Abstract
Purpose of Review
The treatment landscape for chronic lymphocytic leukaemia (CLL) is rapidly evolving, with several targeted agents recently approved. These compounds have dramatically changed the natural history of the disease.
Recent Findings
However, with the array of effective therapies commercially available, the challenge is to define tailored treatment strategies able to realize a balance between treatment efficacy and toxicity or tolerance. New algorithms of treatment are being developed, and it appears that minimal residual disease (MRD) directed therapy will become the norm in the future.
Summary
Clinical trials are looking at various combinations of novel therapies given with a defined, fixed-period of treatment based on MRD analysis. This approach enables patients to have a period of treatment-free remission instead of continuous therapy. In this review, we summarize this evolution of targeted therapies in CLL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
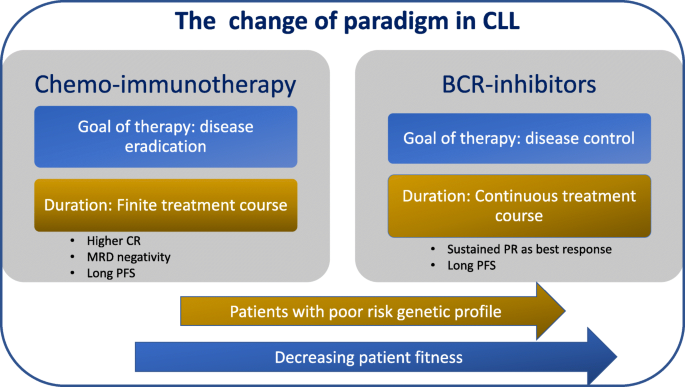
Over the last few years, amazing progresses have been made in the therapy of patients with chronic lymphocytic leukaemia (CLL). At the beginning, chemoimmunotherapy (CIT) led to the identification of increasingly effective combinations, even though the price of these successes translated into a prolonged myelosuppression and long-term bone marrow damage [1••, 2]. More recently, targeted therapy for CLL has radically modified the paradigm of treatment (Fig. 1). Clinical trials of Bruton tyrosine kinase (BTK) inhibitor-based therapies, mainly ibrutinib, have demonstrated that these compounds are highly effective in the disease control, shrinking bulk of disease, especially nodal disease [3, 4••]. However, deep remissions are uncommon with ibrutinib and treatment is given usually continuously, until progression or unacceptable toxicity [5••]. Venetoclax, an oral BCL2 inhibitor, with high capability to induce apoptosis in CLL cells by a p53-independent mechanism [6], has been initially approved for treatment of relapsed/refractory (R/R) patients including patients with del(17p) [7••, 8••]. Of note the efficacy of venetoclax is high in patients progressing after B cell receptor (BCR) inhibitors’ failure [9, 10•].
All these targeted agents, initially tested in patients with R/R disease, have been subsequently shown to be effective for previously untreated patients, and in this setting, combinations with monoclonal antibodies have been studied [11••, 12, 13••, 14, 15••].
Targeted agents are now being extensively studied in fixed time-duration regimes, which have the benefit of potentially reduced toxicity, resistance, and cost. The challenge is to use agents which deepen response for a fixed duration time mimicking the schedule of CIT (Table 1). A key question with this approach is whether a fixed duration treatment would be used for all patients or whether the duration of treatment should be defined on an individual patient basis, depending on their response. To this end, the assessment of minimal residual disease (MRD) in CLL is increasingly being used to assess response and even to provide a tailored individual patient’s treatment.
The Lesson of Clinical Trials of Ibrutinib
Ibrutinib was initially studied in the setting of R/R, heavily pre-treated, high-risk patients who experienced with chemotherapy a dismal clinical outcome [3]. Results of RESONATE phase 3 clinical trial [16••] enabled the Food and Drug Administration (FDA), in February 2014, to approve ibrutinib for the treatment of patients with CLL who had received at least one prior therapy. A few months later, ibrutinib also received breakthrough therapy designation for its use in CLL patients with del 17p. Results of RESONATE trial have been recently updated [5••] providing the longest follow-up analysis never reported with an oral target inhibitor in CLL. With up to 6 years of follow-up, extended ibrutinib treatment yielded sustained efficacy in patients with R/R CLL and small lymphocytic lymphoma (SLL) when compared with ofatumumab. Also safety remained acceptable, with low rates of treatment discontinuation due to adverse events, thus establishing long-term benefit and tolerability of ibrutinib in the treatment of R/R CLL [5••, 17].
