Abstract
Denervating the human kidney to improve blood pressure control is an old therapeutic concept first applied on a larger scale by surgeons in the 1920s. With the advent of modern pharmacology and the development of powerful drugs to lower blood pressure, approaches to directly target the sympathetic nerves were more or less abandoned. Over the past 2–3 years, however, we have witnessed enormous renewed interest in novel and minimally invasive device-based approaches to specifically target the renal nerves. The enthusiasm is fueled by promising results from proof-of-concept studies and clinical trials demonstrating convincing blood pressure–lowering effects in the majority of treated patients, and perhaps even more so by observations indicating potential additional benefits relating to common comorbidities of hypertension, such as impaired glucose metabolism, renal impairment, left ventricular hypertrophy, and others. Herein we review the current findings and assess whether these high hopes are justified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sympathetic nervous system acts as an important regulator of many bodily functions. The neural control of the kidney is of particular relevance for volume and sodium homeostasis and renin release, all of which are key components in blood pressure regulation. Indeed, all essential renal structures, including the renal vasculature, the tubules, and the juxtaglomerular apparatus, are innervated by sympathetic nerve fibers [1]. The consequences of activation of renal sympathetic nerves are volume retention via sodium reabsorption [2], a reduction in renal blood flow [3, 4], and activation of the renin-angiotensin-aldosterone system through stimulatory effects on the juxtaglomerular apparatus and subsequent renin release [5].
Aside from these postganglionic efferent sympathetic fibers, the renal nerves also contain sensory afferent fibers [6] to communicate with integral structures in the central nervous system. Activation of afferent nerve fibers can occur in the presence of renal ischemia, hypoxia, oxidative stress, and other triggers [7–9]. Importantly, by modulating posterior hypothalamic activity, renal afferents directly influence sympathetic outflow to the kidneys and other highly innervated organs involved in cardiovascular control [10, 11]. Accordingly, abrogation of renal afferent nerves has been demonstrated to reduce both blood pressure and organ-specific damage caused by chronic sympathetic overactivity in various experimental models [12, 13]. Together with the demonstration that renal sympathetic efferents are generally highly activated in human hypertension, the renal nerve is an ideal therapeutic target.
Evidence for Sympathetic Activation in Human Hypertension
There is regional differentiation with regard to sympathetic outflow to innervated organs such that outflow to one may be increased, while outflow to another may be unchanged or even inhibited. It is therefore important to study regional norepinephrine (NE) kinetics to account for this phenomenon. Studies employing radiotracer dilution methodology to measure overflow of NE from the kidneys to plasma indeed revealed increased renal NE spillover rates in patients with essential hypertension [14, 15]. In addition, elevated NE spillover from the heart is often present, particularly in young hypertensive subjects. Together with the demonstration of increased muscle sympathetic nerve activity [14, 15], this is commensurate with the hemodynamic profile commonly encountered in hypertension, including increased heart rate, cardiac output, and peripheral and renovascular resistance [16]. Interestingly, activation of cardiorenal sympathetic nerve activity is even more pronounced in heart failure, a common clinical consequence of long-term and sustained blood pressure elevation. An exaggerated increase of NE overflow from the heart and the kidneys to plasma has been demonstrated in heart failure patients [17], which can be attenuated significantly by intravenous infusion of the centrally acting α2-adrenoceptor agonist clonidine [18]. Complementary to the beneficial effects of antiadrenergic therapy in the heart, inhibition of sympathetic outflow to the kidneys counteracts salt and water retention, a hallmark of heart failure. Accordingly, a recent study demonstrated renal sympathetic activation to be a strong negative predictor of all-cause mortality and heart transplantation in patients with congestive heart failure [19], indicating that therapeutic efforts to reduce sympathetic outflow to the kidneys may have the potential to improve survival in patients with heart failure.
