER stress-associated effects on the endothelium may be operative. It is also likely that circulating inflammatory cells exposed to ER stress may secrete factors that negatively impact endothelial function and this hypothesis has not yet been directly tested. Moreover, recent work has suggested that neurons undergoing a stress response may signal to non-neuronal cells (in a cell-nonautonomous manner) to influence proteostasis (protein homeostasis) in other tissues and cells (rev. in [108, 109]). These studies call into question the original assumption that neurogenic htn does not directly influence peripheral endothelial dysfunction.
Future Directions and Therapeutic Considerations
Ultimately, the purpose of these studies is to obtain a greater understanding of endothelial pathophysiology to develop targeted treatment strategies for htn. It will be important to evaluate the influences of other highly conserved proteostasis networks, including the heat shock response, mitochondrial UPR, ubiquitin-proteosome pathway, autophagy, and the integrated stress response [107]. It is likely that there is significant cross-talk among these pathways adding to the complexity of observations and conclusions. A closer evaluation of genomics and metabolomics may clarify cross-talk of these networks. Selective activation of ER stress pathways may also provide therapeutic opportunities. Selective activators of PERK [110], IRE1α, and ATF6 are being developed (rev. in [108]) and will need to be tested in relevant hypertension models. Finally, it will be important to perform detailed and robust human studies to verify findings derived from animal and cellular model systems.
Conclusions
These studies provide evidence in human, animal, and cellular models that ER stress is activated in the endothelium and can alter endothelium-dependent relaxation and contraction factors that ultimately influence expression of hypertension. ER stress is likely one of many players in a complex interplay among molecular pathways that influence the expression of htn.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cimellaro A, Perticone M, Fiorentino TV, Sciacqua A, Hribal ML. Role of endoplasmic reticulum stress in endothelial dysfunction. Nutr Metab Cardiovasc Dis. 2016;26(10):863–71. doi:10.1016/j.numecd.2016.05.008.
Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–4.
Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73(6):1197–206.
Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189(5):783–94.
Arensdorf AM, Diedrichs D, Rutkowski DT. Regulation of the transcriptome by ER stress: non-canonical mechanisms and physiological consequences. Front Genet. 2013;4:256.
Hotamisligil GS, Davis RJ. Cell signaling and stress responses. Cold Spring Harb Perspect Biol. 2016;8(10). doi:10.1101/cshperspect.a006072.
Cybulsky AV. The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int. 2013;84(1):25–33.
Battson ML, Lee DM, Gentile CL. Endoplasmic Reticulum Stress and the Development of Endothelial Dysfunction. Am J Physiol Heart Circ Physiol. 2016; doi:10.1152/ajpheart.00437.2016.
O'Riordan E, Chen J, Brodsky SV, Smirnova I, Li H, Goligorsky MS. Endothelial cell dysfunction: the syndrome in making. Kidney Int. 2005;67(5):1654–8. doi:10.1111/j.1523-1755.2005.00256.x.
Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333(6051):1891–4.
Carrara M, Prischi F, Nowak PR, Kopp MC, Ali MM. Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. elife 2015;4. doi:10.7554/eLife.03522.
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2(6):326–32.
Ventoso I, Kochetov A, Montaner D, Dopazo J, Santoyo J. Extensive translatome remodeling during ER stress response in mammalian cells. PLoS One. 2012;7(5):e35915.
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904.
Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339(Pt 1):135–41.
Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278(37):34864–73.
Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF). J Biol Chem. 2014;289(6):3352–64.
Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24(6):1243–55.
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–33.
Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40(1):14–21.
Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279(19):20108–17. doi:10.1074/jbc.M314219200.
Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74(4):743–56.
Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–59.
Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27(1):53–66.
Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol Cell. 2009;35(5):551–61.
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–6.
Kim I, Shu CW, Xu W, Shiau CW, Grant D, Vasile S, et al. Chemical biology investigation of cell death pathways activated by endoplasmic reticulum stress reveals cytoprotective modulators of ASK1. J Biol Chem. 2009;284(3):1593–603.
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–31.
Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4(12):e423.
Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281(40):30299–304.
Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–9.
Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33(6):679–91.
Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5(3):e9575.
Karali E, Bellou S, Stellas D, Klinakis A, Murphy C, Fotsis T. VEGF signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol Cell. 2014;54(4):559–72.
Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–75.
Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–31.
Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science. 2012;338(6108):818–22.
Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16(2):250–64.
Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10(11):3787–99.
