Abstract
Mathematical models of HIV prevention interventions often provide critical insights related to programmatic design and economic efficiency. One recent dynamic model by Long et al. highlights that a combination prevention approach – with testing, treatment, circumcision, microbicides and PrEP – may decrease transmissions by over 60 % and may be very cost-effective in South Africa. In this analysis, the authors introduce the critical concept of joint effectiveness of preventions programs and demonstrate how some programs operate synergistically (HIV screening coupled with early treatment) while others may create redundancies (microbicides coupled with pre-exposure prophylaxis). Whether combination HIV prevention programs perform with additive, multiplicative or maximal effectiveness will be important to consider in anticipation of their combined transmission impact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Once frustrated by a history of negative prevention trials and of “treatment vs. prevention” antagonism [1], the world of HIV prevention has had recent remarkable successes [2••, 3, 4, 5••]. With reductions in transmission demonstrated through HIV screening, early treatment, circumcision, and pre-exposure prophylaxis (PrEP, among others), research now focuses on how to effectively, efficiently, and affordably coalesce and deploy each of these proven interventions into populations – the question of “combination prevention.”
The combination prevention question may best be answered with a randomized, controlled clinical trial (RCT), the research method long-considered to deliver the most credible form of scientific evidence [6–8]. However, combination HIV prevention trials are notorious for their obstacles – challenges which include large sample size requirements, rare outcomes (incidence), poor adherence to the intervention, loss to follow-up, and enormous costs. The number of worthwhile HIV prevention trials clearly outstrips the available resources.
Despite these challenges, several large randomized combination prevention trials have recently been selected in partnership with major funding agencies: the Botswana Combination Prevention Program (Centers for Disease Control and Prevention and PEPfAR) [9] and the PopART Trial (South Africa and Zambia, National Institutes of Health, HPTN) [10]. These trials each develop a combination prevention package to be examined in an intervention group that is randomized and compared to a control group receiving the current standard of prevention care. The trials are all in their early stages of development (none yet enrolling), envision 4-5-year horizons, and together total over $60 million in costs. One additional trial – the French Agence Nationale de Recherches Sur le Sida (ANRS) Treatment as Prevention “TasP” Trial – is slightly ahead of the others, launched in a pilot phase in South Africa in 2011 [11].
In situations where rare events, long-term outcomes, ethical considerations, and costs make trials difficult, model-based analyses may offer important insights and are increasingly used as an alternative form of information. Outcomes from such models are inherently linked to their embedded assumptions associated with model structure and parameterization.
Portfolios of Biomedical HIV Interventions in South Africa [12•]
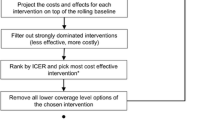
A recent paper by Long et al. entitled “Portfolios of Biomedical HIV Interventions in South Africa: A Cost-effectiveness Analysis” sought to examine individual and combination prevention packages through a cost-effectiveness lens [12•]. Model outcomes included HIV prevalence and incidence, discounted (3 % per annum) quality-adjusted life expectancy, costs and incremental cost-effectiveness ratios (ICERs). The authors built a dynamic HIV transmission model to evaluate the following prevention strategies, alone and in combination: HIV screening and counseling (annually), early antiretroviral therapy (ART, to start at CD4 <350/μl), male circumcision (75 % uptake of adult men within 5 years), vaginal microbicide (used by 50 % of women) and pre-exposure prophylaxis (PrEP, used by 50 % of those at-risk).
Outcomes for Single Interventions
In this paper, when individual prevention strategies were examined in isolation, the largest fraction of infections averted over a ten-year horizon occurred with PrEP (28 %), followed by HIV screening (16 %) and early ART (15 %), and then by microbicide (14 %) and circumcision (12 %). Though it provided the least individual prevention benefit, circumcision was the only intervention that was cost-saving, compared to the status quo over a 10-year horizon. PrEP averted the most infections and had an incremental cost-effectiveness ratio of $9000/quality-adjusted life year (QALY). While this is reported as “very cost-effective” in South Africa, the PrEP ICER was nearly ten-fold higher than all of the other individual programs examined.