Renal Denervation as a Therapeutic Concept
Given the described role of both afferent and efferent renal nerves, efforts to therapeutically denervate the kidneys appear obvious. Indeed, renal denervation has been applied successfully to prevent hypertension in a variety of experimental models [20–23]. In humans, surgical approaches using splanchnicectomy and radical sympathectomy were already applied in the 1920s and 1930s to reduce blood pressure in severely hypertensive patients [24, 25]. Although these approaches were not directly targeted at the renal nerves and plagued by high periprocedural complication rates, improvements in blood pressure control and survival were demonstrated in a substantial number of treated patients. Form a pathophysiologic point of view, surgical renal denervation has been shown to be an effective means of reducing sympathetic outflow to the kidneys and increasing urine output (natriuresis and diuresis) and reducing renin release without adversely affecting other functions of the kidney, such as glomerular filtration rate (GFR) and renal blood flow. The experience from kidney transplantations in humans, in the process of which sympathetic nerves of the kidneys are severed, is perhaps the most persuasive evidence to demonstrate that the denervated kidney is capable of maintaining electrolyte and volume homeostasis, suggesting that selective ablation of renal nerves is unlikely to result in adverse consequences.
Catheter-Based Renal Nerve Ablation for Resistant Hypertension
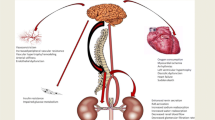
Against this background, a recent safety and proof-of concept study for the first time applied a novel catheter-based technique to selectively denervate the kidneys in patients with treatment-resistant hypertension [26••]. In this approach, renal nerve ablation is achieved percutaneously via the lumen of the renal artery using a catheter connected to a radiofrequency (RF) generator. After the treatment catheter (Symplicity, Ardian, Palo Alto, CA) is introduced, several discrete RF ablations (typically 4–8, depending on the individual renal artery anatomy) are applied and separated both longitudinally and rotationally within each renal artery (Fig. 1). Catheter tip temperature and impedance are constantly monitored during ablation, and RF energy delivery is regulated according to a predetermined algorithm.
Schematic illustration of the percutaneous catheter-based approach to functionally denervate the human kidney. Similar to a routine angiogram, access to the renal artery is obtained via a sheath in the femoral artery. The treatment catheter is then introduced into the renal artery and discrete radiofrequency (RF) ablation treatments lasting 2 min each are applied along the renal artery as illustrated. Up to six ablations are performed in each artery, which are separated both longitudinally and rotationally to achieve circumferential coverage of the renal artery. Catheter tip temperature and impedance are constantly monitored during ablation and RF energy delivery is regulated according to a predetermined algorithm
Vascular and Renal Safety
As for any novel interventional procedure, safety aspects are key to allow for procession to larger clinical trials. Vascular safety analysis in the first proof-of-concept study including 45 patients with treatment-resistant hypertension consisted of renal angiography at 14–30 days after the procedure and MR angiographies at 6 months postprocedure [26••]. There were no instances of renal artery aneurysm or stenosis or other major long-term adverse events. Importantly, renal function remained unchanged. Taken together, these data were indicative of a favorable vascular and renal safety profile. While the ablation procedure is typically accompanied by diffuse visceral nonradiating abdominal pain, this does not persist beyond the RF energy application and can be managed with intravenous narcotics and sedatives [26••].
More recently, results from the first randomized controlled clinical trial including a total of 106 patients were published [27••]. Inclusion criteria were similar to those of the initial safety and proof-of concept trial, with patients required to have a baseline systolic office blood pressure ≥160 mm Hg (≥150 mm Hg for patients with type 2 diabetes) despite compliance with three or more antihypertensive medications. Patients were then randomly assigned to undergo renal nerve ablation treatment (n = 52) or to continue with conventional drug treatment as part of the control group (n = 54).
The two groups had similar baseline characteristics and antihypertensive regimen with the exception of estimated GFR (eGFR), which was lower in the active treatment group (77 mL/min vs 86 mL/min; P = 0.013). Again, renal safety could be confirmed as demonstrated by virtually unchanged mean eGFR in the control and the treatment group at 6-month follow-up [27••].
Effects on Blood Pressure
Aside from vascular and renal safety, efficacy of the procedure in relation to the blood pressure–lowering effect was essential. The initial 45 patients had a mean age of 58 ± 9 years and an average blood pressure of 177/101 ± 20/15 mm Hg at baseline. This was despite concurrent use of a mean of 4.7 ± 1.5 antihypertensive agents. The main findings of this study was that renal nerve ablation was associated with a significant and sustained reduction in both systolic and diastolic office blood pressure up to 12 months follow-up with mean (±95% CI) decreases of -14/-10 ± 4/3, -21/-10 ± 7/4, -22/-11 ± 10/5, -24/-11 ± 9/5 and -27/-17 ± 16/11 mm Hg at 1, 3, 6, 9, and 12 months, respectively.