After multiple studies in relapsed CLL [18,19,20], in 2016, ibrutinib was approved as a single agent given continuously for first-line treatment in older patients with CLL. According to extended follow-up results of a phase 3 trial, ibrutinib resulted in a long-term progression-free survival (PFS) benefit versus chemotherapy [21•]. The quality of response to ibrutinib continued to improve over time in the study, including a substantial increase in the proportion of patients achieving complete response (CR).
Until very recently, however, we did not have convincing evidence supporting a shift from chemotherapy to ibrutinib in upfront. Data from RESONATE-2 [11••, 21•], showing the superiority of ibrutinib as a monotherapy over chlorambucil, were not conclusive because chlorambucil as a single agent is a very uncommonly treatment for CLL nowadays. Only recently, we have had data that inform decision for this patient subset. The ALLIANCE A041202 [13••] is a phase 3 trial which randomized previously untreated older patients with CLL to either ibrutinib, ibrutinib with rituximab, or bendamustine/rituximab (BR). The study showed that there was a significant improvement of PFS in both ibrutinib arms over BR arm. Of note, rituximab did not add any significant benefit to ibrutinib, single agent. Looking at different subsets of patients on this trial, those patients who achieved greater benefit were patients with unmutated IgHV. The ibrutinib-based regimens did not lead to an overall survival (OS) gain over BR. This is most likely due to the fact that the study allowed for a crossover and most of the patients who progressed after BR were suitable to then go on to receive ibrutinib.
The iLLUMINATE is an industry-sponsored study comparing ibrutinib plus obinutuzumab to chlorambucil plus obinutuzumab in untreated CLL/SLL patients ≥ 65 or younger patients with comorbidities [14]. PFS at 30 months in the ibrutinib plus obinutuzumab group was significantly longer than that in chlorambucil plus obinutuzumab group (79% vs 31%). In this study, however, the benefit of adding obinutuzumab is not clear because a third arm with ibrutinib monotherapy was missed.
The real question is whether the results of these trials are practice-changing. For elderly untreated patients with TP53 mutation or del(17p), ibrutinib is the mandatory regimen. The improved PFS observed with ibrutinib-containing regimens in patients with unmutated IgHV suggests that such an approach is a reasonable choice in this setting. However, when selecting the best treatment strategies for patients without TP53 mutation or del(17p), several variables should be considered commencing with patients’ preferences for different treatment features and ending with benefit–risk trade-offs and out-of-pocket cost. Without evidence of a survival benefit with ibrutinib in elderly CLL patients, CIT (i.e. BR or chlorambucil plus obinutuzumab) might also be a reasonable option in some patients. In the ALLIANCE study [13••], the median PFS is 41 months with BR. This regimen provides approximately 3 and a half years until disease progression and possibly even more time until the next therapy is needed. Since ibrutinib is a great salvage regimen associated with long remissions (i.e. median PFS 44 months), the chance for patients treated with a sequencing of BR and ibrutinib is to reach a PFS of 85 months.
With respect to dilemma of upfront therapy in young, fit CLL patients without significant medical problems, relevant information for clinical practice comes from ECOG-ACRIN trial [15••]. In the ECOG study, there was a significant PFS benefit for the ibrutinib-rituximab regimen over fludarabine, cyclophosphamide, and rituximab (FCR). Interestingly, the advantage of ibrutinib-rituximab involved also OS. However, when focusing on the IgHV-mutated group, no difference in terms of PFS or OS was found between ibrutinib and FCR. Given the fact that we have long-term data with FCR suggesting a plateau with a functional cure for about half of patients with mutated IgHV, FCR should be still the preferred therapy in this setting. In contrast, ECOG study provides a strong evidence in favour of ibrutinib-rituximab for patients with unmutated IgHV, even in the younger population.
