Abstract
Infection with the human immunodeficiency virus (HIV), and subsequent treatment with antiretroviral therapy (ART), is often associated with perturbations in lipid profiles. Furthermore, persistent inflammation, in spite of suppression of viral replication by ART, likely contributes to modifications in lipid composition and function, exacerbating risk for development of cardiovascular disease (CVD). Increased levels of several pro-inflammatory lipid species, including oxidized low-density lipoprotein (LDL) and high-density lipoprotein (HDL), have been measured in HIV-infected persons and are associated with markers of immune activation. The mechanisms linked to this bidirectional relationship in which inflammation increases lipid levels and promotes their modification, and these modified lipid species perpetuate inflammatory processes, require further investigation. Treatment with statins and other lifestyle modifications, including improvement in dietary intake and exercise, are critical to reducing CVD risk. Well-designed clinical trials that take into account the complex relationships among lipids and inflammation within persons infected with HIV need to be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
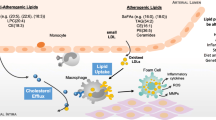
Human immunodeficiency virus (HIV) infection [1], its treatment [2], and chronic inflammation [3•] can all alter lipid and metabolic profiles [4, 5]. Advances in the effectiveness and availability of modern antiretroviral therapy (ART) combinations have prolonged the lives of persons living with HIV, but these patients are at an increased risk of cardiovascular disease (CVD) with CVD events occurring at younger ages for this population [6]. Careful monitoring and treatment of lipid levels, and measurement of emerging particle subclasses and function, are likely more informative in persons infected with HIV than among persons who are not infected, due to the complex and multidirectional relationships among diet, genetic factors, ART, viral replication, chronic inflammation, and lipid metabolism (Fig. 1).
Infection with HIV, and subsequent treatment with ART, is often associated with perturbations in lipid levels. Alterations in lipid profiles among HIV+ persons are complicated further by the bidirectional relationships among lipid metabolism and transport and persistent inflammation and immune activation. The effects of lipid-lowering agents, including statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors), on lipid profiles and levels of chronic inflammation are currently being explored in persons living with HIV
HIV Infection and ART Alter Lipid Profiles by Traditional Lipid Measurement, Decreasing HDL and Increasing LDL and Triglycerides
Infection with HIV [1] and subsequent treatment with antiretroviral therapy [2, 7, 8] has been associated with changes in lipid concentrations, including decreased levels of high-density lipoprotein (HDL) cholesterol and increased levels of low-density lipoprotein (LDL) cholesterol, total cholesterol (TC), and triglycerides. Environmental factors, including diet, genetic factors, and ART-induced adipose tissue dysfunction and dyslipidemia, all likely contribute to metabolic disease in chronic HIV infection [2]. The altered lipid profiles associated with chronic ART-treated HIV infection may, in part, explain the increased risk of cardiovascular disease (CVD) events that has been reported for HIV+ persons, compared to uninfected controls [4, 9]. The metabolic effects of specific combinations of ART have been extensively reviewed [2, 10]. In brief, modern ART regimens, that often include integrase inhibitors, are more “lipid friendly” than older drug combinations that rely more heavily on protease inhibitors (PI) and certain nucleoside reverse transcriptase inhibitors (NRTIs) [2, 9], and may have fewer adverse cardiovascular complications [11]. Yet, even among HIV+ persons receiving regimens that are not known to adversely modulate lipid profiles, alterations in lipid concentration and composition are often observed [2].
Traditional Lipid Panels May not Adequately Assess Lipid Changes and CVD Risk in HIV Infection
While traditional lipid measurements of concentration (total HDL, LDL, and triglycerides) are performed routinely in the clinic, altered lipoprotein species and lipid particle sizes are closer correlates of cardiovascular disease risk than these commonly measured lipid fractions in both HIV-1-infected [12, 13] and -uninfected populations [14, 15]. Lipoprotein subclass profiles change following initiation of ART [13, 16] and during treatment interruption [17]. Patients undergoing antiretroviral treatment interruption, compared to those undergoing continuous therapy, demonstrate significant decreases in total, large, and TG concentrations, but due to concurrent decreases in HDL particle concentrations, the overall lipid profile remained atherogenic within these individuals [17]. Further, in patients switching from a PI-based ART regimen to one using an integrase inhibitor, significant improvements in the lipid profiles of these patients (i.e., decreased LDL, TC, TG, and an increase in the apolipoprotein A1/apolipoprotein B (ApoA1/ApoB ratio)) have been reported [16].
