Abstract
Purpose of Review
Inflammation has been shown to be an important factor in the development and progression of heart failure (HF), regardless of the etiology. There have been many studies that demonstrated roles of inflammatory biomarkers in diagnosis, prognosis of chronic and acute HF patients, and also markers of cardiotoxicity from chemotherapy. These cytokines are high-sensitivity C-reactive protein (hsCRP), myeloperoxidase (MPO), soluble growth stimulation expressed gene 2 (sST2), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNFα), growth differentiation factor-15 (GDF-15), endothelin-1 (ET-1), and galectin-3. In this review, we discuss the past and present insights of those inflammatory biomarkers in order to gain more understanding in pathogenesis of HF, risk stratification of HF patients, and early detection of cardiotoxicity from cancer therapy.
Recent Findings
Many inflammatory cytokines have been shown to be associated with mortality of both chronic and acute HF patients, and some of them are able to track treatment responses, especially sST2 and galectin-3, which are the only two inflammatory biomarkers recommended to use in clinical setting by the recent standard HF guidelines, while some studies described ET-1 and MPO as potential predictors of cardiotoxicity from cancer drugs.
Summary
The prognostic implications of inflammatory biomarkers in HF patients have been demonstrated more consistently in chronic than acute HF, with some suggestions of ET-1 and MPO in patients receiving chemotherapy. However, further studies are necessary for the use of inflammatory biomarkers in routine clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
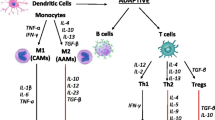
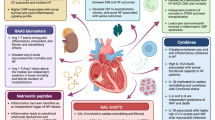
Heart failure (HF) is a complex clinical syndrome characterized by typical signs and symptoms of reduced cardiac output and/or elevated intracardiac filling pressure caused by impaired cardiac function and/or structural abnormality [1]. There has been tremendous progress in medical treatments that improve morbidity and mortality in patients with HF with reduced ejection fraction (HFrEF) [2], but not in patients with HF with preserved ejection fraction (HFpEF) [3]. Meanwhile, prognosis of overall HF patients is still poor with more than a 50% mortality rate in 5 years after the diagnosis [4]. Apart from neurohormonal activation, inflammation has been shown to be an independent key factor in the development and progression of a variety of both HFrEF and HFpEF over the past decades [5,6,7,8,9]. Many inflammatory cytokines are secreted from the myocardium in response to a precipitating event causing myocardial damage and injury [10], resulting in deterioration of myocardial function, and subsequently furthering the progression of HF [11,12,13]. The mechanisms of inflammatory responses as causes of development and progression of HF are not well understood, but clinical studies over the past decades demonstrated the prognostic implications of elevated inflammatory mediators and adverse clinical outcomes [10, 14•, 15, 16]. It is important to recognize that the vast majority of published work described case series (most were retrospective and observational in nature) without designation of treatment decisions based on their quantification, hence largely limiting our ability to translate their insights into clinical practice. We will review current insights into inflammatory biomarkers available in clinical practice and summarize the data supporting their potential clinical utilization in chronic and acute HF. We will also review specific HF conditions such as prediction of cardiotoxicity from cancer drugs and responses to treatments of HF.
High-Sensitivity C-Reactive Protein
C-reactive protein (CRP) is an acute phase proinflammatory cytokine produced by hepatocytes in response to the signal from interleukin-6 (IL-6) [17, 18]. The association between elevated CRP in HF patients was described in 1956 by Elster et al. and was the first study to reveal elevated concentration of CRP levels in 30 of 40 HF patients [19]. Subsequently, several studies corroborated the elevated CRP in HF patients [20, 21]. Over the past two decades, CRP testing has become more available in standard clinical laboratories with high-sensitivity assay for CRP at relatively low cost [22]. Also, high-sensitivity CRP (hsCRP) has emerged as a potential risk predictor of adverse outcomes in HF patients.