Outcomes for Multiple Interventions
The authors next examined combination sets of interventions: 1) screening + early ART, which work synergistically -- the more ongoing screening, the larger the pool eligible for early treatment; 2) “biomedical programs” (circumcision, microbicide and PrEP) which operate at less than the sum of the efficacy of their parts – because a transmission event cannot happen more than once per person; and 3) the “combination portfolio” with all five interventions included together. The “combination portfolio” resulted in the largest fraction of infections averted (62 %), followed by the “biomedical programs” (44 %), and finally by screening + early ART (34 %). Incrementally comparing these three combination prevention sets, the authors found that “biomedical prevention” was a dominated strategy – that is, it was more expensive and conferred less prevention benefit than the “combination portfolio.” The screening + early ART strategy had an incremental cost-effectiveness ratio of $1000/QALY, compared to the status quo. Compared to screening and early ART (because “biomedical programs” was dominated), the “combination portfolio” had an incremental cost-effectiveness ratio of $9900/QALY, again “very cost-effective” in South Africa.
Discussion
Long et al. provide a strong modeling analysis that demonstrates the epidemic impact, costs and cost-effectiveness of alternative prevention services both alone and when delivered as a package. The paper highlights that a large combination portfolio – inclusive of HIV screening, early treatment, circumcision, microbicides and PrEP – could collectively decrease new HIV infections by 62 % over ten years and provide very good value for money in South Africa.
One unique feature of this paper is that it examined the “joint effectiveness” of interventions. This concept acknowledges that two prevention programs may work neither additively nor synergistically when simultaneously employed, thereby potentially creating programmatic redundancy. Suppose program X reduces incidence by 20 % and program Y reduces incidence by 30 %. If the effects of these two programs are additive, the resultant incidence of running both programs together is I * (1 – 0.2 – 0.3) = 0.5 I. If their effects are multiplicative, the resultant incidence of running both programs together is I * (1 – 0.2) * (1 – 0.3) = 0.56 I. Finally in the most conservative scenario, only the maximal effectiveness applies (i.e., the maximal effectiveness of any single one of the N programs examined), in this case I*(1 – 0.3) = 0.7 I. While the authors examine multiplicative effectiveness in the base case, they also consider outcomes under maximal effectiveness assumptions and thereby demonstrate the potential for a marked decrease in anticipated prevention effects.
Why is “joint effectiveness” a critical issue in the design and powering of combination prevention trials? Consider a scenario where we divide a population in half: in one half (A), the “test and treat approach” is employed (frequent testing and immediate treatment) whereby nearly all HIV-infected persons are virologically suppressed; in the other half (B), all at-risk persons are PrEP recipients. If at-risk persons in B (on PrEP), only interact with HIV-infected persons in B who are not necessarily virologically suppressed, PrEP will have a multiplicative effect with “test and treat” provided to those in A. However, if all at-risk people in B (on PrEP) only interact with HIV-infected persons in A who are fully suppressed due to “test and treat,” the used resources for PrEP are largely wasted. Data regarding populations “mixing” are critical to the outcomes and value of combination prevention programs but are a seldom collected or reported behavioral data point.
A less innovative but important point in understanding cost-effectiveness analyses related to prevention is one of discounting. Standard methods of cost-effectiveness analysis, as employed in the Long paper, suggest that costs and outcomes over time should be discounted, generally at 3 % per annum [13•]. At an annual discount rate of 3 %, a promise to pay $100 five years from now has a present value of $86 ($100 / (1 + 0.03)^5 = $86). If those $100 are paid 10 years from now, the present value of the promise is $74 ($100 / (1 + 0.03)^10 = $74). Because of this discounting effect, large prevention investments required today that only pay off in the more distant future – such as transmission events that may not be averted and treated until years from now – are generally less attractive than those investments might be if applied to interventions that might have a more immediate pay off. For example, Long et al. used PrEP and early ART costs each at $800 annually [12•]. The impact of the PrEP intervention will only be realized later, when averted transmissions occur and then present to care; the benefits of early ART will be realized immediately as clinical benefit to the treated patient as well as later when averted transmissions occur.