In addition to measurement of ApoA1 and ApoB, emerging NMR spectroscopic studies [18, 19] have demonstrated a close relationship between HDL particle size and HDL function, measured by HDL cholesterol efflux capacity (CEC). CEC permits understanding of how HDL derived from a patient sample performs reverse cholesterol transport, a process whereby cholesterol from peripheral tissues is removed from the body, excreted in the bile. This CEC can be measured reliably using patient serum and a validated assay; however, CEC remains only for research purposes. The potential prognostic clinical value of CEC is growing; CEC has been related to future CV events [20], CVD by coronary angiography [18], as well as to non-calcified plaque burden [21] in non-HIV patients. Among HIV-infected persons with “normal” lipid profiles based on traditional measures, an increase in LDL particle number, and a decrease in large HDL particles and reduced CEC, suggested that traditional lipid panels may not adequately describe the atherogenic potential of lipid profiles in HIV infection [22•].
Until recently, the dynamic changes in lipid profile composition, HDL function, and inflammatory biomarker levels have not been adequately described following ART initiation. In a substudy of the AIDS Clinical Trials Group (ACTG) A5248 Study [23, 24], authors examined complex lipid phenotypes, including lipid particle number and size, and HDL function by HDL efflux capacity, longitudinally following ART initiation using a raltegravir (RAL)-based regimen. RAL is an HIV-1 integrase inhibitor that is thought to be relatively “lipid-neutral.” Participants initiating RAL had rapid reductions in viremia [23] without normalization of plasma levels of inflammation [24]. Significant increases in TC (13 mg/dL; p < 0.001), LDL (8 mg/dL; p = 0.03), and HDL (7 mg/dL; p < 0.001) were seen following 48 weeks of ART. The HDL increase was accompanied by increases in HDL particle number (4 umol/L; p < 0.001) and HDL efflux (7 % increase; p < 0.001), but these beneficial HDL changes did not reach levels measured in a demographically matched HIV− group [25]. Retrospective studies among HIV+ donors who experienced a CVD event, that measure cholesterol particle size, concentration, and function, may provide an incremental increase in the ability of clinicians to identify HIV+ persons at risk for a CVD event.
Chronic Inflammation in HIV Infection: Cause or Consequence of Altered Lipid Profiles?
Alterations in lipid profiles among HIV+ persons are complicated further by the persistent inflammation and immune activation that are often reported in HIV infection, even when viral replication is suppressed by ART [26, 27]. While combination ART increases dramatically the expected lifespan of HIV-infected persons, cardiovascular disease risk is increased in this population [28–32]. Increased immune activation in HIV infection likely contributes to increased venous and arterial thromboses [6–8, 33–41] and may be a cause or consequence of altered lipid profiles. The Strategies for Management of Anti-Retroviral Therapy (SMART) study found that plasma levels of interleukin-6 (IL-6), C-reactive protein (hsCRP), and D-dimer products of fibrinolysis are independent predictors of mortality, including deaths related to CVD, in HIV infection [42, 43]. The SMART study also demonstrated that total, large, and small HDL particles, but not very low-density LDL (VLDL) or LDL particles, were associated with risk for CVD events, and that there was an inverse relationship between HDL particle numbers and IL-6 and D-dimer levels [13]. Piconi et al. have also reported that LDL and ApoA1 are better correlates of CVD risk in ART-treated HIV+ persons than are inflammatory markers (CRP, TNF-α) [44]; yet, it is difficult to separate the affects lipid profiles and inflammation on CVD risk, as they are closely linked.