In keeping with the results from the first trial, a significant difference in the primary end point of seated office blood pressure of 33/11 mm Hg (P < 0.001 for both systolic and diastolic blood pressure) was noted between the renal denervation group and the control group in the Symplicty-2 trial [27••]. Home blood pressure recordings confirmed the observed office blood pressure changes with a reduction in home blood pressure by 20/12 ± 17/11 mm Hg in the renal denervation group and an increase of 2/0 ± 13/7 mm Hg in the control group (P < 0.001). Blood pressure control (defined as systolic blood pressure <140 mm Hg) was achieved in 39% of patients in the denervation group and in 3% of patients in the control group. However, it is also important to note that there is substantial variability with regard to the blood pressure effects, and that the procedure fails to reduce blood pressure in about 10% of treated patients. Whether this may be related to age of patients, duration of hypertension, established target organ damage, the number of ablation treatments, or other factors is currently unclear.
Initial experiences reported from a variety of countries that have started to introduce this novel technology are largely confirmative with regard to the safety of the procedure and the magnitude of the effect on office blood pressure (typically ranging between 20 and 30 mm Hg systolic).
Mechanisms
From a mechanistic point of view, documentation of the effectiveness of the procedure with regard to a reduction in renal sympathetic nerve activity was paramount. Radiotracer dilution methodologies were therefore applied to assess overflow of NE from the kidneys into the circulation before and after the procedure. These analyses revealed a substantial reduction in mean norepinephrine spillover by 47% (95% CI, 28%–65%) 1 month after bilateral denervation. Furthermore, it is also noteworthy that renal denervation decreased renin secretion and increased renal blood flow [28••], confirming successful targeting of efferent renal nerves.
While the contribution of afferent nerves cannot be measured directly in humans, the demonstration of a substantial and progressive reduction in central sympathetic outflow from baseline through to 12-month follow-up is perhaps indicative of similar alterations in afferent fiber signaling that may well play an important role in the blood pressure effects associated with this procedure [28••]. Further support for a role of afferent nerves in this scenario may be derived from experimental studies demonstrating that rats subjected to renal surgical denervation experience functional reinnervation of the renal vasculature that begins to occur 14–24 days after denervation, with complete return of neural function by 8 weeks [29]. It is therefore possible that some efferent sympathetic reinnervation may occur in patients after renal denervation, although the magnitude and time course of this potential response are unknown. Interestingly, in contrast to efferent nerves, the afferent nerves do not appear to have the capacity to regrow [30], thereby perhaps explaining the sustained blood pressure–lowering effect of renal denervation over time via the removal of renal afferent activity and the subsequent effects on central sympathetic outflow [28••]. Indeed, a very recent analysis summarizing the experience from longer-term follow-up of the initial cohort (n = 45) and similar patients subsequently treated with catheter-based renal denervation in a nonrandomized and uncontrolled fashion (total n = 153) demonstrated the sustained efficacy of renal nerve ablation, with postprocedure office blood pressure being reduced by 32/14 mm Hg at 24-month follow-up [31••].
Effects on Hypertension Comorbidities
Impaired Glucose Metabolism
While the available evidence indicates that catheter-based renal denervation has a favorable safety profile and results in substantial and sustained blood pressure reduction in patients with drug-resistant hypertension, the benefit of renal denervation may not be restricted to blood pressure lowering alone. Hypertension is frequently associated with metabolic alterations such as overweight and obesity, impaired fasting glucose, impaired glucose tolerance, and insulin resistance, and sympathetic activation has clearly been identified as an important contributor to this detrimental clinical scenario [32]. Inhibition of the sympathetic nervous system would therefore be expected to improve glycemic control [33]. Indeed, in a group of patients who had renal denervation (n = 37) or served as controls (n = 13), detailed assessment of glucose metabolism was performed by assessing fasting glucose, insulin, C-peptide, HbA1c, calculated insulin sensitivity (HOMA-IR), and glucose levels during oral glucose tolerance test (OGTT) at baseline and at 1- and 3-month follow-up. In addition to the blood pressure fall observed in the treatment group (-32/-12 mm Hg) after 3 months, fasting glucose (from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL [P = 0.039]), insulin levels (from 20.8 ± 3.0 μIU/mL to 9.3 ± 2.5 μIU/mL [P = 0.006]), C-peptide levels (from 5.3 ± 0.6 ng/mL to 3.0 ± 0.9 ng/mL [P = 0.002]) and the HOMA-IR (from 6.0 ± 0.9 to 2.4 ± 0.8 [P = 0.001]) also improved significantly after 3 months. Additionally, mean 2-hour glucose levels during OGTT were reduced significantly by 27 mg/dL (P = 0.012), while there were no significant changes in blood pressure or any of the metabolic markers in the control group.