In chronic HF, the prognostic implications of hsCRP have been well established. In two large observational studies, hsCRP was found to be independently associated with twofold increased cardiovascular mortality [23, 24]. Similar findings were also found in a study on a cohort of HF patients with left ventricular systolic dysfunction (LVSD); elevated hsCRP was found to be independently associated with increased mortality after adjusting with N-terminal pro-brain natriuretic peptide (NT-proBNP) [25]. Furthermore, along with other biomarkers, such as troponin T and NT-proBNP, hsCRP improved the prediction of 1-year mortality in HF patients [26]. In addition, hsCRP was described as a potential predictor of favorable outcomes from statin treatment in chronic HF patients [27].
In relatively small studies in acute HF patients, baseline hsCRP levels have been shown to be associated with increased mortality at 30 days [28], at 90 days [29], and at 1 year [30] and having higher readmission rates [31]. However, a larger cohort of patients from the biomarker sub-study of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial suggested that baseline hsCRP levels were not associated with 30-day mortality, readmission rate, or 180-day mortality. Instead, only persistently elevated or increase in hsCRP from baseline to 30 days was associated with 180-day mortality [32]. Nevertheless, a recent study of a cohort of 4,269 patients with acute HF revealed that CRP was independently associated with 120-day mortality [33]. The discrepancies among the studies do not warrant the use of hsCRP as a predictor for adverse clinical outcomes in acute HF, and further investigation is warranted. Currently, there are no guideline recommendations supporting routine hsCRP testing in acute or chronic HF.
Myeloperoxidase
Myeloperoxidase (MPO) is an enzyme generated from activated leukocytes in response to inflammation and oxidative stress, through the formation of numerous reactive oxidants and diffuse radical species, which are capable of causing tissue oxidative damage by lipoprotein peroxidation, scavenging of nitric oxide, and inhibition of nitric oxide synthase [34,35,36,37] and subsequently resulting in atherogenesis, plaque vulnerability, and ventricular remodeling [38,39,40,41]. These pathways contribute to the development of coronary artery disease, vascular diseases, and HF. MPO is an FDA-cleared ELISA (enzyme-linked immunosorbent assay) available in many clinical laboratories. However, it is important to highlight the fact that quantification of MPO levels by commercially available assays reflect MPO mass concentration rather than activity; thus, circulating levels detected by current assays do not reflect acute inflammatory status per se.
In terms of clinical correlation, some studies demonstrated MPO as a strong risk predictor of coronary artery disease [42,43,44,45,46]. In chronic HF, the study of Tang et al. [47] revealed that elevated MPO was observed in HF patients compared to healthy controls; plasma MPO levels were strongly and independently associated with the prevalence of HF and able to identify the HF patients with 72% sensitivity and 77% specificity. Another study also described MPO as a predictor of future development of HF in healthy elderly [48]. In addition, along with plasma CRP and NT-proBNP, MPO can improve the specificity to detect HF patients up to 94.3% [49]. Furthermore, plasma MPO levels were also shown to be correlated with echocardiographic parameters indicating severe HF and long-term adverse clinical outcomes [50, 51].
On the other hand, in acute HF with more limited data, there are still controversies in diagnostic and prognostic implications of MPO. A study in a cohort of 412 patients presenting with dyspnea demonstrated that there was no significant difference in plasma MPO levels between the patients with and without acute HF, and plasma MPO levels were not associated with 1-year mortality of those patients [52]. In contrast, a study in a larger cohort of patients presenting with dyspnea suggested that plasma MPO levels independently predicted 1-year mortality in acute HF patients [53]. The use of MPO in acute HF patients is still unclear and yet to be determined.
