Abstract
Modern guidelines based on a large international consensus indicate that treatment of newly diagnosed acute promyelocytic leukemia (APL) requires distinguishing at presentation low-intermediate (<10 × 109/L WBC) from high-risk (>10 × 109/L WBC) disease. The concomitant use of all-trans retinoic acid (ATRA) and anthracycline based chemotherapy, with inclusion of AraC in consolidation for hyperleucocytic patients, has remained the standard of care for the past two decades. The advent of arsenic trioxide (ATO) and results from a large randomized trial, have recently challenged the standard ATRA-chemotherapy approach suggesting that at least patients in the low-intermediate category may be cured without chemotherapy using the ATRA-ATO combination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia characterized by bone marrow infiltration with dysplastic promyelocytes and a severe bleeding diathesis. The disease frequently has a rapid and aggressive clinical course, and it was once regarded as the most rapidly fatal human leukemia. The genetic hallmark of APL is the chromosomal translocation t(15;17) that fuses together the promyelocytic leukemia (PML) and retinoic acid receptor alpha (RARA) genes, located on chromosomes 15 and 17, respectively. The resulting hybrid protein is PML/RARA [1]. A life-threatening coagulopathy accompanies frequently the disease at presentation and both a rapid diagnosis and the prompt initiation of specific therapy with all-trans retinoic acid (ATRA) and supportive care is critical to counteract the bleeding diathesis and the associated high risk of early hemorrhagic death [2].
In the last two decades, the combinatorial approach of differentiation therapy with ATRA and anthracycline-based chemotherapy has transformed APL into a highly curable disease, with cure rates now exceeding 80 % [1, 3]. Arsenic trioxide (ATO) alone or in association with ATRA has also been shown to exert high anti-leukemic activity in APL, both in newly diagnosed and relapsed disease, with the advantage of carrying considerably less toxicity compared to chemotherapy [4]. A recent Italian-German randomized study reported that the simultaneous ATO plus ATRA combination is at least not inferior and possibly superior to a standard ATRA plus chemotherapy regimen in the front-line treatment of patients with low/intermediate risk APL (conventionally defined as those with WBC counts at diagnosis <10 × 109/L) [5•]. While the results of an independent, recently completed similar study conducted in the UK should be available soon, the updated National Cancer Comprehensive Network (NCCN) guidelines have now included the ATO plus ATRA combination in the recommended treatment options for newly diagnosed non-high risk APL and for APL patients considered unfit for chemotherapy [6]. Present investigational efforts are centred on exploring the use of ATO in other clinical settings including high-risk, children and elderly patients. This review article focuses on current recommendations and debated issues in the front-line treatment of APL.
Results with All-Trans Retinoic Acid Plus Chemotherapy
The advent of ATRA and its inclusion in association with chemotherapy in the front-line approach has produced a dramatic improvement in APL cure rates [3]. Studies reported in the early 90s showed that patients receiving ATRA followed by anthracycline-based chemotherapy had significantly better outcomes as compared to patients treated with chemotherapy alone [7, 8]. Moreover, the concomitant administration of ATRA and chemotherapy was shown to be superior to ATRA followed by chemotherapy in a large European APL trial [8], and this benefit was confirmed in a number of large multicenter studies conducted in Europe, USA and Japan, which reported extremely high CR rates (90–95 %) and cure rates of up to 85 % [8–11, 12•]. The GIMEMA and PETHEMA trials conducted in Italy and Spain, respectively, demonstrated high anti-leukemic efficacy of idarubicin combined to ATRA for induction therapy [10, 11], while other approaches included the use of daunorubicin and cytarabine, with no substantial differences in CR rates [8, 9, 12•]. Recently, the MRC trial AML15 reported the superiority of the PETHEMA based on anthracyclines plus ATRA for remission induction and consolidation therapy, compared to the more intensive MRC UK schedule including cytarabine, etoposide and anthracyclines plus ATRA for induction and consolidation therapy [12]. As to the relapse rates, the European APL study reported an increased incidence of relapses when omitting cytarabine from induction therapy [13] while the MRC AML15 trial showed no difference in relapse rates when patients were treated with induction regimens not containing cytarabine [12]. Concerning consolidation therapy, it has to be noted the chemotherapy combinations and schedules are not homogeneous among the various studies;however, at least two cycles of anthracycline-based chemotherapy are generally recommended as consolidation [14].