The Challenge of Chemo-Free Time-Limited Therapy
Although ibrutinib has been established as a reliable and convenient orally administered agent in the frontline setting for patients with treatment-naïve CLL, the indefinite course of therapy can pose a challenge. In a real-world analysis, intolerance (particularly cardiac dysrhythmias and increased risk of bleeding) was shown to be the main reason for discontinuation [18,19,20]. It is also important to be aware of the “financial toxicities” associated with a recommended “life-long” treatment. Another relevant problem with ibrutinib is the development of ibrutinib-resistant CLL clones which occurs in about 20% of patients [22, 23, 24••, 25]. In the majority of patients progressing on ibrutinib, BTK or phospholipase Cg2 (PLCG2) resistance mutations predate clinical progression by up to 15 months. Resistance to ibrutinib generally correlates with progressive CLL or Richter transformation (RT). Early progression (i.e. < 12 months) being generally related to development of RT while progression occurring beyond 12 months is more likely to be determined by BTK or PLCG2 mutations [24••, 25].
In this context, it is important to identify novel patient-tailored treatment strategy harmonizing treatment efficacy and toxicity or tolerance [26]. Because of aforesaid limitations of ibrutinib therapy, a fixed duration time chemotherapy-free regimen seems to provide a reasonable approach for patients with pre-existing medical conditions. The phase 3 CLL14 trial addresses the issue investigating the efficacy of the fixed duration venetoclax–obinutuzumab combination given for 12 months compared to the previously established regimen of chlorambucil–obinutuzumab in patients with untreated CLL and coexisting conditions [12]. The 2-year PFS for the venetoclax–obinutuzumab group was significantly higher compared with the chlorambucil–obinutuzumab group: 88% compared with 64%. This benefit also included the patients with TP53 deletion/mutation in addition to patients with unmutated IgHV. Three months following treatment completion, a higher number of patients in the venetoclax–obinutuzumab group had achieved MRD negativity in peripheral blood (PB, 76% vs 35%) and in bone marrow (BM, 57% vs 17%). The median OS was not reached in either group. The differences in grade 3 or 4 neutropenia, infections, and all-cause mortality were not statistically significant between the two arms. Tumour lysis syndrome (TLS) was reported in three patients in the venetoclax–obinutuzumab group and in five patients in the chlorambucil–obinutuzumab group. The superiority in PFS benefit favouring the venetoclax–obinutuzumab group matched the acceptable toxicity profile and resulted in the approval of venetoclax–obinutuzumab in patients with untreated CLL and multiple comorbidities by the FDA in May 2019.
Although venetoclax adds an important option to frontline treatment of CLL, without studies of direct comparison between ibrutinib and venetoclax, it is difficult to establish the relative efficacy of these two agents. The German CLL group has announced the launch of the upcoming CLL17 trial investigating the efficacy and safety of single-agent ibrutinib compared with venetoclax–obinutuzumab compared with ibrutinib plus venetoclax. When results of this trial will be available, some of the ambiguities around frontline novel agents in CLL will be further deciphered, potentially translating into more positive change for patients with CLL.
MRD: a Novel Endpoint with Targeted Agents
In both treatment-naïve or R/R CLL patients, the highly selective BCL2 inhibitor venetoclax, alone or in association with an anti-CD20 monoclonal antibody, induces deep responses, including undetectable minimal residual disease (uMRD) [12, 27]. As shown in studies of CIT [1••, 28, 29], also with venetoclax uMRD translates into a longer PFS [27]. Therefore, a question is whether combination strategies, based on the association of agents which target different pathways (i.e. BCR and BCL-2), may improve the depth of response obtained with single agents. Preclinical studies have suggested synergism between ibrutinib and venetoclax [30]. These agents have complementary activity in controlling CLL across anatomical compartments. Ibrutinib is also more active in lymph nodes, while venetoclax is more active in blood and marrow [3, 30, 31]. These observations have represented a strong rationale for testing in venetoclax-ibrutinib combinations of clinical trials.
In the CLARITY study, R/R CLL patients were treated with a combination of ibrutinib and venetoclax with primary and secondary endpoints MRD eradication after 12 and 6 months of combination therapy, respectively [32]. The overall response rate (ORR) was 89% in 53 enrolled patients, and 36% of them reached MRD negativity after 12 months of combination therapy. The same combination evaluated in 80 previously untreated high-risk and older patients with CLL led to an 88% CRs, 61% showing uMRD [33].