Soluble Growth Stimulation Expressed Gene 2
Growth stimulation expressed gene 2 (ST2) encodes a transmembrane protein for interleukin-1 receptor family (ST2 ligand or ST2L) and a truncated soluble form of ST2 (soluble ST2 or sST2) secreted from cardiac myocytes and cardiac fibroblasts, triggered by mechanical strain in the heart [54, 55]. Both ST2L and sST2 bind to interleukin-33 (IL-33), a cytokine produced by cardiac fibroblasts, causing contrary effects. Binding of IL-33 and ST2L results in cardioprotective effects against hypertrophic remodeling and myocardial fibrosis by antagonizing angiotensin-II signaling and promoting anti-apoptotic factors, respectively. In contrast, sST2 binds to IL-33, acts as a decoy receptor, and inhibits the protective effects of IL-33 and ST2L in cardiac myocytes [56, 57]. Recently, a study in an experimental model of heart failure claimed that the lungs are another relevant source of sST2 in response to cardiogenic pulmonary edema [58]. Also, in a study of healthy elderly, sST2 was associated with increased incidence of HF [59]. ST2 is an FDA-cleared ELISA assay available in many clinical laboratories.
The role of sST2 as a predictor of adverse clinical outcomes in chronic HF patients has been widely demonstrated since the early 2000s. A study of Weinberg et al. [55] in a cohort of 139 patients with chronic HF revealed that baseline sST2 levels correlated with BNP levels, and changes in serum sST2 levels at 2 weeks from baseline independently predicted subsequent transplantation and mortality rates. Similarly, results from studies in larger cohorts of chronic HF patients suggested that sST2 levels were associated with functional capacity [60], sudden cardiac death [61, 62], and short- and long-term mortality [63, 64], even when the patients were treated with optimized medications [60]. In addition, meta-analysis of studies on prognostic value of sST2 in 5,051 chronic HF patients revealed that sST2 was associated with both all-cause and cardiovascular mortality [65]. Interestingly, Gaggin et al. [66] demonstrated that sST2 levels might be able to identify patients who show better responses to higher dose of beta-blockers.
Unlike other inflammatory biomarkers, the diagnostic and prognostic implications of sST2 in acute HF patients have been validated in several studies. When Januzzi et al. [67] studied 593 patients presenting with acute dyspnea using receiver operator characteristic (ROC) analysis, baseline sST2 levels were found to have an area under the curve (AUC) of 0.74 for the diagnosis of HF. Moreover, in many studies, baseline sST2 levels have been shown to be a strong independent predictor of mortality in acute HF patients [68,69,70,71,72]. Also, changes in serum sST2 levels were shown to be associated with increased mortality in the setting of acute HF [73, 74]. In addition to the chronic HF setting, the prognostic implication of sST2 in the acute HF setting was also corroborated by meta-analysis of studies of 4,835 acute HF patients [75]. Moreover, a study on the effect of spironolactone on the 30-day mortality and rehospitalization of patients with acute HF [76] revealed that subgroup of patients with higher levels of sST2 showed significant benefits from spironolactone.
Since there has been robust evidence of sST2 as a biomarker for prognosis and monitoring in both acute and chronic HF patients, sST2 was included in the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) focused update of HF guidelines [77••] and has been increasingly recognized as a biomarker in HF [78].
Interleukin-6
IL-6 is a proinflammatory cytokine secreted by T lymphocytes in response to a variety of stresses in the body, including HF [7, 79]. IL-6 affects myocardium by binding to the IL-6 receptor (IL-6 R) on cell membranes of myocytes and transduces signals through the glycoprotein (gp) 130 receptor system [80]. It also leads to the deterioration of myocardial function by three potential main pathways that contribute to the development of HF. Firstly, IL-6 has an indirect negative inotropic effect on myocardium by upregulating nitric oxide synthase [81]. Secondly, IL-6 downregulates myocardial sarcoplasmic reticulum Ca2+ ATPase (SERCA) [82]. Thirdly, IL-6 subsequently causes myocardial hypertrophy and apoptosis via Janus kinase (JAK) and mitogen-activated protein kinase (MAPK), respectively [83]. Likewise, in clinical studies of healthy subjects, the role of IL-6 in the development of HF has been reported for decades. The study of Vasan et al. [16] revealed that elevated IL-6 was independently associated with a three-fold increased risk of incident HF in a cohort of healthy elderly without history of cardiovascular disease from the Framingham Heart Study. Similarly, in another cohort of healthy individuals, IL-6 was shown to be inversely related with a decline in LV systolic function, determined by cardiac magnetic resonance imaging (MRI) [84]. These findings supported that IL-6 may play an important role in the pathogenesis of HF.