Further support for such a beneficial role comes from investigations of yet another group of patients commonly characterized by overweight or obesity, sympathetic nervous system activation, insulin resistance, and blood pressure elevation—namely women with polycystic ovary syndrome. Using euglycemic hyperinsulinemic clamps, it was demonstrated that insulin sensitivity improved by 17.5% in the absence of any weight changes at 3 months after renal denervation. Of note, glomerular hyperfiltration and urinary albumin excretion were also reduced, indicating that the benefits of renal denervation may also extend to renal structure and function, as suggested previously [34–36].
While the effects of renal denervation on glucose metabolism described above are primarily of descriptive nature, there is ample evidence highlighting potential mechanisms through which inhibition of sympathetic activation could improve glucose metabolism. In the human forearm, increased NE release results in a substantial reduction in forearm blood flow [37]. This is associated with a markedly reduced uptake of glucose, demonstrating the adverse effect of sympathetic activation on the ability of the cell to transport glucose across its membrane [37]. Experimental and clinical data indicate that the rate of diffusion of a substance decreases with the square of the distance to its target [38], that there is a direct relationship between the sympathetic nerve firing rate to skeletal muscle tissue and insulin resistance [39], and that insulin resistance is inversely related to the number of open capillaries [40]. Furthermore, this situation may be enhanced if insulin resistance is already established, a state in which the ability of insulin to increase muscle perfusion has been demonstrated to be reduced by approximately 30% [41]. In view of these data, it is perhaps not surprising that a procedure that reduces sympathetic nerve activity can also result in improvements in glucose metabolism.
Chronic Renal Failure and End-Stage Renal Disease
Hypertension is present in the majority of patients with chronic and end-stage renal failure [42] and plays a key role in the progression of renal dysfunction and in the exceedingly high rate of cardiovascular events [43, 44]. Although such a role of hypertension is widely accepted, control of blood pressure in this population group is often poor [45, 46]. Hypervolemia and activation of the renin-angiotensin-aldosterone system are important factors contributing to the increase in blood pressure [47], and previous research into therapeutic strategies therefore focused primarily on interventions targeting volume control and the renin-angiotensin-aldosterone system. Indeed, RAS inhibition has been demonstrated to slow progression of renal disease and proteinuria [48].
Despite convincing evidence of increased sympathetic activity in various forms of hypertension, including essential hypertension [15], obesity-related hypertension [49], hypertension associated with obstructive sleep apnea [50], and preeclampsia [51], its involvement in the development of hypertension, progression of renal failure, and cardiovascular prognosis in patients with renal disease has been somewhat neglected in the past. This may be surprising given that elevated plasma NE levels have clearly been demonstrated to be predictive of both survival and incidents of cardiovascular events in patients with end-stage renal disease [52]. Accordingly, progression of renal failure can be delayed by the centrally acting sympatholytic agent moxonidine [53]. Moxonidine has also been demonstrated to reduce microalbuminuria in normotensive patients with type 1 diabetes in the absence of any significant blood pressure changes [54]. Furthermore, bilateral nephrectomy is associated with consistent reductions in blood pressure, total systemic vascular resistance [55], and a substantial decrease in left ventricular (LV) mass of 54 g within 4 months following bilateral nephrectomy [56]. This supports the concept of heightened sympathetic outflow, particularly to the heart, being a main contributor to hypertensive LV hypertrophy [57].