CAPTIVATE (PCYC-1142) is a multi-centre phase 2 study (NCT02910583) evaluating the combination of ibrutinib plus venetoclax in first-line treatment of CLL/SLL patients younger than 70 years [34]. Enrolled patients received single-agent ibrutinib lead-in for 3 cycles followed by ibrutinib plus venetoclax for 12 cycles. In total, 151/162 enrolled patients (92%) completed ibrutinib lead-in and all 12 cycles of ibrutinib plus venetoclax. uMRD was achieved at any time after baseline in more than 70% of patients in both PB and BM.
The triplet combination of umbralisib (a novel PI3K inhibitor) and ublituximab (U2) plus venetoclax demonstrated a tolerable toxicity profile in patients with R/R CLL in a phase 1–2 clinical trial producing an ORR of 90% (CR, 29%) and a 2-year PFS of 90% [35]. Undetectable MRD was achieved in PB and BM in 58% of patients.
Efforts are under way to examine the ibrutinib, venetoclax, obinutuzumab (IVO) combination triplet that is highly active R/R CLL with a high number of patients achieving uMRD, particularly given that this was in the relapsed setting [36].
The first-line triplet regimen consisting of acalabrutinib, venetoclax, and obinutuzumab (AVO) induced uMRD in the BM after only 8 monthly cycles of therapy in 48% of patients with CLL, which improved to 75.0% after cycle 16 [37]. The safety profile of AVO was favourable. Notably, the rate of infusion-related reactions was markedly lower with AVO when compared with historical data for obinutuzumab alone or with chemotherapy. In addition, pretreatment with acalabrutinib and obinutuzumab reduced the risk of TLS at the time of venetoclax administration.
Two phase 3 studies are evaluating a time-limited combination including ibrutinib, venetoclax, and obinutuzumab (IVO) with the current standard of indefinite ibrutinib (plus obinutuzumab) (Clinical-Trials.gov identifier:NCT03701282; ClinicalTrials.gov identifier: NCT03737981).
Thus, the MRD measurement might become an appropriate tool to assess efficacy and direct therapeutic decisions also in the clinical management of CLL, with a modulation of treatment on the basis of patient need. However, the predictive value of MRD status, durability of response, fixed treatment durations and, importantly, criteria for selection of patients for the optimal combinations are still unanswered questions.
Evolving Role of BTK Inhibitors in CLL
Adverse events (AEs) have led to discontinuation of ibrutinib in 9–14% of patients in clinical studies and about in 22% of patients in routine clinical practice [5••, 11••, 18,19,20, 21•, 38]. In this respect, it is of interest to note that with acalabrutinib, a potent, highly selective covalent BTK inhibitor, toxicities which are associated with ibrutinib were less frequently observed. Among 33 R/R CLL patients who had discontinued ibrutinib because of intolerance, no acalabrutinib dose reductions were needed, although treatment grade 3/4 AEs occurred, most commonly neutropenia (12%) and thrombocytopenia (9%) [39]. Of note, acalabrutinib, based on findings from the randomized phase 3 ELEVATE-TN [40•] and ASCEND [41•] trials, received approval from the FDA for the treatment of adult patients with CLL or SLL. These results allowed acalabrutinib approval for both indications (i.e. treatment naïve and R/R CLL patients) in November 2019 by FDA [42].
Of note, ELEVATE-TN study examined chlorambucil/obinutuzumab versus acalabrutinib alone versus acalabrutinib plus obinutuzumab [39]. Acalabrutinib alone and acalabrutinib/obinutuzumab had significantly improved PFS along with a 2-year PFS rate of about 90%.
In the acalabrutinib monotherapy arm (n = 179), the most common AEs of any grade (30%) included headache (36.9%) and diarrhoea (34.6%). These results are encouraging for a patient population that is known to face multiple comorbidities and where tolerability is a critical factor in their treatment. There appears also to be a beneficial trend with the addition of obinutuzumab to acalabrutinib, which demonstrates, for the first time, the potential PFS benefit of adding obinutuzumab to a BTK inhibitor in a randomized trial.
Other second-generation irreversible BTK inhibitors (i.e. zanubrutinib, tirabrutinib) are also being studied now [43, 44]. In particular, a phase 3 study comparing zanubrutinib to ibrutinib (NCT03734016) will further assess the safety and efficacy of this novel second-generation irreversible BTK inhibitor in R/R CLL. Results of the safety and efficacy for zanubrutinib in treatment-naïve patients with del(17p) CLL/SLL who are enrolled in the non-randomized arm C of the SEQUOIA (BGB-3111-304) trial have been recently presented [45]. The ORR was 92.2%. Importantly, only two patients had disease progression due to RT, and only one patient died due to grade 5 pneumonia. In this study, including one of the largest prospective cohorts of treatment-naïve patients with del(17p) CLL/SLL zanubrutinib was active and generally well tolerated.