The prognostic implications of IL-6 in HF patients have been described in many studies, especially in the chronic HF setting, where IL-6 has been shown to be correlated with severity of HF [85, 86] and also an independent predictor of mortality in patients with chronic HF [87,88,89]. Recently, these findings were supported by a study in a large cohort of 2,329 chronic HF patients which revealed that baseline IL-6 levels independently predicted all-cause, cardiovascular, and non-cardiovascular mortality in 2 years [90•]. However, despite the results of IL-6 on mortality, there was no association between baseline IL-6 levels and HF hospitalization. Also, adding IL-6 to the published risk prediction model for this cohort [91] failed to improve discrimination in this model.
The challenge in interpreting many of these findings is that the large majority of studies utilized research assays that varied in sensitivities and reproducibility. Nevertheless, IL-6 is now available clinically in some specialty laboratory services, and therapeutic targeting of IL-6 signaling pathways (tocilizumab) have been developed in treating rheumatoid arthritis. Meanwhile, there is much limited evidence of the association between IL-6 and adverse clinical outcomes in acute HF compared to chronic HF, and most of the results were from relatively small cohorts. The study of Chin et al. [92] on 77 patients with acute HF demonstrated baseline IL-6 levels predicted 6-month mortality. Furthermore, the study in a larger cohort of 423 acute HF patients also revealed the independent association between IL-6 levels at 48 hours after admission and 1-year mortality [93]. Nevertheless, the prognostic value for IL-6 in predicting adverse clinical outcomes in acute HF patients still needs to be investigated in larger cohorts of patients in an acute setting.
Tumor Necrosis Factor-Alpha
Tumor necrosis factor-alpha (TNFα) is a proinflammatory cytokine that has been shown to play important roles in inflammatory cascades and accounts for pathogenesis of HF. The pathophysiology of TNFα in HF has been described in many pathways such as downregulating myocardial SERCA [13], promoting cardiac hypertrophy [94], enhancing cardiac remodeling by upregulation of angiotensin II type I receptor in cardiac fibroblasts [95], and by matrix metalloproteinases in both cardiac myocytes and fibroblasts [96]. In clinical studies, the association between TNFα and HF was first observed in the study of Levine et al. [97], which demonstrated that serum TNFα levels were significantly higher in chronic HF patients compared to healthy controls. Subsequent studies also revealed that TNFα levels were correlated with development of HF in healthy subjects [16, 98], severity [7, 99, 100], and mortality of chronic HF patients [101,102,103]. Only one study described the prognostic implication of TNFα in acute HF patients, which showed the independent association between TNFα levels at 48 hours and mortality at 1 year [93]. Like IL-6, TNFα is now available in some specialty clinical laboratories, yet the majority of studies utilized research-based assays.
The evidences of TNFα and HF raised the hypothesis that TNFα inhibitors might improve clinical outcomes in chronic HF patients and potentially be the next target of HF treatments. In contrast, many clinical trials failed to demonstrate any improvement on death or HF hospitalization [104, 105]. Interestingly, in the clinical trial of infliximab, a monoclonal antibody of TNFα, on moderate to severe chronic HF patients, high doses of infliximab were associated with higher mortality than placebo and low-dose infliximab [106].
Growth Differentiation Factor-15
Growth differentiation factor-15 (GDF-15) is one of the transforming growth factor beta superfamily cytokines [107], secreted from cardiac myocytes in response to a number of stresses, for instance, ischemia [108], and mechanical stretch by signaling of angiotensin-II [109]. GDF-15 has been shown to be a strong predictor for mortality in patients with coronary artery disease [110, 111]. In chronic HF, the study of Kempf et al. [112] was the first to demonstrate that GDF-15 independently predicted mortality in a cohort of 455 patients. Afterwards, the prognostic utilities of GDF-15 were corroborated in large cohort studies of chronic HF patients with both HFrEF and HFpEF [113,114,115]. GDF-15 is available clinically in Europe as a commercial assay, but not yet in the United States.