Very recent findings from a pilot study applying catheter-based renal denervation for the first time in patients with moderate to severe chronic kidney disease (mean eGFR, 31 mL/min per 1.73 m2) have shown that 1) renal function as assessed by eGFR was not compromised in these patients and 2) that blood pressure reductions that were achieved are similar to those seen in patients with normal renal function [58••]. Furthermore, augmentation index, an indicator of vascular stiffness, was also significantly reduced after 3 months. Perhaps important in this context is the demonstration that the dipping pattern has also improved in these patients. These findings provide the first evidence of a favorable short-term safety profile and beneficial blood pressure effects of catheter-based renal nerve ablation in patients with stage 3/4 chronic kidney disease and resistant hypertension.
Left Ventricular Hypertrophy
Additional factors that may translate into better outcomes after renal denervation include improvements in cardiac baroreflex sensitivity (from 7.8 to 11.7 ms/mm Hg) and a reduction in LV mass from 184 to 169 g (78.8–73.1 g/m2) at 12-month follow up compared to baseline [28••]. These initial findings have now been confirmed in a larger cohort of 46 patients with resistant hypertension for which they underwent renal denervation [59••]. The authors were able to demonstrate that renal denervation was not only associated with a substantial reduction in systolic and diastolic blood pressure (-22.5/-7.2 mm Hg at 1 month and -27.8/-8.8 mm Hg at 6 months [P < 0.001 at each time point]), but also significantly reduced mean interventricular septum thickness from 14.1 ± 1.9 mm to 13.4 ± 2.1 mm and 12.5 ± 1.4 mm (P < 0.007), and LV mass index from 112.4 ± 33.9 g/m2 to 103.6 ± 30.5 g/m2 and 44.7 ± 14.9 g/m2 (94.9 ± 29.8 g/m2) (P < 0.001) at 1 month and 6 months, respectively. Diastolic function was also improved, as assessed by mitral valve lateral E/E, which decreased after renal denervation from 9.9 ± 4.0 to 7.9 ± 2.2 at 1 month and 7.4 ± 2.7 at 6 months (P < 0.001), indicating reduction of LV filling pressures. Isovolumic relaxation time shortened (baseline, 109.1 ± 21.7 ms vs 85.6 ± 24.4 ms at 6 months [P < 0.006]), whereas ejection fraction significantly increased after renal denervation (baseline, 63.1 ± 8.1% vs 70.1 ± 11.5% at 6 months [P < 0.001]). No such changes were observed in a matched group of 18 control patients. Interestingly, the beneficial effects appeared to be somewhat independent from blood pressure effects, with LV hypertrophy being improved even in those patients who only had a minor or no blood pressure response. These data may indicate that the effects of renal denervation go beyond that of merely reducing blood pressure, and may contribute to regression of hypertensive end-organ damage.
Future Perspectives
Optimal treatment of hypertension remains one of the biggest challenges in clinical medicine. The development and clinical implementation of safe and effective antihypertensive drugs from various classes has advanced the field enormously and continues to prevent and reduce cardiovascular morbidity and mortality worldwide. Nevertheless, many hypertensive patients remain uncontrolled, which exposes them to increased cardiovascular risk. Alternative approaches to curb the burden of hypertension are warranted and may perhaps unexpectedly come from “left field.” Indeed, there appears to be a new and refreshing vibe in the hypertension world centered around device-based approaches, in particular catheter-based renal denervation, the new kid on the block. This approach has a great deal of appeal in that it is based on solid pathophysiologic principles, has been established in a large number of experimental models of hypertension and cardiorenal disease, and most importantly has thus far been demonstrated to be very safe and effective in lowering blood pressure in the limited number of clinical trials in human resistant hypertension. The wealth of review articles on this topic, by far outnumbering original scientific papers, is perhaps testament to this enthusiasm in the scientific world. Furthermore, about a dozen alternative approaches to target the renal nerves directly are currently being developed or tested in preclinical and clinical studies, indicating that the medical device industry also sees enormous potential for this approach.
Conclusions
Are our hopes justified? Is renal denervation the future of hypertension treatment? Only time and larger-scale clinical trials will tell and inform us about the long-term safety, the long-term effectiveness of the procedure with regard to reduction in blood pressure, target organ damage, improvement in hypertension comorbidities, and its potential role in other conditions characterized by sympathetic activation (eg, renal failure, heart failure, metabolic syndrome, diabetes, and others). Currently, reasonably solid data are available only for patients with hypertension resistant to pharmacotherapy, which cannot necessarily be extrapolated to other forms of hypertension or conditions referred to above. However, at this point in time, no clouds have appeared in the sky, so let us dream on.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Barajas L. Innervation of the renal cortex. Fed Proc. 1978;37(5):1192–201.