Inflammation Modulates Lipid Particles and Lipid Transport
Lipids are important in CVD associated with HIV infection, and persistent inflammation may also be critical or even synergistic, in CVD risk via lipid-mediated pathways. Levels of inflammatory markers (IL-6 and hsCRP) before initiation of ART are inversely related to improvements in HDL particle number and ApoA1 following 6 months of ART [45]. Chronic inflammatory conditions, including systemic lupus erythematosus, rheumatoid arthritis [46], and psoriasis [47], much like HIV infection, have been associated with increases in atherogenic lipid profiles and decreases in HDL levels and function. Several mechanisms for modulating cholesterol homeostasis in the blood compartment and within the walls of the blood vessels are closely regulated by inflammation and the acute phase response [3•, 48]. Reverse cholesterol transport (RCT), where HDL removes atherogenic lipid molecules from atherosclerotic plaques for clearance by the liver [49], is impaired by inflammation [48]. Macrophages within the vascular wall shuttle atherogenic lipid molecules onto HDL or ApoA1 for reverse cholesterol efflux though a number of ATP-binding cassette transporters (ABC transporters), initiating RCT, and leading to cholesterol excretion [50, 51]. Exposure to Toll-like receptor ligands (TLRs), including lipopolysaccharide (LPS), can modulate expression of ABC transporters and reduce efficiency of reverse cholesterol transport [48]. This may be of particular importance in HIV infection, as plasma levels of LPS and other microbial products are increased in HIV-infected persons as a result of microbial translocation [52–55]. Maisa et al. recently reported that monocytes exposed to pooled serum from ART-treated persons infected with HIV more readily become foam cells than cells exposed to pooled serum from HIV− donors, and that monocytes from HIV+ donors also had lower levels of ABCA1 gene expression [56•], suggesting that both monocyte and HDL function may contribute to decreased cholesterol efflux in HIV infection, likely driven by chronic immune activation.
Inflammation and Oxidative Modulation of Lipids
Chronic innate immune activation in ART-treated HIV infection [26] may promote increases in LDL levels by altering how lipids are processed and transported, and immune activation likely enhances modification of these increased lipid molecules through the activity of reactive oxygen species (ROS) or enzymes such as lipoprotein-associated phospholipase A2 (Lp-PLA2) [57], rendering them more “inflammatory.” Modified lipid species, including oxidized forms of LDL (oxLDL) [58, 59] and HDL (HDLox) [60, 61], may contribute directly to monocyte [62] and endothelial cell activation in HIV disease, placing them on the mechanistic pathway for increased inflammation, immune activation, and CVD risk. Results from the ACTG substudy A5260s demonstrate that levels oxidized HDL are related to markers of inflammation and immune activation at baseline (IL-6, hsCRP, %CD38 + HLA − DR + CD8+ T cells, sCD163) and following 96 weeks of ART (markers as before, including sCD14) [63]. Oxidized HDL can induce cellular activation through the scavenger receptor CD36 on monocytes/macrophages [61].
OxLDL levels are increased in HIV infection [62], confirming a previous report [64]. Also, in HIV+ individuals, levels of oxLDL, but not LDL, were related to sCD14 and the proportion of CD14 + CD16+ “inflammatory” monocytes that express tissue factor (TF) [62]. In a separate study, initiation of ART resulted in decreases in oxLDL levels by week 12 (p = 0.02), but these levels rose subsequently, concurrent with LDL increases; interestingly, sCD14 levels also fell rapidly, plateaued by week 24, but rose between weeks 24 and 48 [25]. Oxidized LDL can activate monocytes and endothelial cells through interactions with the lectin-like oxidized low-density lipoprotein receptor-1 (Lox-1) [65–67] or the heterotrimer of CD36/TLR4/TLR6 [58, 59, 68] increasing expression of adhesion molecules [65, 68], chemokines, and cytokines [58, 59]. Monocytes from HIV+ individuals exposed to oxLDL resulted in greater production of IL-1β than did oxLDL stimulated cells from uninfected donors [69]. Primary blood monocytes increase TF expression in response to oxLDL [68], which may contribute to activation of the extrinsic coagulation cascade and increases in D-dimer levels [70, 71].
Oxidized LDL-induced activation of monocytes and macrophages may be an important contributor of blood vessel inflammation in HIV infection, as markers of monocyte/macrophage activation (sCD14 and sCD163) have been linked to oxLDL levels [62], coronary calcium [72], mortality [73, 74], and with non-calcified coronary artery plaques [75], with perivascular fat [76], and with arterial inflammation in HIV-infected subjects [77]. Macrophage-induced vascular inflammation may be generated, in part, by the activity of Lp-PLA2 [57], an enzyme produced by macrophages that can cleave oxLDL into lysophosphatidylcholine (LPC) and oxidized-free fatty acids (oxFA). Levels of Lp-PLA2 are related to both oxLDL levels and carotid atherosclerosis [78], and risk for coronary heart disease in HIV-uninfected individuals [79]. A relationship between oxLDL levels and carotid intima-media thickness (cIMT) in HIV+ persons has been reported [80]. Levels of oxLDL in carotid plaques from HIV− donors are directly related to macrophage infiltration, and plaque oxLDL levels can be 70 times greater than levels in circulation [81]. Modulation of pro-inflammatory lipid levels and myeloid cell activation are targets for therapeutic intervention in chronic HIV infection.