Finally, Long et al. demonstrate that the combination portfolio is “very cost-effective” in South Africa, according to the standards suggested by the WHO [14]. The WHO’s Commission on Macroeconomics and Health has offered that an intervention be considered “very cost-effective” if its incremental cost-effectiveness ratio is less than the per capita gross domestic product (GDP) based on purchasing power parity (PPP) of the country of interest, 10,520 for South Africa, 2010 [15]. Because the cost-effectiveness benchmark is set by country, it is critical to recognize that combination prevention interventions deemed “very cost-effective” in South Africa, may not be so (even when accounting for alternative cost and care structures) in other sub-Saharan nations such as Malawi and Mozambique (2010 per capita PPP GDPs of 820 and 1010, respectively) [15].
Conclusions
In a modeling analysis examining the dynamics of HIV transmission in South Africa, Long et al. found that a combination prevention package may lead to a large reduction in transmission and may be very cost-effective. The analysis is novel in its examination of the potential redundancies in certain prevention programs and in its demonstration that the efficacy of all prevention programs may not be additive. Caution should be taken when generalizing the cost-effectiveness findings to other international settings, where resources may be more severely limited than those in South Africa.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Marseille E, Hofmann PB, Kahn JG. HIV prevention before HAART in sub-Saharan Africa. Lancet. 2002;359(9320):1851–6.
•• Abdool Karim Q, Abdool Karim SS, Frohlich JA et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. The first clinical trial demonstrating the efficacy of PrEP in women in South Africa.
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99.
•• Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. The landmark trial demonstrating the efficacy of early ART for prevention of HIV transmission.
Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–92.
Kaptchuk TJ. The double-blind, randomized, placebo-controlled trial: gold standard or golden calf? J Clin Epidemiol. 2001;54(6):541–9.
LeLorier J, Gregoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337(8):536–42.
Botswana Harvard AIDS Institute Partnership: Mochudi prevention project. 2011. http://www.hsph.harvard.edu/bhp/research/hiv_prevention/mochudi_prevention.html. Accessed 24 May 2013.
HIV Prevention Trials Network. HPTN 071, population effects of antiretroviral therapy to reduce HIV transmission (PopART): a cluster-randomized trial of the impact of a combination prevention package on population-level HIV incidence in Zambia and South Africa. 2013. http://www.hptn.org/research_studies/hptn071.asp. Accessed 23 May 2013.
Agence Nationale de Recherches sur le Sida et les Hépatites Virales. ANRS 12249 TASP. 2012. http://www.anrs.fr/index.php/content/download/3809/20567/file/ANRS-12249.pdf. Accessed 23 May 2013.
• Long EF, Stavert RR. Portfolios of biomedical HIV interventions in South Africa: a cost-effectiveness analysis. J Gen Intern Med. 2013. The paper being reviewed in this commentary: a modeling paper on combination HIV prevention.
• Gold M, Siegel J, Russell L, et al. Cost effectiveness in health and medicine. New York: Oxford University Press; 1996. A frequently cited book on the gold-standard for methods in cost-effectiveness.
Macroeconomics and Health: Investing in Health for Economic Development Geneva: World Health Organization, Commission on Macroeconomics and Health. 2001.
World Economic Outlook Database. International Monetary Fund. 2013. http://www.imf.org/external/pubs/ft/weo/2013/01/weodata/index.aspx. Accessed 23 May 2013.
Acknowledgments
The author would like to thank A. David Paltiel, PhD for his critical review of the manuscript and Yoriko Nakamura for her technical assistance.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Rochelle P. Walensky has been a consultant for Le Clair Ryan.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walensky, R.P. Combination HIV Prevention: The Value and Interpretation of Mathematical Models. Curr HIV/AIDS Rep 10, 195–198 (2013). https://doi.org/10.1007/s11904-013-0167-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-013-0167-7