Bell-Reuss E, Trevino DL, Gottschalk CW. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest. 1976;57(4):1104–7.
Kirchheim H, Ehmke H, Persson P. Sympathetic modulation of renal hemodynamics, renin release and sodium excretion. Klin Wochenschr. 1989;67(17):858–64.
Kon V. Neural control of renal circulation. Miner Electrolyte Metab. 1989;15(1–2):33–43.
Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation. 1977;56(5):691–8.
Campese VM. Neurogenic factors and hypertension in chronic renal failure. J Nephrol. 1997;10(4):184–7.
Ye S, et al. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens. 1998;11(6 Pt 1):723–8.
Ye S, et al. Renal injury caused by intrarenal injection of phenol increases afferent and efferent renal sympathetic nerve activity. Am J Hypertens. 2002;15(8):717–24.
Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int. 2000;57 Suppl 75:S2–6.
Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25(4 Pt 2):878–82.
Campese VM, Kogosov E, Koss M. Renal afferent denervation prevents the progression of renal disease in the renal ablation model of chronic renal failure in the rat. Am J Kidney Dis. 1995;26(5):861–5.
DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11(2):197–200.
DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41(3 Pt 2):621–4.
Esler M, et al. Mechanism of elevated plasma noradrenaline in the course of essential hypertension. J Cardiovasc Pharmacol. 1986;8 Suppl 5:S39–43.
Schlaich MP, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43(2):169–75.
Esler M, Jennings G, Lambert G. Noradrenaline release and the pathophysiology of primary human hypertension. Am J Hypertens. 1989;2(3 Pt 2):140S–6S.
Hasking GJ, et al. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73(4):615–21.
Aggarwal A, et al. Regional sympathetic effects of low-dose clonidine in heart failure. Hypertension. 2003;41(3):553–7.
Petersson M, et al. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J. 2005;26(9):906–13.
DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension. 2004;43(2):147–50.
DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77(1):75–197.
Alexander BT, et al. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45(4):754–8.
Kassab S, et al. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25(4 Pt 2):893–7.
Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152(16):1501–4.
Morrissey DM, Brookes VS, Cooke WT. Sympathectomy in the treatment of hypertension; review of 122 cases. Lancet. 1953;1(6757):403–8.
•• Krum H, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81. This study presents the first data on the safety and efficacy of a novel catheter based approach to functionally denervate the human kidney demonstrating a favorable safety profile and substantial and sustained reductions blood pressure.
•• Esler MD, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9. Initial proof-of-concept studies are now supported by this first randomized controlled clinical trial demonstrating that compared to an untreated control group there is substantial BP reduction associated with renal denervation.
•• Schlaich MP, et al. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361(9):932–4. This study reports on a patient treated with catheter based renal nerve ablation demonstrating a reduction in central sympathetic outflow indicative of a potential involvement of afferent nerve fibers in the sustained blood pressure reduction associated with the procedure and additional benefit by regression of LV hypertrophy.
Kline RL, Mercer PF. Functional reinnervation and development of supersensitivity to NE after renal denervation in rats. Am J Physiol. 1980;238(5):R353–8.
Arrowood JA, et al. Evidence against reinnervation of cardiac vagal afferents after human orthotopic cardiac transplantation. Circulation. 1995;92(3):402–8.
•• Catheter-based renal sympathetic denervation for resistant hypertension. durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–7. This study demonstrates that the blood pressure effects achieved by renal denervation are sustained up to at least 2 years.
Lambert GW, et al. Sympathetic nervous activation in obesity and the metabolic syndrome–causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126(2):159–72.
Straznicky, N.E., et al., Baseline Sympathetic Nervous System Activity Predicts Dietary Weight Loss in Obese Metabolic Syndrome Subjects. J Clin Endocrinol Metab, 2011.
Schlaich MP, et al. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009;54(6):1195–201.
Schlaich MP, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20(5):933–9.