Inflammation and Lipid Subclass by Lipidomics
Oxidized forms of HDL and LDL provide “model” pro-inflammatory lipids that can be measured for a gross estimate of lipids that may contribute to inflammation in HIV infection. Several lipid subclasses are pro-inflammatory and contribute to CVD in the general population [82–84]. Cholesterol crystals, a potential byproduct of inefficient RCT, can activate macrophages, inducing inflammasome activation and production of IL-1β [85], a cytokine that is associated ischemic heart disease [86]. Advances in liquid chromatography electrospray ionization tandem mass spectrometry (LC ESI-MS/MS) can provide information on the biosynthesis and metabolism of over 1000 individual lipid species, many of which can module inflammation [87, 88]. Recently, Wong et al. measured the lipidome of 113 donors, including an HIV-uninfected group, and a subgroup of HIV+ donors with samples available within 1 year of CVD diagnosis [89]. Eighty-three individual lipid species, among several lipid classes, were associated with HIV infection, including: ceramides (Cer), phosphatidylcholine (PC), and several species of diacy- and triacylglycerols (DG and TG). Further analyses described an association among DGs, TGs, Cer, and cholesterol esters, with risk for CVD in HIV+ individuals. Similar lipid profiles are related to CVD risk [90], including non-calcified coronary plaque [91, 92] and diabetes [93] in HIV− donors. Many of these lipid species have pro-inflammatory properties. Ceramides induce NFkB and pro-inflammatory cytokines in mice [94] and are related to mortality in chronic heart failure in humans [95]. Several lipid species not reported by the Wong study, including eicosanoids, molecules derived from oxidation of ω-3 and ω-6, are involved in pro-inflammatory signaling [96], and their levels should be assessed samples from HIV+ and well-matched HIV− donors. Currently, high-resolution longitudinal analyses of changes in lipid species among persons infected with HIV have not been reported and deserve consideration.
Lipid-Lowering Therapies, Especially Statins, Are an Important Secondary Treatment Strategies in ART+ HIV+ Patients
Perturbations in lipid profiles and the increased risk for CVD reported in chronic HIV infection [1, 4, 6–8, 97] have generated significant interest in modulating lipid levels and inflammation in HIV+ persons, especially though the use of statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) [98]. The effects of statin use among HIV-infected individuals have recently been reviewed [99], and overall, statin use was well-tolerated and improved lipid profiles. Recently, two double blind, placebo controlled trials among HIV-infected participants (with LDL cholesterol <130 mg/dL) have demonstrated significant beneficial effects on markers of inflammation and CVD risk [100–102, 103•]. Nou et al. report that significant reductions in non-calcified plaque volumes were measured by coronary CT angiography among HIV+ participants receiving atorvastatin [103•]. The Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV (SATURN-HIV) randomized 147 HIV+ ART+ participants with normal LDL (<130 mg/dL), but with elevated markers of inflammation/immune activation (hsCRP > 2.0 mg/dL or CD8 + CD38 + HLA − DR + ≥19 %) to treatment with 10 mg of rosuvastatin or placebo, and changes in several biomarkers of CVD risk and immune activation were measured over 96 weeks. Results from SATURN-HIV also demonstrated, for the first time in ART-treated subjects, that rosuvastatin treatment reduced levels of T cell and monocyte activation markers [101, 102], including reduction in monocyte tissue factor expression, and sCD14, a marker of monocyte activation linked to morbidity in HIV+ patients [73, 74]. This study also showed that statin therapy reduced vascular inflammation [100, 101] and markers of cardiac strain, and may preserve renal function in ART-treated HIV+ subjects [104].