Nonetheless, there is much limited evidence in acute HF patients with several studies on the ability of GDF-15 to predict adverse clinical outcomes. Cotter et al. [116] revealed that only increases in serum GDF-15 at 2 and 14 days, but not baseline levels, were independently associated with cardiovascular death at 180 days. In addition, another study of Boulogne et al. [117] showed that GDF-15 levels were not associated with death or cardiovascular rehospitalization. Further studies are needed to determine the role of GDF-15 as a prognostic indicator in acute HF patients.
Endothelin-1
Although technically not an inflammatory biomarker, endothelin-1 (ET-1) has been recognized as a potent vasoconstrictor produced by various cells, including cardiac myocytes and fibroblasts, triggered by angiotensin-II, epinephrine, cortisol, inflammatory cytokines, hypoxia, vascular shear stress, etc. [118]. There are two types of ET receptors. ET receptor A, located in vascular smooth muscle cells, has higher affinity for ET-1 than other receptors and is responsible for sustained vasoconstriction [119] and, ET receptor B which is located in both vascular and endothelial smooth muscles cells. In contrast to ET receptor A, binding of ET-1 and ET receptor B causes vasodilation [120, 121]. In HF, ET receptor A is upregulated, while ET receptor B is not, resulting in vasoconstriction and subsequent ventricular remodeling [118]. Like IL-6 and TNFα, ET-1 assays are available in specialty clinical laboratories.
The clinical utilities of ET-1 in chronic HF patients have been widely studied in the last decades. Cody et al. [122] reported that ET-1 levels were correlated with pulmonary capillary wedge pressure and pulmonary vascular resistance. Subsequent studies revealed that ET-1 was a strong predictor of both short- and long-term mortality [123,124,125,126,127,128] and also might be superior to natriuretic peptides [129]. Furthermore, the association between ET-1 and mortality has been consistently demonstrated in studies on cohorts of acute HF patients [130,131,132]. These findings shed light on the potential benefits of ET receptor antagonists on HF patients and have been recommended in patients with pulmonary arterial hypertension [133]. However, most clinical trials have yet to demonstrate any benefits towards decreased mortality or hospitalization of both chronic and acute HF patients [134,135,136], including patients with HFpEF [137].
Galectin-3
Galectin-3 is a member of beta-galactoside-binding lectin family, secreted from a variety of organs, including myocardial tissue, following injury, inflammation, and mechanical stress [138]. Galectin-3 has been shown to play important roles in the pathogenesis of HF through activation of cardiac fibroblasts and macrophages and promotes cardiac fibrosis and ventricular remodeling [139]. The role of galectin-3 in the development of new-onset HF was corroborated by two studies on large cohorts of healthy individuals and demonstrated that galectin-3 independently predicted incident HF and subsequent mortality [140, 141]. Galectin-3 is an FDA-cleared assay available in many clinical laboratories.
In terms of prognostic indicators in HF patients, the association between serum galectin-3 levels and mortality in chronic HF patients with either HFrEF or HFpEF was consistently demonstrated in many studies [142,143,144,145,146]. Nevertheless, there are still discrepancies among the data in acute HF patients. Previously in relatively small studies, galectin-3 was shown to be associated with mortality [147,148,149,150] and HF rehospitalization [151, 152]. In contrast, recently, a study on a cohort of 1,161 patients with acute HF revealed that there was no correlation between galectin-3 levels and mortality among those patients [153]. Furthermore, similar to hsCRP, galectin-3 was also found to be a predictor for statin responses in the same cohort of patients with chronic HF [154].
Although the data in acute HF patients remain unclear, the meta-analysis of prognostic implications of galectin-3 in all HF patients showed that galectin-3 significantly predicted cardiovascular mortality [155]. Moreover, as well as sST2, galectin-3 was also recommended in the latest ACC/AHA HF guidelines [77••] to be used as a predictor of death and hospitalization. Importantly, galectin-3 tracks strongly with renal function, and the majority of studies supporting its prognostic value did not fully adjust for renal function.