Cunard R. Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J Clin Med. 2015;4(4):715–40. doi:10.3390/jcm4040715.
•• Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, et al. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122(11):3960–4. doi:10.1172/JCI64583. This reference is the first study to demonstrate that brain ER stress plays a role in chronic hypertension.
Chao YM, Lai MD, Chan JY. Redox-sensitive endoplasmic reticulum stress and autophagy at rostral ventrolateral medulla contribute to hypertension in spontaneously hypertensive rats. Hypertension. 2013;61(6):1270–80. doi:10.1161/HYPERTENSIONAHA.111.00469.
Young CN, Li A, Dong FN, Horwath JA, Clark CG, Davisson RL. Endoplasmic reticulum and oxidant stress mediate nuclear factor-kappaB activation in the subfornical organ during angiotensin II hypertension. Am J Phys Cell Phys. 2015;308(10):C803–12. doi:10.1152/ajpcell.00223.2014.
Xia H, de Queiroz TM, Sriramula S, Feng Y, Johnson T, Mungrue IN, et al. Brain ACE2 overexpression reduces DOCA-salt hypertension independently of endoplasmic reticulum stress. Am J Phys Regul Integr Comp Phys. 2015;308(5):R370–8. doi:10.1152/ajpregu.00366.2014.
Jo F, Jo H, Hilzendeger AM, Thompson AP, Cassell MD, Rutkowski DT, et al. Brain endoplasmic reticulum stress mechanistically distinguishes the saline-intake and hypertensive response to deoxycorticosterone acetate-salt. Hypertension. 2015;65(6):1341–8. doi:10.1161/HYPERTENSIONAHA.115.05377.
•• Walsh LK, Restaino RM, Neuringer M, Manrique C, Padilla J. Administration of tauroursodeoxycholic acid prevents endothelial dysfunction caused by an oral glucose load. Clin Sci (Lond). 2016;130(21):1881–8. doi:10.1042/CS20160501. This reference is important because it is the first study that investigates the effect of an ER stress inhibitor (TUDCA) on vascular function in humans.
Kaplon RE, Chung E, Reese L, Cox-York K, Seals DR, Gentile CL. Activation of the unfolded protein response in vascular endothelial cells of nondiabetic obese adults. J Clin Endocrinol Metab. 2013;98(9):E1505–9. doi:10.1210/jc.2013-1841.
Tampakakis E, Tabit CE, Holbrook M, Linder EA, Berk BD, Frame AA, et al. Intravenous lipid infusion induces endoplasmic reticulum stress in endothelial cells and blood mononuclear cells of healthy adults. J Am Heart Assoc. 2016;5(1). doi:10.1161/JAHA.115.002574.
• Lenna S, Townsend DM, Tan FK, Kapanadze B, Markiewicz M, Trojanowska M, et al. HLA-B35 upregulates endothelin-1 and downregulates endothelial nitric oxide synthase via endoplasmic reticulum stress response in endothelial cells. J Immunol. 2010;184(9):4654–61. doi:10.4049/jimmunol.0903188. This manuscript is important because it is the first that investigates the mechanism underlying the association of a genetic factor (HLA-B35) with endothelial dysfunction and ER stress in patients with pulmonary hypertension.
Lenna S, Farina AG, Martyanov V, Christmann RB, Wood TA, Farber HW, et al. Increased expression of endoplasmic reticulum stress and unfolded protein response genes in peripheral blood mononuclear cells from patients with limited cutaneous systemic sclerosis and pulmonary arterial hypertension. Arthritis Rheum. 2013;65(5):1357–66. doi:10.1002/art.37891.
Lenna S, Assassi S, Farina GA, Mantero JC, Scorza R, Lafyatis R, et al. The HLA-B*35 allele modulates ER stress, inflammation and proliferation in PBMCs from limited cutaneous systemic sclerosis patients. Arthritis Res Ther. 2015;17:363. doi:10.1186/s13075-015-0881-1.
Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016;126(9):3207–18. doi:10.1172/JCI86249.
•• Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, et al. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32(7):1652–61. doi:10.1161/ATVBAHA.112.249318. This manuscript is important because it demonstrates for the first time how ER stress regulates htn and investigates mechanisms underlying this key observation.
Spitler KM, Webb RC. Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension. 2014;63(3):e40–5. doi:10.1161/HYPERTENSIONAHA.113.02558.
Liang B, Wang S, Wang Q, Zhang W, Viollet B, Zhu Y, et al. Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-alpha2 in vivo. Arterioscler Thromb Vasc Biol. 2013;33(3):595–604. doi:10.1161/ATVBAHA.112.300606.
• Spitler KM, Matsumoto T, Webb RC. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2013;305(3):H344–53. doi:10.1152/ajpheart.00952.2012. This manuscript is important because it shows that an ER stress inhibitor improves endothelial-derived contractile factors in the aortae of SHR due to reduced oxidative stress, cPLA2 phosphorylation and reduced COX-1 expression.
Choi SK, Lim M, Byeon SH, Lee YH. Inhibition of endoplasmic reticulum stress improves coronary artery function in the spontaneously hypertensive rats. Sci Rep. 2016;6:31925. doi:10.1038/srep31925.
Padilla J, Jenkins NT. Induction of endoplasmic reticulum stress impairs insulin-stimulated vasomotor relaxation in rat aortic rings: role of endothelin-1. J Physiol Pharmacol. 2013;64(5):557–64.
Choi SK, Lim M, Yeon SI, Lee YH. Inhibition of endoplasmic reticulum stress improves coronary artery function in type 2 diabetic mice. Exp Physiol. 2016;101(6):768–77. doi:10.1113/EP085508.
Loinard C, Zouggari Y, Rueda P, Ramkhelawon B, Cochain C, Vilar J, et al. C/EBP homologous protein-10 (CHOP-10) limits postnatal neovascularization through control of endothelial nitric oxide synthase gene expression. Circulation. 2012;125(8):1014–26. doi:10.1161/CIRCULATIONAHA.111.041830.
Galan M, Kassan M, Kadowitz PJ, Trebak M, Belmadani S, Matrougui K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta. 2014;1843(6):1063–75. doi:10.1016/j.bbamcr.2014.02.009.
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–95.
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–77.
Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol. 2011;301(5):F919–31. doi:10.1152/ajprenal.00068.2011.
• Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY, et al. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arterioscler Thromb Vasc Biol. 2014;34(4):830–6. doi:10.1161/ATVBAHA.113.301938. This manuscript is important because it demonstrates how a commonly used pharmacologic agent, Metformin improves endothelial function by inhibiting ER stress, increasing NO and activating the AMPK/PPARd pathway in obese diabetic mice.
Lu Y, Qian L, Zhang Q, Chen B, Gui L, Huang D, et al. Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life Sci. 2013;92(24–26):1165–73. doi:10.1016/j.lfs.2013.05.002.
• Cheang WS, Wong WT, Zhao L, Xu J, Wang L, Lau CW, et al. PPARdelta Is Required for Exercise to Attenuate Endoplasmic Reticulum Stress and Endothelial Dysfunction in Diabetic Mice. Diabetes. 2017;66(2):519–28. doi:10.2337/db15-1657. This manuscript is important because it describes how exercise improves vascular function, by activating AMPK and PPARdδ which inhibit endothelial ER stress.
Lu Y, Cheng J, Chen L, Li C, Chen G, Gui L, et al. Endoplasmic reticulum stress involved in high-fat diet and palmitic acid-induced vascular damages and fenofibrate intervention. Biochem Biophys Res Commun. 2015;458(1):1–7. doi:10.1016/j.bbrc.2014.12.123.
Li J, Wang Y, Wang Y, Wen X, Ma XN, Chen W, et al. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J Mol Cell Cardiol. 2015;86:62–74. doi:10.1016/j.yjmcc.2015.07.010.
Kim JA, Jang HJ, Hwang DH. Toll-like receptor 4-induced endoplasmic reticulum stress contributes to impairment of vasodilator action of insulin. Am J Physiol Endocrinol Metab. 2015;309(9):E767–76. doi:10.1152/ajpendo.00369.2015.
Li Y, Yang J, Chen MH, Wang Q, Qin MJ, Zhang T, et al. Ilexgenin a inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol Res. 2015;99:101–15. doi:10.1016/j.phrs.2015.05.012.
Santos CX, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV, Lopes LR. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid Redox Signal. 2014;20(1):121–34. doi:10.1089/ars.2013.5262.
Oslowski CM, Hara T, O'Sullivan-Murphy B, Kanekura K, Lu S, Hara M, et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012;16(2):265–73.
Mohamed IN, Hafez SS, Fairaq A, Ergul A, Imig JD, El-Remessy AB. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia. 2014;57(2):413–23. doi:10.1007/s00125-013-3101-z.
Kassan M, Vikram A, Kim YR, Li Q, Kassan A, Patel HH, et al. Sirtuin1 protects endothelial Caveolin-1 expression and preserves endothelial function via suppressing miR-204 and endoplasmic reticulum stress. Sci Rep. 2017;7:42265. doi:10.1038/srep42265.
• Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26(11):2490–6. doi:10.1161/01.ATV.0000242903.41158.a1. This is an early manuscript linking the UPR with inflammation in endothelial cells.
D'Apolito M, Du X, Pisanelli D, Pettoello-Mantovani M, Campanozzi A, Giacco F, et al. Urea-induced ROS cause endothelial dysfunction in chronic renal failure. Atherosclerosis. 2015;239(2):393–400. doi:10.1016/j.atherosclerosis.2015.01.034.
Zeng W, Guo YH, Qi W, Chen JG, Yang LL, Luo ZF, et al. 4-Phenylbutyric acid suppresses inflammation through regulation of endoplasmic reticulum stress of endothelial cells stimulated by uremic serum. Life Sci. 2014;103(1):15–24. doi:10.1016/j.lfs.2014.03.007.
Zhou QG, Fu XJ, Xu GY, Cao W, Liu HF, Nie J, et al. Vascular insulin resistance related to endoplasmic reticulum stress in aortas from a rat model of chronic kidney disease. Am J Physiol Heart Circ Physiol. 2012;303(9):H1154–65. doi:10.1152/ajpheart.00407.2012.
Yum V, Carlisle RE, Lu C, Brimble E, Chahal J, Upagupta C, et al. Endoplasmic reticulum stress inhibition limits the progression of chronic kidney disease in the dahl salt-sensitive rat. Am J Physiol. 2017;312(1):F230–F44. doi:10.1152/ajprenal.00119.2016.
• Wang Z, do Carmo JM, Aberdein N, Zhou X, Williams JM, da Silva AA, et al. Synergistic Interaction of Hypertension and Diabetes in Promoting Kidney Injury and the Role of Endoplasmic Reticulum Stress. Hypertension. 2017;69(5):879–91. doi:10.1161/HYPERTENSIONAHA.116.08560. This manuscript is important as it hypothesizes that endothelial ER stress accounts for the synergistic ability of Type 2 diabetes and hypertension to promote kidney dysfunction.
Mahfoudh-Boussaid A, Zaouali MA, Hadj-Ayed K, Miled AH, Saidane-Mosbahi D, Rosello-Catafau J, et al. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1alpha in ischemic kidney: the role of nitric oxide. J Biomed Sci. 2012;19:7. doi:10.1186/1423-0127-19-7.
Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol. 2004;10(12):1699–708.
Lai WK, Kan MY. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab. 2015;67(1):1–12. doi:10.1159/000437098.
Outinen PA, Sood SK, Pfeifer SI, Pamidi S, Podor TJ, Li J, et al. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94(3):959–67.
Zhang C, Cai Y, Adachi MT, Oshiro S, Aso T, Kaufman RJ, et al. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J Biol Chem. 2001;276(38):35867–74. doi:10.1074/jbc.M100747200.
Zhang C, Kawauchi J, Adachi MT, Hashimoto Y, Oshiro S, Aso T, et al. Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem Biophys Res Commun. 2001;289(3):718–24. doi:10.1006/bbrc.2001.6044.
Cai Y, Zhang C, Nawa T, Aso T, Tanaka M, Oshiro S, et al. Homocysteine-responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter response element. Blood. 2000;96(6):2140–8.
Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11(Suppl 1):S56–64. doi:10.1038/sj.cdd.4401451.
Hossain GS, van Thienen JV, Werstuck GH, Zhou J, Sood SK, Dickhout JG, et al. TDAG51 is induced by homocysteine, promotes detachment-mediated programmed cell death, and contributes to the cevelopment of atherosclerosis in hyperhomocysteinemia. J Biol Chem. 2003;278(32):30317–27. doi:10.1074/jbc.M212897200.
Wang XC, Sun WT, Yu CM, Pun SH, Underwood MJ, He GW, et al. ER stress mediates homocysteine-induced endothelial dysfunction: modulation of IKCa and SKCa channels. Atherosclerosis. 2015;242(1):191–8. doi:10.1016/j.atherosclerosis.2015.07.021.
Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177–86. doi:10.1016/j.ijcard.2016.11.040.
Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, et al. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One. 2013;8(1):e54625. doi:10.1371/journal.pone.0054625.
Haas MJ, Jafri M, Wehmeier KR, Onstead-Haas LM, Mooradian AD. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radic Biol Med. 2016;99:1–10. doi:10.1016/j.freeradbiomed.2016.07.020.
Zhu L, Jia F, Wei J, Yu Y, Yu T, Wang Y, et al. Salidroside protects against homocysteine-induced injury in HUVECs via the regulation of endoplasmic reticulum stress. Cardiovasc Ther. 2016; doi:10.1111/1755-5922.12234.
Kil JS, Jeong SO, Chung HT, Pae HO. Piceatannol attenuates homocysteine-induced endoplasmic reticulum stress and endothelial cell damage via heme oxygenase-1 expression. Amino Acids. 2017;49(4):735–45. doi:10.1007/s00726-016-2375-0.
Cheang WS, Ngai CY, Tam Y, Tian XY, Wong WT, Zhang Y, et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Sci Rep. 2015;5:10340.
Sim WC, Han I, Lee W, Choi YJ, Lee KY, Kim DG, et al. Inhibition of homocysteine-induced endoplasmic reticulum stress and endothelial cell damage by l-serine and glycine. Toxicol in Vitro. 2016;34:138–45. doi:10.1016/j.tiv.2016.04.004.
Liu L, Liu J, Huang Z, Yu X, Zhang X, Dou D, et al. Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem Biophys Res Commun. 2015;458(4):796–801. doi:10.1016/j.bbrc.2015.02.028.
Song J, Li J, Hou F, Wang X, Liu B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism. 2015;64(3):428–37. doi:10.1016/j.metabol.2014.11.008.
Ye M, Qiu H, Cao Y, Zhang M, Mi Y, Yu J, et al. Curcumin improves palmitate-induced insulin resistance in human umbilical vein endothelial cells by maintaining Proteostasis in endoplasmic reticulum. Front Pharmacol. 2017;8:148. doi:10.3389/fphar.2017.00148.
Hu HJ, Jiang ZS, Qiu J, Zhou SH, Liu QM. Protective effects of hydrogen sulfide against angiotensin II-induced endoplasmic reticulum stress in HUVECs. Mol Med Rep. 2017;15(4):2213–22. doi:10.3892/mmr.2017.6238.
Choy KW, Mustafa MR, Lau YS, Liu J, Murugan D, Lau CW, et al. Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARdelta signaling pathway. Biochem Pharmacol. 2016;116:51–62. doi:10.1016/j.bcp.2016.07.013.
Murugan D, Lau YS, Lau CW, Mustafa MR, Huang Y. Angiotensin 1-7 protects against angiotensin II-induced endoplasmic reticulum stress and endothelial dysfunction via mas receptor. PLoS One. 2015;10(12):e0145413. doi:10.1371/journal.pone.0145413.
Maamoun H, Zachariah M, McVey JH, Green FR, Agouni A. Heme oxygenase (HO)-1 induction prevents endoplasmic reticulum stress-mediated endothelial cell death and impaired angiogenic capacity. Biochem Pharmacol. 2017;127:46–59. doi:10.1016/j.bcp.2016.12.009.
Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res. 2007;101(9):919–27. doi:10.1161/CIRCRESAHA.107.154781.
Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319(5865):916–9. doi:10.1126/science.1141448.
Martinez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017; doi:10.1111/acel.12599.
van Oosten-Hawle P, Morimoto RI. Organismal proteostasis: role of cell-nonautonomous regulation and transcellular chaperone signaling. Genes Dev. 2014;28(14):1533–43. doi:10.1101/gad.241125.114.
Axten JM. Protein kinase R(PKR)-like endoplasmic reticulum kinase (PERK) inhibitors: a patent review (2010-2015). Expert Opin Ther Pat. 2016:1–12. doi:10.1080/13543776.2017.1238072.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Metabolic Syndrome
Rights and permissions
About this article
Cite this article
Cunard, R. Endoplasmic Reticulum Stress, a Driver or an Innocent Bystander in Endothelial Dysfunction Associated with Hypertension?. Curr Hypertens Rep 19, 64 (2017). https://doi.org/10.1007/s11906-017-0762-x
Published:
DOI: https://doi.org/10.1007/s11906-017-0762-x