The formulation of the so called “Sanz score” [15] in the year 2000 allowed the stratification of APL patients at diagnosis in low/intermediate-risk of relapse (WBC < 10 × 109/L) and high-risk (WBC > 10 × 109/L) groups and was the basis for the design of modern, risk-adapted regimens for APL treatment. High-risk APL has also been associated with higher risk of severe bleeding and early death [1]. Even after the advent of ATRA, patients with elevated WBC still remain at very high risk of early hemorrhagic death with most studies reporting increased early mortality rates (10–20 %) in patients with elevated WBC at presentation [16]. Thus, it is critical that, together with anti-leukemic therapy, massive supportive care with plasma and platelet transfusion is provided in this setting, particularly during the first two weeks after diagnosis [14].
The possibility to stratify patients upfront has offered the opportunity to modulate the intensity of post-remission therapy for non-high risk patients in order to spare unnecessary toxicity. In this regard, the feasibility and efficacy of a risk-adapted approach to post-remission therapy in APL was demonstrated by two independent PETHEMA and GIMEMA trials. In both studies, high-risk patients received consolidation therapy including cytarabine while low-risk and intermediate-risk patients received three courses of anthracycline-based consolidation. The trials have demonstrated an advantage in terms of relapse rates when including cytarabine in consolidation schedules only for high-risk patients whereas this drug could be safely omitted in the low-intermediate group [17, 18•].
In summary, based also on very long-term updates of several of the above studies [18•, 19–22], the simultaneous administration of ATRA and anthracycline-based chemotherapy followed by the same approach for at least two consolidation cycles remains the most established standard approach for newly diagnosed APL patients. In addition, substantial evidence indicates that patients in the high-risk category should also receive high-dose of AraC together with anthracycles and ATRA in the consolidation phase.
Role of Maintenance Therapy and CNS Prophylaxis
Although the results of randomized comparative studies conducted in the 90s pointed to a benefit from using maintenance therapy with ATRA and low-dose chemotherapy in APL [23, 24], the role of maintenance has been recently questioned especially for non-high risk patients who are in molecular complete remission (CRm) after consolidation [25•, 26]. CRm is conventionally defined as conversion from PCR-positive from PCR-negative for the PML/RARA transcript in the marrow using PCR tests with sensitivity <10−4 [27].
A GIMEMA study in which APL patients in CRm after induction and consolidation were randomized for maintenance, showed similar disease-free survival rates for those patients in the observation arm compared to those receiving ATRA plus low-dose chemotherapy maintenance [25•]. In addition, a JALSG study including patients in CRm after induction and intensive consolidation chemotherapy, showed significantly poorer 6-year disease-free survival and overall survival when six courses of intensive maintenance chemotherapy where administered, as compared with observation only [26].
As to the need for CNS prophylaxis as part of front-line therapy in APL, this issue has remained somewhat controversial [2]. Because the majority of CNS relapses occur in patients presenting with hyperleukocytosis, it has been suggested to include CNS prophylaxis for patients in this particular high-risk setting only [2, 28]. However, due to the risk of severe bleeding, it is strongly recommended that lumbar puncture for CNS prophylaxis is performed only after the achievement of complete remission. At present, however, the benefit of using CNS prophylaxis for high-risk patients has not yet been established. For patients in the low-intermediate risk category, whose risk of developing CNS relapse is extremely low, there is a general consensus to avoid CNS prophylaxis [14].