Considerations of Inflammatory Biomarkers in Cardiotoxicities and Treatment Responses
The role of inflammatory biomarkers as predictors for cardiotoxicity from cancer drugs has emerged in the last decades. ET-1 was the first inflammatory biomarker found to be associated with increased risk of cardiotoxicity [156,157,158]. Subsequently, Ky et al. [159] showed that higher risk of cardiotoxicity at 15 months follow-up was associated with only increases in serum MPO levels at 3 months, but not with CRP, GDF-15, or galectin-3. Similarly, in another study, Putt et al. [160] revealed that increases in serum MPO and GDF-15 predicted cardiotoxicity, while hsCRP and galectin-3 did not. Other studies also failed to demonstrate the prognostic implications of sST2, IL-6, TNFa, and galectin-3 [161, 162]. Therefore, ET-1 and MPO seem to be potentially useful inflammatory biomarkers for prediction of cardiotoxicity, and further studies should focus on validating these findings.
The ability for inflammatory biomarkers to track treatment responses has been explored, especially in statins [27, 154] and inhibitors of the renin–angiotensin–aldosterone systems [23, 32, 66, 73, 76, 127, 132, 142]. While retrospective and post hoc in nature, the large majority of studies appear to indicate that lower (rather than higher) levels of inflammatory biomarkers were tracked with favorable treatment responses. No studies to date utilized inflammatory biomarkers in therapeutic decisions in the setting of HF.
Conclusion
The role of inflammation in HF has been increasingly recognized over the past decades. Table 1 summarizes the many studies that demonstrated the clinical utility of both the diagnostic and prognostic indicators of many inflammatory biomarkers on chronic and acute HF patients. These biomarkers could improve the diagnosis and risk stratification in HF patients. However, sST2 and galectin-3 are the two clinically available inflammatory biomarkers that might be useful in risk stratification with data specifically for HF patients. Also, they were recommended by the recent standard HF guidelines, while ET-1 and MPO have potential to predict cardiotoxicity from chemotherapy. However, few if any studies linked inflammatory biomarkers with treatment choices and responses, thus making their incorporation into clinical practice somewhat challenging.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
von Lueder TG, Kotecha D, Atar D, Hopper I. Neurohormonal blockade in heart failure. Card Fail Rev. 2017;3(1):19–24.
Bonsu KO, Arunmanakul P, Chaiyakunapruk N. Pharmacological treatments for heart failure with preserved ejection fraction-a systematic review and indirect comparison. Heart Fail Rev. 2018;23(2):147–56.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):16.
Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119(1):159–76.
Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71.
Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the studies of left ventricular dysfunction (SOLVD). J Am Coll Cardiol. 1996;27(5):1201–6.
Van Linthout S, Tschope C. Inflammation - cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14(4):251–65.
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4(1):44–52.
Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90(4):464–70.
Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2(3):243–9.
Thaik CM, Calderone A, Takahashi N, Colucci WS. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest. 1995;96(2):1093–9.
Wu CK, Lee JK, Chiang FT, Yang CH, Huang SW, Hwang JJ, et al. Plasma levels of tumor necrosis factor-alpha and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med. 2011;39(5):984–92.
• Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116(7):1254–68 An updated review of the “inflammatory hypothesis” of heart failure emphasizing not only inflammatory process but the important balance of innate immunity.
Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93(4):704–11.
Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–91.
Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12(5):1179–86.
Liuzzo G, Santamaria M, Biasucci LM, Narducci M, Colafrancesco V, Porto A, et al. Persistent activation of nuclear factor kappa-B signaling pathway in patients with unstable angina and elevated levels of C-reactive protein evidence for a direct proinflammatory effect of azide and lipopolysaccharide-free C-reactive protein on human monocytes via nuclear factor kappa-B activation. J Am Coll Cardiol. 2007;49(2):185–94.
Elster SK, Braunwald E, Wood HF. A study of C-reactive protein in the serum of patients with congestive heart failure. Am Heart J. 1956;51(4):533–41.