Both studies also report that statin use reduces levels of Lp-PLA2 and oxLDL [100, 101, 103•, 105•]. Atorvastatin use decreased oxLDL levels, and this change was associated with a reduction in non-calcified plaque volume, independent of viremia, CD4 count, or LDL levels [103•]. Hileman et al. report that rosuvastatin treatment-induced changes in oxLDL were directly related to decreases in sCD14 and TF expression on patrolling monocytes (CD14Dim CD16+), and improvements in cIMT [105•]. The association between changes in oxLDL levels and improvements in cIMT remained after adjusting for other known risk factors including age, sex, race, smoking, BMI, HOMA-IR, hepatitis C status, nadir CD4+ cell count, and PI use, placing oxLDL on the causal pathway of CVD progression in HIV infection [105•]. Statin pre-treatment also maintained the expression levels of the anti-inflammatory transcriptional regulator, Kruppel-like factor 2 (KLF-2), in an aortic endothelial cell line exposed to oxLDL in vitro, potentially providing another link to statin use and improvement in vascular inflammation and function [106]. These results add to the growing interest in the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE, NCT02344290), where 6500 HIV-infected participants will be randomized to pitavastatin or placebo, and will be followed for clinical endpoints. Modulation of lipid composition and function by other methods should also be considered. Recently, sevelamar, a phosphate-lowering drug, lowered LDL, oxLDL, and soluble TF levels in HIV-infected participants [107]. Studies that explore the beneficial effects of exercise and dietary interventions in dyslipidemic ART+ participants, with a focus on changes in the microbiome, lipidome, and inflammatory markers, should also be explored further.
Conclusion
HIV is associated with primary and treatment associated lipid disturbances. Furthermore, persistent inflammation in treated HIV patients contributes to modifications of lipid composition and function, further potentially exacerbating CVD. Treatment with statins and other lifestyle modifications are critical to mitigate CVD risk, but the optimal strategies remain to be determined with ongoing clinical trials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–52.
Lake JE, Currier JS. Metabolic disease in HIV infection. Lancet Infect Dis. 2013;13:964–75.
Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16. This excellent review describes the complex relationship between inflammation and lipid metabolism in health and disease.
Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62.
Rose H, Hoy J, Woolley I, et al. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199:79–86.
Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12.
Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35.
Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS (London, England). 2003;17:1179–93.
Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr (1999). 2008;49 Suppl 2:S79–85.
Willig AL, Overton ET. Metabolic consequences of HIV: pathogenic insights. Curr HIV/AIDS Rep. 2014.
Monforte A, Reiss P, Ryom L, et al. Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS (London, England). 2013;27:407–15.
Bucher HC, Richter W, Glass TR, et al. Small dense lipoproteins, apolipoprotein B, and risk of coronary events in HIV-infected patients on antiretroviral therapy: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr (1999). 2012;60:135–42.
Duprez DA, Kuller LH, Tracy R, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–9.
Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. Jama. 2009;302:412–23.
McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case–control study. Lancet. 2008;372:224–33.
Saumoy M, Sanchez-Quesada JL, Martinez E, et al. LDL subclasses and lipoprotein-phospholipase A2 activity in suppressed HIV-infected patients switching to raltegravir: spiral substudy. Atherosclerosis. 2012;225:200–7.
Lampe FC, Duprez DA, Kuller LH, et al. Changes in lipids and lipoprotein particle concentrations after interruption of antiretroviral therapy. J Acquir Immune Defic Syndr (1999). 2010;54:275–84.
Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35.
Mehta NN, Li R, Krishnamoorthy P, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–21.
Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93.
Salahuddin T, Natarajan B, Playford MP, et al. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J. 2015;36:2662–5.
Munger AM, Chow DC, Playford MP, et al. Characterization of lipid composition and high-density lipoprotein function in HIV-infected individuals on stable antiretroviral regimens. AIDS Res Hum Retrovir. 2015;31:221–8. This manuscript describes an atherogenic lipid profile in HIV-infected donors who had a benign lipid profile based on traditional lipid measurements. These results suggest a more in depth assessment of lipid profiles in persons infected with HIV may be of value in determining CVD risk.
Andrade A, Rosenkranz SL, Cillo AR, et al. Three distinct phases of HIV-1 RNA decay in treatment-naive patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J Infect Dis. 2013;208:884–91.
Funderburg NT, Andrade A, Chan ES, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naive patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One. 2013;8, e83514.
Funderburg NTX, Playford D, Andrade M, Kuritzkes A, Lederman D, Mehta MM, et al. Treatment of HIV disease with a raltegravir-based regimen increases LDL levels, but improves HDL composition and function. 17th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV Barcelona, Spain, 2015.
Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83.
Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr Opin HIV AIDS. 2013.
d’Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS (London, England). 2004;18:1811–7.
Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS (London, England). 2008;22:1615–24.
Mangili A, Gerrior J, Tang AM, et al. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis. 2006;43:1482–9.
Mooser V. Atherosclerosis and HIV in the highly active antiretroviral therapy era: towards an epidemic of cardiovascular disease? AIDS (London, England). 2003;17 Suppl 1:S65–9.
Periard D, Cavassini M, Taffe P, et al. High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis. 2008;46:761–7.
Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000;11:41–6.
Matta F, Yaekoub AY, Stein PD. Human immunodeficiency virus infection and risk of venous thromboembolism. Am J Med Sci. 2008;336:402–6.
Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8.
Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS (London, England). 2006;20:2275–83.
Hsue PY, Giri K, Erickson S, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109:316–9.
Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9.
Crum-Cianflone NF, Weekes J, Bavaro M. Thromboses among HIV-infected patients during the highly active antiretroviral therapy era. AIDS patient care and STDs. 2008.
Fultz SL, McGinnis KA, Skanderson M, Ragni MV, Justice AC. Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans. Am J Med. 2004;116:420–3.
Klein SK, Slim EJ, de Kruif MD, et al. Is chronic HIV infection associated with venous thrombotic disease? A systematic review. Neth J Med. 2005;63:129–36.
Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5, e203.
Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44.
Piconi S, Parisotto S, Rizzardini G, et al. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS (London, England). 2013;27:381–9.
Baker JV, Neuhaus J, Duprez D, et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS (London, England). 2011;25:2133–42.
Liao KP, Playford MP, Frits M, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc. 2015;4.
Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006;2:99–106.
McGillicuddy FC, de la Llera MM, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–45.
Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–63.
Rader DJ, Tall AR. The not-so-simple HDL story: is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–6.
Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–75.
Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006.
Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndromes. Blood. 2012.
Jiang W, Lederman MM, Salkowitz JR, Rodriguez B, Harding CV, Sieg SF. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in alpha/beta interferon responsiveness and production. J Virol. 2005;79:4109–19.
Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS (London, England). 2008;22:2035–8.
Maisa A, Hearps AC, Angelovich TA, et al. Monocytes from HIV-infected individuals show impaired cholesterol efflux and increased foam cell formation after transendothelial migration. AIDS (London, England). 2015;9:1445–57. The authors provide evidence that monocytes/macrophages from HIV-infected donors are more likely to become foam cells when exposed to pooled serum samples than are cells from HIV-uninfected donors and that these changes in macrophage function may be related to TNF-alpha.
Akerele OA, Cheema SK. Fatty acyl composition of lysophosphatidylcholine is important in atherosclerosis. Med Hypotheses. 2015.
Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20.
Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61.
Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J. 2001;15:2073–84.
Thorne RF, Mhaidat NM, Ralston KJ, Burns GF. CD36 is a receptor for oxidized high density lipoprotein: implications for the development of atherosclerosis. FEBS Lett. 2007;581:1227–32.
Zidar DA, Juchnowski S, Ferrari B, et al. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr. 2015.
Kelesidis TJ, McComsey N, Brown GA, Wang TT, Yang X, Stein OO, et al. Oxidized HDL is associated with biomarkers of inflammation and immune activation in untreated and treated HIV infection. In: Infectious diseases society of America, ID Week. (San Diego, CA).
Duong M, Petit JM, Martha B, et al. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV Clin Trials. 2006;7:41–7.
Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25:419–29.
Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–95.
Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20:1116–22.
Owens 3rd AP, Passam FH, Antoniak S, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–68.
Jalbert E, Crawford TQ, D’Antoni ML, et al. IL-1Beta enriched monocytes mount massive IL-6 responses to common inflammatory triggers among chronically HIV-1 infected adults on stable anti-retroviral therapy at risk for cardiovascular disease. PLoS One. 2013;8, e75500.
Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–7.
Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–7.
Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS (London, England). 2014;28:969–77.
Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210:1228–38.
Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90.
Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–36.
Longenecker CT, Jiang Y, Yun CH, et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int J Cardiol. 2013;168:4039–45.
Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. Jama. 2012;308:379–86.