Conclusions
Recent trials in upfront suggest that ibrutinib or an ibrutinib combination with an anti-CD20 monoclonal antibody should be considered standard of care for most patients with CLL [13••, 14, 15••, 46]. Acalabrutinib was shown as a single agent to have very impressive results, and the association with obinutuzumab seems to improve the depth of response [39]. These results suggest that acalabrutinib is the more direct competitor of ibrutinib. A number of questions remain, however, with MRD, in particular regarding criteria for selection of patients for the optimal combinations [47]. On the basis of the available evidence, it can be hypothesised that a combination of targeted agents, having uMRD as endpoint and given for limited time, could be most useful in patients with high-risk disease (e.g. pre-treated patients or patients with TP53 aberrations or complex karyotypes) in whom rapid eradication of the disease is desirable to prevent the emergence of resistant clones and to prolong, possible, survival. In contrast, a sequential continuous approach might be satisfactory for patients with low-risk disease, especially in older patients (aged > 70 years) with an acceptable burden of comorbidities.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–15 Long-term follow-up of the randomized CLL8 trial reporting safety and efficacy of FC and FCR treatment of previously untreated CLL patients.
Kutsch N, Bahlo J, Robrecht S, et al. Long term follow-up data and health-related quality of life in frontline therapy of fit patients treated with FCR versus BR (CLL10 trial of the GCLLSG). Hemasphere. 2020;4(1):e336.
Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
•• Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007 First published phase 3 study showing the combination of idelalisib and rituximab, as compared with placebo and rituximab, significantly improved progression-free survival, response rate, and overall survival among patients with relapsed CLL who were less able to undergo chemotherapy.
•• Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63 The Authors report the longest follow-up of CLL patients receiving an oral targeted agent.
Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12:1691–700.
•• Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–20 The first trial investigating time-fixed therapy with oral inhibitor in CLL. Based on these results the EMA approved the combination of venetoclax with rituximab in R/R CLL patients.
•• Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36:1973–80 The Authors report results of a phase 2 trial enrolling patients harbouring del(17p), mostly refractory to CIT. Based on these results venetoclax was firstly approved as monotherapy for CLL patients with del(17p) or TP53 mutation.
Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19:65–75.
• Mato AR, Hill BT, Lamanna N, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28(5):1050–6 Retrospective, non-randomized study on large cohort of patients treated with BTKi or BCL2i.
•• Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37 First published phase 3 study establishing ibrutinib as a frontline therapy for patients with CLL.
Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumabin chronic lymphocytic leukemia. Blood. 2017;129:2702–5.
•• Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–28 Phase 3 study establishing superior PFS with ibrutinib compared with chemoimmunotherapy in older CLL patients and establishing lack of additional efficacy of rituximab combined with ibrutinib.
Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicenter, randomized, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56.
•• Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–43 The study showed a significant PFS benefit for the ibrutinib-rituximab regimen over FCR in unmutated IGHV patients.
•• Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23 First randomized study comparing ibrutinib with ofatumumab in previously treated patients with advanced progressive CLL. Based on the results ibrutinib was approved for R/R CLL patients.
Brown JR, Hillmen P, O'Brien S, et al. Extended follow-up and impact of high risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83–91.
UK CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–72.
Winqvist M, Asklid A, Andersson PO, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 2016;101(12):1573–80.
Coutré SE, Furman RR, Flinn IW, et al. Extended treatment with single-agent Ibrutinib at the 420 mg dose leads to durable responses in chronic lymphocytic leukemia/small lymphocytic lymphoma. Clin Cancer Res. 2017;23(5):1149–55.
• Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;4(3):787–98 The longest follow-up of first-line ibrutinib treatment for CLL patients.
Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80–7.
Woyach JA. How I manage ibrutinib-refractory chronic lymphocytic leukemia. Blood. 2017;129(10):1270–4.
•• Woyach JA, Ruppert AS, Guinn D, et al. BTKC481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017;35(13):1437–43 Analysis of CLL patients treated with ibrutinib across four prospective studies characterizing prevalence of BTK and PLCG2 mutations and chronological relationship with disease progression.
Quinquenel A, Fornecker LM, Letestu R, et al. Prevalence of BTK and PLCG2 mutations in a real-life CLL cohort still on ibrutinib after three years: a FILO group study. Blood. 2019;134(7):641–4.
Molica S, Giannarelli D, Shanafelt TD. Comparison of venetoclax plus rituximab with B-cell receptor inhibitors in patients with relapsed/refractory chronic lymphocytic leukemia: a systematic review and network meta-analysis. Leuk Lymphoma. 2020 Apr;61(4):955–8.
Kater AP, Seymour JF, Hillmen P, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269–77.
Bottcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30(9):980–8.
Kovacs G, Robrecht S, Fink AM, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL study group. J Clin Oncol. 2016;34(31):3758–65.
Deng J, Isik E, Fernandes SM, et al. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic. Leukemia. 2017;31(10):2075–84.
Roberts AW, Ma S, Kipps TJ, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukaemia is influenced by disease and response variables. Blood. 2019;134(2):111–22.
Hillmen P, Rawstron AC, Brock K, et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study. J Clin Oncol. 2019;37(30):2722–9.
Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–103.
Tam CS, Siddiqi T, Allan JN, et al. Ibrutinib (Ibr) plus venetoclax (ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): Results from the MRD cohort of the phase 2 CAPTIVATE study. Blood. 2019;134(1):35.
Davids MS, Kim HT, Nicotra A, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 2019;6(1):e38–47.
Rogers KA, Huang Y, Ruppert AS, et al. Phase 1b study of obinutuzumab, ibrutinib, and venetoclax in relapsed and refractory chronic lymphocytic leukemia. Blood. 2018;132(15):1568–72.
Lampson BL, Tyekucheva S, Crombie JL, et al. Preliminary safety and efficacy results from a phase 2 study of acalabrutinib, venetoclax and obinutuzumab in patients with previously untreated chronic lymphocytic leukemia (CLL). [Abstract]. Blood. 2019;134(S1):32.
Coutré SE, Byrd JC, Hillmen P, et al. Long- term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3(12):1799–807.
Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3(9):1553–62.
• Sharman JP, Banerji V, Fogliatto LM, et al. ELEVATE TN: Phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL). [Abstract]. Blood. 2019;134(S1):31 Based on these results FDA approved acalabrutinib for untreated CLL patients.
• Ghia P, Pluta A, Wach M, et al. ASCEND phase 3 study of acalabrutinib vs investigator’s choice of rituximab plus idelasib (IDR) or bendamustine (BR) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). [Abstract]. EHA Library. 2019;LB2606. Based on these results FDA approved acalabrutinib for R/R CLL patients.
U.S. Food and Drug Administration. Project Orbis: FDA approves acalabrutinib for CLL and SLL. https://www.fda.gov/drugs/resources-information-approved-drugs/project-orbis-fda-approves-acalabrutinib-cll-and-sll. [Accessed 26 November 2019].
Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–9.
Walter HS, Rule SA, Dyer MJ, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127(4):411–9.
Tam CS, Robak T, Ghia P, et al. Efficacy and safety of zanubrutinib in patients with treatment-naive chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): initial results from arm C of the Sequoia (BGB-3111-304) trial. [Abstract]. Blood. 2019;134(S1):499.
Molica S, Giannarelli D, Baumann T, Montserrat E. Ibrutinib as initial therapy in chronic lymphocytic leukemia: a systematic review and meta-analysis. Eur J Haematol. 2020. https://doi.org/10.1111/ejh.13387.
Molica S, Giannarelli D, Montserrat E. Minimal residual disease and survival outcomes in patients with chronic lymphocytic leukemia: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2019;19(7):423–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Valentina Gianfelici, Luciano Levato, and Stefano Molica do not declare any conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on T-Cell and Other Lymphoproliferative Malignancies
Rights and permissions
About this article
Cite this article
Gianfelici, V., Levato, L. & Molica, S. The Evolution of Targeted Therapies in Chronic Lymphocytic Leukaemia. Curr Hematol Malig Rep 15, 343–349 (2020). https://doi.org/10.1007/s11899-020-00586-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-020-00586-1