Straznicky, N.E., et al., Exercise augments weight loss induced improvement in renal function in obese metabolic syndrome individuals. J Hypertens. 2011;29(3):553–64.
Jamerson KA, et al. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993;21(5):618–23.
Rasio EA. Passage of glucose through the cell membrane of capillary endothelium. Am J Physiol. 1975;228(4):1103–7.
Grassi G, et al. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22(12):2363–9.
Egan BM. Neurohumoral, hemodynamic and microvascular changes as mechanisms of insulin resistance in hypertension: a provocative but partial picture. Int J Obes. 1991;15 Suppl 2:133–9.
Laakso M, et al. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85(6):1844–52.
Klag MJ, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–8.
Rostand SG, et al. Cardiovascular complications in renal failure [editorial]. J Am Soc Nephrol. 1991;2(6):1053–62.
Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339(12):799–805.
Coresh J, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988-1994). Arch Intern Med. 2001;161(9):1207–16.
Tonelli M, et al. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis. 2001;37(3):484–9.
Lazarus JM, Hampers C, Merrill JP. Hypertension in chronic renal failure. Treatment with hemodialysis and nephrectomy Arch Intern Med. 1974;133(6):1059–66.
Ligtenberg G, et al. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure [see comments]. N Engl J Med. 1999;340(17):1321–8.
Grassi G, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97(20):2037–42.
Narkiewicz K, et al. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32(6):1039–43.
Schobel HP, et al. Preeclampsia – a state of sympathetic overactivity [see comments]. N Engl J Med. 1996;335(20):1480–5.
Zoccali C, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105(11):1354–9.
Vonend O, et al. Moxonidine treatment of hypertensive patients with advanced renal failure. J Hypertens. 2003;21(9):1709–17.
Strojek K, et al. Lowering of microalbuminuria in diabetic patients by a sympathicoplegic agent: novel approach to prevent progression of diabetic nephropathy? J Am Soc Nephrol. 2001;12(3):602–5.
Onesti G, et al. Blood pressure regulation in end-stage renal disease and anephric man. Circ Res. 1975;36(6 Suppl 1):145–52.
Getts RT, et al. Regression of left ventricular hypertrophy after bilateral nephrectomy. Nephrol Dial Transplant. 2006;21(4):1089–91.
Schlaich MP, et al. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108(5):560–5.
•• Hering D MF, Walton AS, Krum H, Lambert G, Lambert EA, Sobotka PA, Böhm M, Cremers B, Esler M, Schlaich MP. Renal denervation in moderate to severe chronic kidney disease. J Am Soc Nephrol. 2012;in press. This pilot study provides first evidence that renal denervation is safe and effective in patients with moderate to severe chronic kidney disease.
•• Brandt MC, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59(10):901–9. This study demonstrates that renal denervation is not only associated with BP reduction but also with a marked regression of LV hypertrophy in patients with resistant hypertension.
Disclosure
Parts of this work were funded by grants from the National Health and Research Council of Australia (NHMRC) and the Victorian Government’s Operational Infrastructure Support Program.
Dr. Schlaich has served on a board for, served as a consultant for, served on the speaker’s bureau for, received grant support from, and received travel support from Ardian/Medtronic.
Dr. Schlaich and Dr. Esler are supported by career fellowships from the NHMRC.
Dr. Hering is currently supported by Research Fellowship from the Foundation for Polish Science KOLUMB/2010-1.
Dr. Schlaich, Dr. Krum, and Dr. Esler are investigators in ClinicalTrials.gov: NCT00483808 and ClinicalTrials.gov: NCT 00551304, sponsored by Ardian/Medtronic.
Dr. Sobotka is an employee of Ardian/Medtronic.
Dr. Krum has served on the speaker’s bureau for Ardian/Medtronic.
Dr. Esler has had travel expenses/accommodations covered/reimbursed by, has served as a consultant for, has received grant support from, and has served on the speakers’ bureau/developed educational presentations for Ardian/Medtronic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlaich, M.P., Hering, D., Sobotka, P.A. et al. Renal Denervation in Human Hypertension: Mechanisms, Current Findings, and Future Prospects. Curr Hypertens Rep 14, 247–253 (2012). https://doi.org/10.1007/s11906-012-0264-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-012-0264-9