Vickers KC, Maguire CT, Wolfert R, et al. Relationship of lipoprotein-associated phospholipase A2 and oxidized low density lipoprotein in carotid atherosclerosis. J Lipid Res. 2009;50:1735–43.
White HD, Simes J, Stewart RA, et al. Changes in lipoprotein-associated phospholipase A2 activity predict coronary events and partly account for the treatment effect of pravastatin: results from the Long-Term Intervention with Pravastatin in Ischemic Disease study. J Am Heart Assoc. 2013;2, e000360.
Parra S, Coll B, Aragones G, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–31.
Nishi K, Itabe H, Uno M, et al. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22:1649–54.
Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57.
Holvoet P, Theilmeier G, Shivalkar B, Flameng W, Collen D. LDL hypercholesterolemia is associated with accumulation of oxidized LDL, atherosclerotic plaque growth, and compensatory vessel enlargement in coronary arteries of miniature pigs. Arterioscler Thromb Vasc Biol. 1998;18:415–22.
Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–94.
Rajamaki K, Lappalainen J, Oorni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5, e11765.
Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–6.
Weir JM, Wong G, Barlow CK, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54:2898–908.
Chiu S, Williams PT, Dawson T, et al. Diets high in protein or saturated fat do not affect insulin sensitivity or plasma concentrations of lipids and lipoproteins in overweight and obese adults. J Nutr. 2014;144:1753–9.
Wong G, Trevillyan JM, Fatou B, et al. Plasma lipidomic profiling of treated HIV-positive individuals and the implications for cardiovascular risk prediction. PLoS One. 2014;9, e94810.
Martinelli N, Girelli D, Malerba G, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008;88:941–9.
Ueeda M, Doumei T, Takaya Y, et al. Serum N-3 polyunsaturated fatty acid levels correlate with the extent of coronary plaques and calcifications in patients with acute myocardial infarction. Circ J. 2008;72:1836–43.
Ellims AH, Wong G, Weir JM, Lew P, Meikle PJ, Taylor AJ. Plasma lipidomic analysis predicts non-calcified coronary artery plaque in asymptomatic patients at intermediate risk of coronary artery disease. Eur Heart J Cardiovasc Imaging. 2014;15:908–16.
Hodge AM, English DR, O’Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86:189–97.
Wu D, Ren Z, Pae M, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–39.
Yu J, Pan W, Shi R, et al. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can J Cardiol. 2015;31:357–63.
Hardwick JP, Eckman K, Lee YK, et al. Eicosanoids in metabolic syndrome. Adv Pharmacol. 2013;66:157–266.
Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr (1999). 2009;51:268–73.
Longenecker CT, Eckard AR, McComsey GA. Statins to improve cardiovascular outcomes in treated HIV infection. Curr Opin Infect Dis. 2016;29:1–9.
Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol. 2015;115:1760–6.
Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209:1156–64.
Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr (1999). 2015;68:396–404.
Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–95.
Nou E, Lu MT, Looby SE, et al. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with HIV. AIDS (London, England). 2016;30:583–90. The authors provide compelling data suggesting that oxLDL, and changes in oxLDL following statin therapy, is likely on the causal pathway for immune CVD risk in HIV infection.
Longenecker CT, Hileman CO, Funderburg NT, McComsey GA. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: the SATURN-HIV trial. Clin Infect Dis. 2014;59:1148–56.
Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS (London, England). 2016;30:65–73. This work places oxidized LDL on the causal pathway for progression of CVD in ART-treated HIV infection. Changes in oxidized LDL levels were independently associated with changes in carotid intima media thickness, even when correcting for other risk factors.
Panigrahi S, Freeman ML, Funderburg NT, et al. SIV/SHIV infection triggers vascular inflammation, diminished expression of Kruppel-like factor 2 (KLF2) and endothelial dysfunction. J Infect Dis. 2015.
Sandler NG, Zhang X, Bosch RJ, et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210:1549–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nicholas T. Funderburg reports grants from NHLBI and has served as consultant for Gilead Inc.
Nehal N. Mehta declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Complications of Antiretroviral Therapy
Rights and permissions
About this article
Cite this article
Funderburg, N.T., Mehta, N.N. Lipid Abnormalities and Inflammation in HIV Inflection. Curr HIV/AIDS Rep 13, 218–225 (2016). https://doi.org/10.1007/s11904-016-0321-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-016-0321-0