Pilot Studies with ATO in Front-Line Therapy
After the successful results with ATO in the treatment of relapsed APL first reported in China and successively confirmed by several studies in the US and Europe [29–31], several trials were initiated to investigate the role of this agent in front-line therapy. ATO exerts a double dose-dependent action in APL, including induction of partial differentiation and apoptosis of leukemic promyelocytes. Moreover, studies using in vivo models have shown the synergistic effects of combining ATO with ATRA [32, 33, 34••, 35]. A non-comparative study from India reported high CR rates (86 %) with ATO used as a single agent for induction therapy in 72 newly diagnosed APL patients [36]. Similar results were published in Iran, again with ATO being used as monotherapy [37]. In both trials, the early death rate was 14 %. In the Iranian study a significantly higher fraction of patients died among those presenting with WBC higher than 10 × 109/L. Post-remission therapy consisted in the Indian experience of a 4-week consolidation course with ATO followed by six courses of ATO for ten days every month. In the Iranian study, ATO was given for one course and then repeated for a total of four consolidation courses. Long-term updates of these two studies reported 5-year OS of 74 % and 64 % and DFS estimates of 80 % and 67 % for the Indian and Iranian studies, respectively. Importantly, this regimen was significantly more effective in the non-high risk patient category. Gore et al., analyzed the efficacy of a single cycle of ATO consolidation therapy in order to decrease exposure to other cytotoxic agents. Overall, at a median follow-up of three years, estimated DFS was 90 % while OS for all patients was 88 % [38].
ATO was also combined with ATRA as induction therapy in a randomized Chinese trial comparing three options for induction, i.e., ATO monotherapy, ATO plus ATRA and ATRA as single agent, followed by chemotherapy-based consolidation and maintenance [39]. Despite a similar CR rate (90–95 %), time to achieve CR was shorter and the kinetics of reduction of PML/RARA transcript was faster and more profound in the ATO plus ATRA arm as compared to the other groups. Given the superiority of the combination arm, the ATO plus ATRA approach was extended to 85 patients and in a long-term follow-up 5-year OS, EFS and RFS were reported to be 92 %, 89 % and 95 %, respectively [40•].
An entirely chemotherapy free-approach used for low/intermediate risk APL combining ATO and ATRA was reported by Estey and colleagues at the MD Anderson Cancer Center in the US [41]. In this study, a remission induction schedule based on ATO and ATRA was followed by four consolidation courses with the same agents. In the same trial, 18 patients who were classified as high-risk were also included and given one single dose of gemtuzumab ozogamycin in addition to ATO and ATRA for induction. The long-term outcomes of the extended study on 82 patients reported by Ravandi and colleagues showed CR rates of 91 % with an early death rate of 7 % and a 3-year DFS of 85 % [42••].
Besides studies of ATO alone or in combination with ATRA as an alternative to chemotherapy, recent investigation also focused on combining ATO with reduced doses of chemotherapy in the attempt to decrease treatment-related toxicity and increase anti-leukemic efficacy. The opportunity to integrate ATO in the standard ATRA plus chemotherapy strategy was initially explored in a randomized US study reported by Powell and colleagues [43]. After standard ATRA plus chemotherapy induction, patients in this study were randomized to receive post-remission ATRA and chemotherapy courses preceded or not by two cycles of ATO. The results showed better EFS and OS for patients receiving ATO; however, this study could not definitely clarify whether incorporation of ATO into consolidation therapy improved the outcome of standard therapy, as overall survival in the control arm was relatively low compared with results reported by other groups employing ATRA and anthracycline chemotherapy-based schedules.
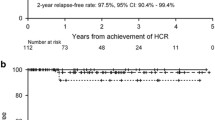
Recently, the Australasian group has reported the results of the APML 4 trial, combining anthracycline chemotherapy, ATO and ATRA for remission induction therapy, followed by two consolidations with ATO plus ATRA without further chemotherapy. Compared with previous studies from the same group, this regimen resulted in improved survival outcomes, with two-year freedom from relapse (FFR), failure-free survival (FFS) and OS of 97.5 %, 88 % and 93 %, respectively [44••].