Kaneko K, Kanda T, Yamauchi Y, Hasegawa A, Iwasaki T, Arai M, et al. C-reactive protein in dilated cardiomyopathy. Cardiology. 1999;91(4):215–9.
Pye M, Rae AP, Cobbe SM. Study of serum C-reactive protein concentration in cardiac failure. Br Heart J. 1990;63(4):228–30.
Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–8.
Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112(10):1428–34.
Lamblin N, Mouquet F, Hennache B, Dagorn J, Susen S, Bauters C, et al. High-sensitivity C-reactive protein: potential adjunct for risk stratification in patients with stable congestive heart failure. Eur Heart J. 2005;26(21):2245–50.
Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JG. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J. 2007;153(6):1048–55.
Dunlay SM, Gerber Y, Weston SA, Killian JM, Redfield MM, Roger VL. Prognostic value of biomarkers in heart failure: application of novel methods in the community. Circ Heart Fail. 2009;2(5):393–400.
McMurray JJV, Kjekshus J, Gullestad L, Dunselman P, Hjalmarson Å, Wedel H, et al. Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the controlled rosuvastatin multinational trial in heart failure (CORONA). Circulation. 2009;120(22):2188–96.
Zairis MN, Tsiaousis GZ, Georgilas AT, Makrygiannis SS, Adamopoulou EN, Handanis SM, et al. Multimarker strategy for the prediction of 31 days cardiac death in patients with acutely decompensated chronic heart failure. Int J Cardiol. 2010;141(3):284–90.
Lourenco P, Paulo Araujo J, Paulo C, Mascarenhas J, Frioes F, Azevedo A, et al. Higher C-reactive protein predicts worse prognosis in acute heart failure only in noninfected patients. Clin Cardiol. 2010;33(11):708–14.
Park JJ, Choi DJ, Yoon CH, Oh IY, Jeon ES, Kim JJ, et al. Prognostic value of C-reactive protein as an inflammatory and N-terminal probrain natriuretic peptide as a neurohumoral marker in acute heart failure (from the Korean Heart Failure registry). Am J Cardiol. 2014;113(3):511–7.
Alonso-Martinez JL, Llorente-Diez B, Echegaray-Agara M, Olaz-Preciado F, Urbieta-Echezarreta M, Gonzalez-Arencibia C. C-reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. 2002;4(3):331–6.
Kalogeropoulos AP, Tang WH, Hsu A, Felker GM, Hernandez AF, Troughton RW, et al. High-sensitivity C-reactive protein in acute heart failure: insights from the ASCEND-HF trial. J Card Fail. 2014;20(5):319–26.
Minami Y, Kajimoto K, Sato N, Hagiwara N. Effect of elevated C-reactive protein level at discharge on long-term outcome in patients hospitalized for acute heart failure. Am J Cardiol. 2018;121(8):961–8.
Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci U S A. 2003;100(25):14766–71.
La Rocca G, Di Stefano A, Eleuteri E, Anzalone R, Magno F, Corrao S, et al. Oxidative stress induces myeloperoxidase expression in endocardial endothelial cells from patients with chronic heart failure. Basic Res Cardiol. 2009;104(3):307–20.
Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(6):1102–11.
Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, et al. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277(48):46116–22.
Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, et al. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197(5):615–24.
Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94(1):437–44.
Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158(3):879–91.
Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, et al. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112(18):2812–20.
Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108(12):1440–5.
Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–604.
Karakas M, Koenig W, Zierer A, Herder C, Rottbauer W, Baumert J, et al. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/KORA Augsburg study. J Intern Med. 2012;271(1):43–50.
Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk prospective population study. J Am Coll Cardiol. 2007;50(2):159–65.
Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. Jama. 2001;286(17):2136–42.
Tang WH, Brennan ML, Philip K, Tong W, Mann S, Van Lente F, et al. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol. 2006;98(6):796–9.
Tang WH, Katz R, Brennan ML, Aviles RJ, Tracy RP, Psaty BM, et al. Usefulness of myeloperoxidase levels in healthy elderly subjects to predict risk of developing heart failure. Am J Cardiol. 2009;103(9):1269–74.
Ng LL, Pathik B, Loke IW, Squire IB, Davies JE. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am Heart J. 2006;152(1):94–101.
Tang WH, Shrestha K, Troughton RW, Borowski AG, Klein AL. Integrating plasma high-sensitivity C-reactive protein and myeloperoxidase for risk prediction in chronic systolic heart failure. Congest Heart Fail. 2011;17(3):105–9.
Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49(24):2364–70.
Shah KB, Kop WJ, Christenson RH, Diercks DB, Kuo D, Henderson S, et al. Lack of diagnostic and prognostic utility of circulating plasma myeloperoxidase concentrations in patients presenting with dyspnea. Clin Chem. 2009;55(1):59–67.
Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. 2010;56(6):944–51.
Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7(10):827–40.
Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107(5):721–6.
Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117(6):1538–49.
Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2(6):684–91.
Pascual-Figal DA, Perez-Martinez MT, Asensio-Lopez MC, Sanchez-Mas J, Garcia-Garcia ME, Martinez CM, et al. Pulmonary production of soluble ST2 in heart failure. Circ Heart Fail. 2018;11(12):005488.
Parikh RH, Seliger SL, Christenson R, Gottdiener JS, Psaty BM, CR dF. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community-dwelling population. J Am Heart Assoc. 2016;5(8):003188.
Felker GM, Fiuzat M, Thompson V, Shaw LK, Neely ML, Adams KF, et al. Soluble ST2 in ambulatory patients with heart failure: association with functional capacity and long-term outcomes. Circ Heart Fail. 2013;6(6):1172–9.
Ahmad T, Fiuzat M, Neely B, Neely ML, Pencina MJ, Kraus WE, et al. Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. JACC Heart Fail. 2014;2(3):260–8.
Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54(23):2174–9.
Daniels LB, Clopton P, Iqbal N, Tran K, Maisel AS. Association of ST2 levels with cardiac structure and function and mortality in outpatients. Am Heart J. 2010;160(4):721–8.
Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–7.
Aimo A, Vergaro G, Passino C, Ripoli A, Ky B, Miller WL, et al. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail. 2017;5(4):280–6.
Gaggin HK, Motiwala S, Bhardwaj A, Parks KA, Januzzi JL Jr. Soluble concentrations of the interleukin receptor family member ST2 and beta-blocker therapy in chronic heart failure. Circ Heart Fail. 2013;6(6):1206–13.
Januzzi JL Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50(7):607–13.
Frioes F, Lourenco P, Laszczynska O, Almeida PB, Guimaraes JT, Januzzi JL, et al. Prognostic value of sST2 added to BNP in acute heart failure with preserved or reduced ejection fraction. Clin Res Cardiol. 2015;104(6):491–9.
Lassus J, Gayat E, Mueller C, Peacock WF, Spinar J, Harjola VP, et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168(3):2186–94.
Manzano-Fernandez S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107(2):259–67.
Pascual-Figal DA, Manzano-Fernandez S, Boronat M, Casas T, Garrido IP, Bonaque JC, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13(7):718–25.
Rehman SU, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52(18):1458–65.
Tang WH, Wu Y, Grodin JL, Hsu AP, Hernandez AF, Butler J, et al. Prognostic value of baseline and changes in circulating soluble ST2 levels and the effects of nesiritide in acute decompensated heart failure. JACC Heart Fail. 2016;4(1):68–77.
van Vark LC, Lesman-Leegte I, Baart SJ, Postmus D, Pinto YM, Orsel JG, et al. Prognostic value of serial ST2 measurements in patients with acute heart failure. J Am Coll Cardiol. 2017;70(19):2378–88.
Aimo A, Vergaro G, Ripoli A, Bayes-Genis A, Pascual Figal DA, de Boer RA, et al. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart Fail. 2017;5(4):287–96.
Maisel A, Xue Y, van Veldhuisen DJ, Voors AA, Jaarsma T, Pang PS, et al. Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH study). Am J Cardiol. 2014;114(5):737–42.
•• