Abstract
Cardiovascular death rates continue to rise for women under age 55, underlying the importance of focusing on female-specific conditions that may increase cardiovascular risk, including pregnancy-related disorders. Hypertension complicates about 5–10 % of pregnancies. Preeclampsia, a pregnancy-specific condition, is characterized by hypertension and proteinuria after 20 weeks of gestation and remains one of the major causes of maternal deaths in the United States. In addition, preeclampsia may have an impact on women’s health beyond their pregnancies, and has been associated with increased risks for future hypertension and cardiovascular disease, such as coronary heart disease and stroke. In this review, we discuss the evidence supporting the association between preeclampsia and future hypertension; possible mechanisms that underlie this association; current approach to women with a history of preeclampsia; and future research that is needed in this field in order to deliver optimal and timely medical care to the affected women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertensive pregnancy disorders cover a spectrum of conditions, including preeclampsia (de novo or superimposed on preexisting chronic hypertension), chronic and gestational hypertension (Table 1) [1]. Preeclampsia and related conditions, its convulsive form, eclampsia, and HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome, remain leading causes of fetal and maternal mortality and morbidity. Over the last 50 years, much progress has been made in improving treatment of preeclampsia with respect to blood pressure control and prevention of eclamptic seizures. However, the etiology and pathogenesis of this condition remain elusive, resulting in a failure to develop specific preventive and treatment strategies. Delivery remains the mainstay of therapy for severe forms and anticipated life-threatening complications, frequently resulting in preterm delivery, and fetal/neonatal complications related to immaturity. A study of the number of delivery hospitalizations in the United States in women with hypertensive disorders in pregnancy in 1986–2006 showed that this number is increasing, and that these hospitalizations are associated with a substantial burden of severe obstetric morbidity, particularly in the presence of severe preeclampsia/eclampsia. [2•]
There is accumulating evidence suggesting that endothelial dysfunction, caused by placental factors that enter the maternal circulation, may play a central role in the pathogenesis of preeclampsia. The fact that hypertension rapidly resolves upon the removal of products of conception has led to several theories implicating structural and/or functional changes in the developing placenta as factors causing preeclampsia. In preeclampsia, the placental spiral arteries fail to lose their musculoelastic layers, ultimately leading to decreased placental perfusion [3, 4]. Placental hypoxia is viewed frequently as an early event that may cause placental production of soluble factors leading to endothelial dysfunction [5]. The clinical syndrome of preeclampsia ensues, which has been attributed to complex interactions among maternal constitutional factors (e.g., pre-existing metabolic abnormalities), placenta-derived products (exemplified by the imbalance between pro-angiogenic and anti-angiogenic factors, favoring the latter), and the exaggerated adaptive mechanisms that normally occur during pregnancy (features of the metabolic syndrome, an inflammatory response, and a hypercoagulable state). Decreased placental perfusion coupled with maternal hypertension result in serious maternal and fetal complications, most notably placental abruption and infarction, fetal growth restriction, and intrauterine fetal demise. In addition, hypertensive pregnancy disorders may have an impact on women’s health well beyond the affected pregnancies. Women with a history of hypertensive pregnancy disorders, particularly preeclampsia that develops remote from term, are at an increased risk for cardiovascular disease (CVD) later in life, including coronary heart disease, stroke, thromboembolic disease, heart failure, and arrhythmias [6, 7••]. The increased risk for hypertension in these women may contribute to their overall increased rates of CVD years after their affected pregnancies.
Preeclampsia and Future Hypertension: Review of the Evidence
The earliest report of an association between ‘hypertensive toxemia of pregnancy’, as preeclampsia was once called, and chronic cardiovascular disease was by Corwin in 1927 [8]. In this study, 165 women, average age 29 years, with “hypertensive toxemia of pregnancy” were followed up for a period of 6 months to six years post-partum. Of these, 37 % showed persistent hypertension, defined as a systolic blood pressure >140 mm Hg. This view was challenged by the classic work of Leon Chesley [9, 10], who reported that the prevalence of hypertension in 206 primiparous eclamptic women an average of 33 years later was similar to that in age-matched women from 11 epidemiological studies of blood pressure. Chesley’s work has been given considerable weight since the women he followed had eclampsia, and were nulliparas, thus minimizing the heterogeneity in diagnosis. Chesley further suggested that, among women with eclampsia, those who are multiparous are different and should be analyzed separately, as the prevalence of chronic hypertension was increased among 64 multiparous women who survived eclampsia. In fact, Chesley recognized that pregnancy hypertension in multiparous women or in those without proteinuria (i.e., gestational hypertension) was most likely an early manifestation of an increased risk for later essential or renal hypertension. Although remarkable for the serious effort on the part of Dr. Chesley to track down women decades after delivery, Chesley’s study design raised methodological questions–most specifically, what is the appropriate control group with which to compare women with hypertension in pregnancy? The control group in Chesley’s study was a historical control group of women from previously published epidemiologic studies, and thus may have included women with hypertensive pregnancy disorders which may have led to bias towards the null hypothesis.
Chesley’s study had a profound impact and, for many years, authors of chapters and review articles concluded that hypertensive diseases of pregnancy are limited to the affected pregnancy, and have few, if any, long-term maternal effects. There have been multiple epidemiological studies since that time that have supported an association between hypertensive pregnancy disorders and future chronic hypertension (Table 2) [11], ischemic heart disease, stroke, venous thromboembolism, and cardiovascular death [6, 12]. In 1986, Sibai et al. [13] reported a significantly higher incidence of hypertension in patients with histories of preeclampsia or eclampsia during their first pregnancies compared to matched normotensive controls. The risk was increased further by a history of recurrent preeclampsia or eclampsia, and early preeclampsia (<30 weeks gestation). Most of the differences were noticed in individuals who had been followed up for at least 10 years. In the work of Selvaggi et al. [14], half of the participating women with histories of preeclampsia were hypertensive 10 years after delivery, compared with only one-third who were hypertensive 5 years after delivery, further supporting the importance of an adequate follow-up after the affected pregnancies when assessing this association.
As most of the published studies clearly have been underpowered [11], a systematic review and meta-analysis assessing the risks of CVD in women with histories of preeclampsia and eclampsia was performed by Bellamy et al. [6]. They conducted a search of Medline and Embase between 1960 and December 2006; both positive and negative studies were included in the analysis. A total of 13 studies involving 21,030 women examined the risk of hypertension subsequent to a preeclamptic or eclamptic pregnancy. Over a mean follow-up of 14.1 years, the authors found that 1885 of 3658 women with preeclampsia developed hypertension later in life, providing a relative risk of 3.7.
It is important to acknowledge the limitations of published studies related to their retrospective designs, including inadequate durations of follow-up, difficulties in establishing the diagnosis of preeclampsia in a retrospective fashion, and changes in the definition of and diagnostic criteria for hypertensive pregnancy disorders over time. Making distinctions among different categories of hypertension in pregnancy, and particularly contrasting preeclampsia to other hypertensive pregnancy disorders, is challenging and has been hampered by the lack of a suitable biomarker that clearly distinguishes preeclampsia from chronic or gestational hypertension [15]. It is likely that differences in the clinical presentations of preeclampsia and other hypertensive pregnancy disorders (Table 1) probably result from differences in their underlying mechanisms, which might have varying implications for future hypertension and CVD later in life. For example, although recent clinical guidelines from Australia and New Zealand have minimized the importance of proteinuria for the purposes of clinical care [16], it is possible, and perhaps likely that this clinical feature identifies an important subset of the disease with respect to mechanisms and prognosis. Further complicating the interpretation of some studies is the use of index codes or self-reported events rather than accepted diagnostic criteria to make a diagnosis of preeclampsia and other hypertensive pregnancies. Finally, several studies failed to exclude women with chronic hypertension that occurs before pregnancy, and then persists during pregnancy and after delivery. In these women, chronic hypertension predates pregnancy and does not occur subsequent to, or as a result of, a hypertensive pregnancy disorder. A single cohort study included only women who developed hypertension after the age of 40, with the rationale that most pregnancies are likely to occur by that age. In other words, by looking at the onset of hypertension at age 40, chances are that these women were not hypertensive before their pregnancies, but rather became hypertensive after their pregnancies. Compared with women with histories of normotensive pregnancies, those with histories of hypertensive pregnancies had an increased hazard ratio of 1.53 (95 % CI 1.25–1.87, P < 0.001) for the diagnosis of hypertension after the age of 40, after controlling for race, family history of cardiovascular disease, education, diabetes (time-dependent), smoking status, and body mass index (BMI) [17]. The major limitation of this study was that hypertension in pregnancy was self-reported and, therefore, subject to recall bias.
Studies published to date, despite their limitations, provide compelling evidence that supports the association between preeclampsia and future hypertension and CVD. However, these women are frequently lost to follow-up after their deliveries. If their hypertension either persists or occurs soon after their affected pregnancies, it frequently remains under-recognized and under-treated. The limitations of the fragmented medical care that these women receive have been recognized recently by the guidelines for the prevention of cardiovascular disease in women, which recommend referral of women with a history of hypertensive pregnancy to primary care or cardiology [18••]. Monitoring and control of CVD risk factors, and hypertension, in particular, in the affected women, may prevent future CVD.
The Mechanism(s) Underlying the Association Between Preeclampsia and Future Hypertension
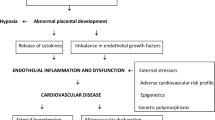
Endothelial dysfunction plays a crucial role in the pathogenesis of both preeclampsia and future hypertension and CVD. Hypertensive pregnancy disorders, including preeclampsia, have been suggested to be a ‘failed stress test’, with pregnancy unmasking underlying endothelial dysfunction and a predisposition to cardiovascular disease. These two conditions share several common risk factors, such as diabetes and obesity, which may lead to preeclampsia and future hypertension and CVD at different times of a woman’s life. An alternative explanation is that preeclampsia itself may induce irreversible vascular and metabolic changes that my increase the overall risk for hypertension and CVD (Fig. 1).
Preeclampsia and Future Hypertension: Common Mechanisms
Preeclampsia and cardiovascular disease share common risk factors (Fig. 1), such as metabolic abnormalities (insulin resistance, dyslipidemia, obesity), inflammation, oxidative stress, and hypercoagulability; these shared risk factors may result in endothelial dysfunction and increased risks for both preeclampsia and future hypertension and CVD [11, 19]. Several potent mediators of endothelial cell dysfunction have been shown to be up-regulated in preeclampsia, including cellular fibronectin [20], von Willebrand factor [21], cell adhesion molecules such as P-selectin [22], vascular endothelial adhesion molecule-1 (VCAM-1), and inter-cellular adhesion molecule-1 (ICAM-1) [23], and cytokines, such as interleukin-6 (IL-6) [24] and tumor necrosis factor-α (TNF-α) [25]. Relative nitric oxide deficiency may contribute to the generalized vasoconstriction seen in preeclampsia [26]. Oxidative stress due to free radical generation contributes to endothelial dysfunction both in preeclampsia and atherosclerosis [27]. Elevated levels of the soluble receptor for vascular endothelial growth factor (VEGF), commonly referred to as sFlt-1 (from fms-like tyrosine-kinase receptor-1), may be the missing link between placental ischemia on one side and endothelial dysfunction, mediated by sFlt-1 neutralization of VEGF and placental growth factor (PIGF), on the other. By antagonizing the pro-angiogenic effects of VEGF, increased levels of sFlt-1 can induce endothelial dysfunction, and thus hypertension and proteinuria [28]. Most published studies suggest that measurements of these markers, although not predictive of late-onset preeclampsia in term pregnancies, may be useful in predicting early onset and severe disease. These findings raise the possibility of using these tests in special patient subgroups. A recent study suggested that angiogenic factors may be useful to facilitate diagnosis of superimposed preeclampsia in women with chronic hypertension [29].
Possible mechanisms underlying the association between preeclampsia and future hypertension. These two conditions share common risk factors, such as oxidative stress and hypercoagulability, which could result in preeclampsia and hypertension/CVD at different times in a woman’s life. Alternatively, preeclampsia may result in irreversible metabolic and vascular changes that may contribute to the risk of developing hypertension/CVD later in life
The interactions among endothelial dysfunction and metabolic abnormalities, oxidative stress, inflammation and a hypercoagulable state, appear quite complex in preeclampsia; these mechanisms may potentiate each other, resulting in cumulative, multisystem vascular damage. The sum effect of these abnormalities is a state of systemic vasoconstriction, resultant ischemia, and multisystem dysfunction.
Metabolic Abnormalities
Several common metabolic abnormalities are shared risk factors for CVD and preeclampsia, including obesity, insulin resistance, and lipid abnormalities [11, 19, 30, 31]. “Acute atherosis” of the placental bed vessels, commonly encountered in preeclampsia, closely resembles atherosclerotic plaques [32••]. Patients who are obese before pregnancy are at a greater risk for preeclampsia [33]. The risk for hypertension in pregnancy and/or preeclampsia is doubled in diabetic compared to non-diabetic controls [34]. The pattern of increased small, dense low-density lipoprotein (LDL) and triglycerides (pattern B) is known as particularly atherogenic and has been described in patients with coronary artery disease [35] and in women with preeclampsia [36, 37]. In addition, elevated levels of leptin (an adipocyte-derived hormone), which are suggestive of resistance to its metabolic effects, are associated with both CVD [38] and preeclamptic pregnancies [39]. Elevated levels of leptin may promote platelet aggregation [40], thus further potentiating the hypercoagulable state which is present in preeclampsia.
Inflammatory Response
Pregnancy is characterized by hormonal modulation of both innate and adaptive immunity (T-cell-mediated cellular immune system and B-cell-mediated humoral immune system), in order to establish maternal immune tolerance to a semi-allogeneic fetus expressing both maternal and paternal antigens. However, while the innate immune response is enhanced, the cellular immune response (which, through activation of cytotoxic T-cells and natural killer cells, may damage trophoblast and result in pregnancy loss), is down-regulated [41, 42]. During normal pregnancy, the maternal immune reaction to fetal antigens manifests as an exaggerated inflammatory response [43, 44]. This inflammatory response is further potentiated in preeclampsia, as evidenced by elevated levels of markers of neutrophil activation compared to normal pregnancy [24, 25, 37, 45–48]. As to the mechanism of leukocyte activation, it is possible that it may occur when leukocytes circulate through the intervillous space and are exposed to oxidized lipid secreted by the placenta [47]. Similar to CVD, elevated C-reactive protein levels have been associated with an increased risk for preeclampsia [48]. Likewise, and similar to atherosclerosis [49], leukocyte adhesion to the endothelium plays an important role in promoting inflammation that may contribute to the development of preeclampsia.
Hypercoagulability
In preeclampsia, the hypercoagulable state of normal pregnancy is further potentiated, as evidenced by an imbalance between fibrinolysis and coagulation, in favor of the latter [11, 19]. Most notably, procoagulant proteins such as tissue plasminogen activator (tPA), plasminogen activator inhibitor (PAI-1), von Willebrand factor [21], fibronectin [50], homocysteine [51], and thrombomodulin [52], are elevated, while levels of anticoagulant proteins, including anti-thrombin III, protein C and protein S [53], are reduced. Women with histories of preeclampsia, compared to women with histories of normotensive pregnancies, have a higher incidence of activated protein C resistance, protein S deficiency, anticardiolipin antibodies, factor V Leiden mutations and hyperhomocysteinemia [54, 55]. Similarly, an increased risk for cardiovascular disease has been associated with elevated levels of homocysteine [56] and PAI-1 [57]. Mechanistically, in the setting of low-pressure placental blood flow, the presence of a maternal hypercoagulable state may trigger deposition of fibrin and formation of thrombi, further worsening endothelial dysfunction and placental ischemia [58].
Preeclampsia and Future Hypertension: A Causative Relationship?
It is possible that hypertensive disorders in pregnancy may induce long-term vascular, renal and metabolic changes that may increase future cardiovascular risk (Fig. 1). The supporting evidence comes from studies showing that, despite normalization of blood pressure post-partum, these seemingly healthy women may demonstrate unfavorable metabolic and vascular changes [19], such as an impaired brachial artery flow-mediated (endothelium-dependent) dilatation, a measure of endothelial dysfunction, three years after the diagnosis of preeclampsia [59]. Microalbuminuria, which may be a marker of endothelial dysfunction and/or renal injury, has also been reported to be more prevalent following a preeclamptic pregnancy [60]. The renal lesion associated with preeclampsia, glomeruloendotheliosis, believed to be reversible, may in fact be associated with subtle alterations in kidney function after pregnancy that may contribute to hypertension later in life. In addition, several studies have indicated that cardiac changes, which occur during hypertensive pregnancies and may contribute to future heart disease, do not revert to normal upon delivery. During a hypertensive pregnancy, there may be eccentric and concentric ventricular remodeling [61•], impaired contractility, and diastolic dysfunction [62]. Increased left atrial size has been reported, as well as global right ventricular systolic-diastolic dysfunction. Echocardiographic studies of women with preeclampsia performed one year post-partum showed an increased risk of altered left ventricular geometry (concentric remodelling, eccentric hypertrophy), impaired left ventricular relaxation with global diastolic dysfunction, and mildly impaired radial systolic function (ejection fraction 45 %–55 %), compared to those with normotensive pregnancies [61]. At six months post-partum, a fourfold increase in the risk of diastolic dysfunction was observed in women who had placental syndromes, which, in addition to preeclampsia, included gestational hypertension, HELLP syndrome, placental abruption and fetal growth restriction [63]. Conceivably, these persistent abnormalities may contribute to the risk of future hypertension and heart disease, including heart failure and arrhythmias, which recently have been associated with maternal placental syndromes [7].
Future Perspectives
The precise relationship between hypertensive disorders of pregnancy and later onset of hypertension remains incompletely resolved. Additional research is needed to address the mechanisms underlying the associations between preeclampsia and future hypertension and CVD, leading to specific diagnostic, preventive, and therapeutic measures. If, indeed, hypertension in pregnancy and preeclampsia, in particular, leave permanent vascular and inflammatory changes that increase the risk for hypertension and CVD later in life, improved screening and treatment of hypertension in pregnancy may not only optimize pregnancy outcomes, but may have a long-term positive impact on women’s cardiovascular health. A growing body of evidence demonstrating persistent cardiovascular abnormalities and inflammatory responses may result in a change in clinical practice, and the current guidelines for the management of hypertensive pregnancy, which recommend lenient blood pressure control in the absence of end organ damage [1]. Carefully designed and adequately powered studies are needed to confirm and address the nature of these associations. Until then, treatment of hypertension in pregnancy should remain a matter of carefully weighing the risk/benefit ratio for each individual patient, with the goal to improve maternal and fetal pregnancy outcomes. After pregnancy, regular (annual) surveillance of blood pressure, urine albumin level, fasting glucose and lipid panel, and treatment according to the national guidelines for CVD prevention in women should be implemented, coupled with primary prevention focusing on lifestyle modifications (smoking cessation, healthy diet, exercise and weight loss), in order to optimize maternal cardiovascular health.
Conclusion
Increasing evidence suggests that preeclampsia is associated with increased risks for hypertension and CVD later in life. Preeclampsia may simply serve as a marker for women at risk or, alternatively, it may induce irreversible vascular and metabolic changes that may increase the risk for future hypertension and CVD in the affected women. Future studies are needed to elucidate the mechanisms underlying these associations. Irrespective of the underlying mechanism, a history of preeclampsia may help identify women at CVD risk early in life, thus offering an opportunity for timely screening, and preventive and treatment strategies. A detailed history of pregnancy outcomes should become a standard portion of the risk assessment visit with a doctor; primary prevention in these women should include lifestyle modification. Their modifiable risk factors should be monitored regularly and treated in a timely fashion.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Anonymous: Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy.[comment]. Am J Obstet and Gynecol 2000, 183(1):S1–S22.
• Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–306. This paper examines trends in the rates of hypertensive disorders in pregnancy in the USA in 1998–2000, and reports that the number of delivery hospitalizations with hypertensive disorders in pregnancy is increasing, and that these hospitalizations are associated with a substantial burden of severe obstetric morbidity.
Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101(8):669–74.
Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–59.
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–72.
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis.[see comment]. BMJ. 2007;335(7627):974.
•• Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98(15):1136–41. This paper was the first to report the association between maternal placental syndromes, including preeclampsia, and a higher risk of premature HF and dysrhythmias, especially when perinatal morbidity is present.
Corwin J, Herrick WW. Relation of hypertensive toxemia of pregnancy to chronic cardiovascular disease. JAMA. 1927;88:457–9.
Chesley SC, Annitto JE, Cosgrove RA. The remote prognosis of eclamptic women. Sixth periodic report. Am J Obstet Gynecol. 1976;124(5):446–59.
Chesley LC. Recognition of the long-term sequelae of eclampsia. Am J Obstet Gynecol. 2000;182(1 Pt 1):249–50.
Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis. 2008;2(4):249–59.
McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Hear J. 2008;156(5):918–30.
Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155(5):1011–6.
Selvaggi L, Loverro G, Schena FP, Manno C, Cagnazzo G. Long term follow-up of women with hypertension in pregnancy. Int J Gynaecol Obstet. 1988;27(1):45–9.
Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine. 1981;60(4):267–76.
Lowe SA, Brown MA, Dekker GA, Gatt S, McLintock CK, McMahon LP, et al. Guidelines for the management of hyertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49:242–6.
Garovic VD, Bailey KR, Boerwinkle E, Hunt SC, Weder AB, Curb JD, et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens. 2010;28(4):826–33.
•• Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–62. These are the first guidelines for for the prevention of cardiovascular disease in women that recognize the association between pregnancy conditions, and preeclampsia in particular, and future cardiovascular disease.
Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3(11):613–22.
Islami D, Shoukir Y, Dupont P, Campana A, Bischof P. Is cellular fibronectin a biological marker for pre-eclampsia? Eur J Obstet Gynecol Reprod Biol. 2001;97(1):40–5.
Friedman SA, Schiff E, Emeis JJ, Dekker GA, Sibai BM. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am J Obstet Gynecol. 1995;172(1 Pt 1):202–3.
Halim A, Kanayama N, el Maradny E, Nakashima A, Bhuiyan AB, Khatun S, et al. Plasma P selectin (GMP-140) and glycocalicin are elevated in preeclampsia and eclampsia: their significances. Am J Obstet Gynecol. 1996;174(1 Pt 1):272–7.
Krauss T, Kuhn W, Lakoma C, Augustin HG. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am J Obstet Gynecol. 1997;177(2):443–9.
Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84(6):937–40.
Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia.[see comment]. Br J Obstet Gynaecol. 1995;102(1):20–5.
Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide. 2000;4(4):441–58.
Parthasarathy S, Santanam N. Mechanisms of oxidation, antioxidants, and atherosclerosis. Curr Opin Lipidol. 1994;5(5):371–5.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia.[see comment]. J Clin Investig. 2003;111(5):649–58.
Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M, et al. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension. 2012;59(3):740–6.
Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The national institute of child health and human development network of maternal-fetal medicine Units. Am J Obstet Gynecol. 1995;172(2 Pt 1):642–8.
Kaaja R. Insulin resistance syndrome in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):41–6.
•• Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–34. This review summarizes normal and pathological vessel remodeling in pregnancy and discuss similarities and differences between preeclampsia and arteriosclerosis.
Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group.[comment]. Am J Obstet Gynecol. 1997;177(5):1003–10.
Dunne F, Brydon P, Smith K, Gee H. Pregnancy in women with Type 2 diabetes: 12 years outcome data 1990-2002. Diabet Med. 2003;20(9):734–8.
Griffin BA. Lipoprotein atherogenicity: an overview of current mechanisms. Proc Nutr Soc. 1999;58(1):163–9.
Hubel CA, Lyall F, Weissfeld L, Gandley RE, Roberts JM. Small low-density lipoproteins and vascular cell adhesion molecule-1 are increased in association with hyperlipidemia in preeclampsia. Metab Clin Exp. 1998;47(10):1281–8.
Sattar N, Bendomir A, Berry C, Shepherd J, Greer IA, Packard CJ. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol. 1997;89(3):403–8.
Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS).[see comment]. Circulation. 2001;104(25):3052–6.
Teppa RJ, Ness RB, Crombleholme WR, Roberts JM. Free leptin is increased in normal pregnancy and further increased in preeclampsia. Metab Clin Exp. 2000;49(8):1043–8.
Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, et al. Platelet activation in obese women: role of inflammation and oxidant stress.[see comment]. JAMA. 2002;288(16):2008–14.
Szekeres-Bartho J. Immunological relationship between the mother and the fetus. Int Rev Immunol. 2002;21(6):471–95.
Muller I, Munder M, Kropf P, Hansch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30(11):522–30.
Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–36.
Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis.[see comment]. Am J Obstet Gynecol. 1998;179(1):80–6.
Austgulen R, Lien E, Vince G, Redman CW. Increased maternal plasma levels of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 1997;71(1):53–8.
Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia.[see comment]. Hypertension. 2004;44(5):708–14.
Cadden KA, Walsh SW. Neutrophils, but not lymphocytes or monocytes, infiltrate maternal systemic vasculature in women with preeclampsia. Hypertens Pregnancy. 2008;27(4):396–405.
Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98(5 Pt 1):757–62.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–9.
Ostlund E, Hansson LO, Bremme K. Fibronectin is a marker for organ involvement and may reflect the severity of preeclampsia. Hypertens Pregnancy. 2001;20(1):79–87.
Powers RW, Evans RW, Ness RB, Crombleholme WR, Roberts JM. Homocysteine and cellular fibronectin are increased in preeclampsia, not transient hypertension of pregnancy. Hypertens Pregnancy. 2001;20(1):69–77.
Shaarawy M, Didy HE. Thrombomodulin, plasminogen activator inhibitor type 1 (PAI-1) and fibronectin as biomarkers of endothelial damage in preeclampsia and eclampsia. Int J Gynaecol Obstet. 1996;55(2):135–9.
Paternoster D, Stella A, Simioni P, Trovo S, Plebani P, Girolami A. Clotting inhibitors and fibronectin as potential markers in preeclampsia. Int J Gynaecol Obstet. 1994;47(3):215–21.
Kupferminc MJ, Fait G, Many A, Gordon D, Eldor A, Lessing JB. Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol. 2000;96(1):45–9.
Lin J, August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet Gynecol. 2005;105(1):182–92. doi:10.1097/01.AOG.0000146250.85561.e9.
Mayer EL, Jacobsen DW, Robinson K. Homocysteine and coronary atherosclerosis. J Am Coll Cardiol. 1996;27(3):517–27.
Potter van Loon BJ, Kluft C, Radder JK, Blankenstein MA, Meinders AE. The cardiovascular risk factor plasminogen activator inhibitor type 1 is related to insulin resistance. Metab Clin Exp. 1993;42(8):945–9.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1(1):111.
Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285(12):1607–12.
North RA, Simmons D, Barnfather D, Upjohn M. What happens to women with preeclampsia? Microalbuminuria and hypertension following preeclampsia. Aust N Z J Obstet Gynaecol. 1996;36(3):233–8.
• Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–15. This study evaluated the postpartum natural history and clinical significance of asymptomatic left ventricular impairment known to occur with preeclampsia, and concludes that the cardiovascular implications of preeclampsia do not end with the birth of the infant and placenta.
Bamfo JE, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32(5):682–6.
Zandstra M, Stekkinger E, van der Vlugt MJ, van Dijk AP, Lotgering FK, Spaanderman ME. Cardiac diastolic dysfunction and metabolic syndrome in young women after placental syndrome. Obstet Gynecol. 2010;115(1):101–8.
Nisell H, Lintu H, Lunell NO, Mollerstrom G, Pettersson E. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol. 1995;102(11):876–81.
Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77(2):154–8.
Marin R, Gorostidi M, Portal CG, Sanchez M, Sanchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy. 2000;19(2):199–209.
Hubel CA, Snaedal S, Ness RB, Weissfeld LA, Geirsson RT, Roberts JM, et al. Dyslipoproteinaemia in postmenopausal women with a history of eclampsia. BJOG: Intl J Obstet Gynaecol. 2000;107(6):776–84.
Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845. doi:10.1136/bmj.326.7394.845.
Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42(1):39–42.
Diehl CL, Brost BC, Hogan MC, Elesber AA, Offord KP, Turner ST, et al. Preeclampsia as a risk factor for cardiovascular disease later in life: validation of a preeclampsia questionnaire. Am J Obstet Gynecol. 2008;198(5):e11–3.
Acknowledgments
The project described was supported by Award Number K08 HD051714 (V.D. Garovic) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and by Award Number P-50 AG44170 (V.D. Garovic) from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. The writing of the manuscript and the decision to submit it for publication were solely the authors’ responsibilities.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garovic, V.D., August, P. Preeclampsia and the Future Risk of Hypertension: The Pregnant Evidence. Curr Hypertens Rep 15, 114–121 (2013). https://doi.org/10.1007/s11906-013-0329-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-013-0329-4