In all of the above studies using ATO, the toxicity profile of this agent has been reported to be mild and relatively manageable. In particular, unlike chemotherapy, ATO seems not to be associated with severe mylosuppression and consequent life-threatening infections. Frequent toxicity linked to ATO includes the occurrence of differentiation syndrome, hyperleucocytosis, increase of hepatic enzymes and QT prolongation [45].
ATO and ATRA vs. ATRA and Chemotherapy
Following the promising results reported by Estey et al., using the chemotherapy-free ATO plus ATRA combination [41], the Italian and German multicenter groups GIMEMA, SAL and AMLSG started in 2007 a randomized trial to compare ATO plus ATRA vs. the standard ATRA and chemotherapy in patients with newly diagnosed, low-intermediate risk APL. Patients in the experimental arm received conventional doses of ATRA plus ATO (0.15 mg/kg) daily until complete remission, followed by four consolidation courses of ATO given five days per week, four weeks on and four weeks off, and ATRA administered daily for two weeks every four weeks for a total of seven courses. The protocol was adopted following the same scheme reported by Ravandi et al. [42••] starting ATO on day 1. The comparative standard arm included ATRA and idarubicin (AIDA scheme) for induction, three chemotherapy and ATRA consolidation courses and two years maintenance with low-dose chemotherapy and ATRA as reported by Lo-Coco et al. [5•]. A total of one hundred and sixty-two patients were enrolled. No significant differences were reported in terms of CR rates after induction between the standard and experimental arm (95 % vs. 100 %). Four patients in the standard arm died during induction, two of them due to differentiation syndrome; one patient in the experimental arm and three in the standard arm died during consolidation. At a median follow-up of 34 months, the primary endpoint was achieved with significantly higher 2-year EFS in the ATRA plus ATO arm as compared to standard chemotherapy (97 % vs. 86 %, P = 0.02). Moreover, the 2-year OS probability was also significantly higher for patients in the ATRA plus ATO arm (99 % vs. 91 %, P = 0.02).As to toxicity, the standard chemotherapy and ATRA approach was associated with significantly more frequent episodes of prolonged myelosuppression, while treatment with ATO-ATRA resulted in more frequent QT prolongation, hyperleucocytosis and hepatic toxicity. The latter side effects observed in the experimental arm were, however, manageable with temporary drug discontinuation and successive dose-adjustments as per protocol recommendation and led to permanent discontinuation of ATO (due to persistent QT prolongation) in only one patient [5•]. In light of these results, the recently updated NCCN guidelines included the ATO plus ATRA protocol as one of the available standard options for newly diagnosed patients with low-intermediate risk APL as well as for patients who are unfit for conventional chemotherapy [6].
A very similar randomized study was recently completed by the UK NCRI which included patients with both low-intermediate and high-risk APL patients. The trial, whose results should be available soon, compared the standard AIDA vs. an ATO-ATRA chemotherapy-free protocol in which ATO was given on a three-days/week schedule at 0.30 mg/Kg/d for induction and on a two-days/week schedule at the same doses in the post-induction phase. For patients with WBC > 10 × 109/L (high-risk), gemtuzumab ozogamycin was added to ATO and ATRA, as reported by Estey and colleagues [41].
Most recently, a Chinese randomized study compared the combination of ATRA with an oral formulation of arsenic tetra-sulfide (As4S4) vs. the intravenous ATO plus ATRA approach. In this non-inferiority study, consolidation therapy consisted in both arms of three cycles of chemotherapy. No difference in CR rates was observed between the two arms and at a median follow-up of 39 months, DFS at 2-years for the oral arm was not inferior to the intravenous ATO arm, while the OS rate at three years was also comparable between the two groups. Finally, the toxicity profile was mild in either arms [46••]. Such results, together with previously reported experience on oral ATO, strongly suggest that future curative treatment of APL patients might be carried out using oral therapy only.
Conclusions and Future Perspectives
While ATRA and chemotherapy has remained for decades the standard front-line therapy for newly diagnosed APL, the recent results of the Italian-German randomized study have challenged this indication, suggesting that the ATRA-ATO chemotherapy-free approach may replace in the near future the standard ATRA and chemotherapy at least for the low-intermediate risk patients. This view would be further strengthened whether the parallel randomized study conducted independently by the NCRI confirm the findings of the Italian-German study. For the time being, the ATO-ATRA approach should be selected for patients with low-intermediate risk APL unfit to chemotherapy and might represent the first recommended choice for therapy-related APL in patients who have been previously exposed to substantial chemotherapy doses.
Further investigation on the chemotherapy-free ATO-ATRA approach using well-designed clinical trials is warranted in other APL settings including pediatric and elderly patients. Both these categories would, in fact, potentially benefit from therapeutic approaches carrying significantly less toxicity without apparently compromising anti-leukemic efficacy. As to the category of high-risk patients, here again the results of the randomised NCRI study should provide important preliminary indications. Given the presumably low number of high-risk cases in that trial, however, large international studies need to be launched to compare standard ATRA and AraC-containing chemotherapy against a combination of ATO, ATRA and minimal chemotherapy in this clinical setting.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–15.
Sanz MA, Tallman MS, Lo-Coco F. Tricks of the trade for the appropriate management of newly diagnosed acute promyelocytic leukemia. Blood. 2005;105(8):3019–25.
Sanz MA, Lo CF. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495–503.
Breccia M, Lo-Coco F. Arsenic trioxide for management of acute promyelocytic leukemia: current evidence on its role in front-line therapy and recurrent disease. Expert Opin Pharmacother. 2012;13:1031–43.
Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–21. This is the first study showing through a randomised comparison that a chemotherapy-free ATO plus ATRA-based approach is at least not inferior to standard ATRA and chemotherapy for non-high risk APL patients.
O’Donnel MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia, version 2.2013. J Natl Compr Canc Netw. 2013;11(9):1047–55.
Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocyticleukemia. N Engl J Med. 1997;337(22):1639.
Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of alltransretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia: The European APL Group. Blood. 1999;94:1192–200.
Lengfelder E, Reichert A, Schoch C, et al. Double induction strategy including high dose cytarabine in combination with all-trans retinoic acid: Effects in patients with newly diagnosed acute promyelocytic leukemia. Leukemia. 2000;14:1362–70.
Sanz MA, Martín G, Rayon C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/ RARa-positive acute promyelocyticleukemia: PETHEMA Group. Blood. 1999;94:3015–21.
Mandelli F, Diverio D, Avvisati G, et al. Molecular remission in PML/RARa-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy: Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90:1014–21.
Burnett AK, Hills RK, Grimwade D, et al. Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: results of the MRC AML15 trial. United Kingdom National Cancer Research Institute Acute Myeloid Leukaemia Subgroup. Leukemia. 2013;27(4):843–51. This randomised trial demonstrates that additional chemotherapy may be spared to APL patients receiving the standard ATRA plus anthracyline-based approach.
Adès L, Chevret S, Raffoux E, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24:5703–10.
Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91.
Sanz MA, Lo Coco F, Martin G, et al. Definition of relapse risk and role of non-anthracycline drugs for consolidation in patients with acute pro- myelocytic leukemia: A joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–53.
Tallman M, Lo-Coco F, Kwaan H, Sanz M, et al. Clinical roundtable monograph. Early death in patients with acute promyelocytic leukemia. Clin Adv Hematol Oncol. 2011;9(2):1–16.
Sanz MA, Martín G, González M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–43.
Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–9. This and other investigations conducted by large multicenter groups support the notion that risk-adapted approaches lead to improved outcome.
Sanz MA, Montesinos P, Vellenga E. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112(8):3130–4.
Burnett AK, Grimwade D, Solomon E, et al. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with alltrans retinoic acid: result of the randomized MRC trial. Blood. 1999;93:4131–43.
Lengfelder E, Reichert A, Schoch C, et al. Double induction strategy including high dose cytarabine in combination with all-trans retinoic acid: effects in patients with newly diagnosed acute promyelocytic leukemia. Leukemia. 2000;14:1362–70.
Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100:4298.
Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all- transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia: The European APL Group. Blood. 1999;94:1192–200.
Tallman MS, Altman JK. Curative strategies in acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:391–9.
Avvisati G, Lo-Coco F, Paoloni FP, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117:4716. The role of maintenance is questioned in this randomised comparative study.
Asou N, Kishimoto Y, Kiyoi H, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia wo have become negative for PML-RAR alpha transcript after consolidation therapy : the Japan Adult Leukemia Study Group (JALSG) APL 97 study. Blood. 2007;110:59.
Grimwade D, Lo CF. Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia. 2002;16(10):1959–73.
Breccia M, Carmosino I, Diverio D, et al. Early detection of meningeal localization in acute promyelocytic leukaemia patients with high presenting leucocyte count. Br J Haematol. 2003;120(2):266–70.
Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19(18):3852–60.
Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–60.
Lengfelder E, Hofmann WK, Nowak D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 2012;26(3):433–42.
Shao W, Fanelli M, Ferrara FF, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90(2):124–33.
Miller Jr WH, Schipper HM, Lee JS, et al. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62(14):3893–903.
Zhang XW, Yan XJ, Zhou ZR, et al. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010;328(5975):240–3. A critical mechanism underlying response to ATO in APL is unravelled in this important study.
Nasr R, Lallemand-Breitenbach V, Zhu J, et al. Therapy-induced PML/RARA proteolysis and acute promyelocytic leukemia cure. Clin Cancer Res. 2009;15(20):6321–6.
Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–32.
Ghavamzadeh A, Alimoghaddam K, Ghaffari SH, et al. Treatment of acute promyelocytic leukemia without ATRA and/or chemotherapy. Ann Oncol. 2006;17:131–4.
Gore SD, Gojo I, Sekeres MA, et al. Single cycle of arsenic trioxide-based consolidation chemotherapy spares anthracycline exposure in the primary management of acute promyelocytic leukemia. J Clin Oncol. 2010;28(6):1047–53.
Shen ZX, Shi ZZ, Fang J, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101(15):5328–35.
Hu J, Liu YF, Wu CF, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106(9):3342–7. The update experience of this Chinese group strongly suggests the curative effect of ATO-containing regimens.
Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107(9):3469.
Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27(4):504. The potentially curative effect of a chemo-free regimen is shown in this study.
Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–7.
Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570–80. The combination of the three most effective agents in APL (i.e., ATRA, ATO and idarubicin) is shown to be highly effective in this study, which pave the way to future trials using “minimal” chemotherapy together with ATO and ATRA in high-risk patients.
Breccia M, Lo-Coco F. Arsenic trioxide for management of acute promyelocytic leukemia: current evidence on its role in front-line therapy and recurrent disease. Expert Opin Pharmacother. 2012;13(7):1031–43.
Zhu HH, Wu DP, Jin J, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31(33):4215–21. This randomised study strongly suggests that oral ATO may replace the IV formulation in the near future.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Francesco Lo-Coco reports personal fees from Teva during the conduct of the study.
Dr. Laura Cicconi declares no potential conflicts of interest relevant to this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo-Coco, F., Cicconi, L. What is the Standard Regimen for Patients with Acute Promyelocytic Leukemia?. Curr Hematol Malig Rep 9, 138–143 (2014). https://doi.org/10.1007/s11899-014-0206-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-014-0206-5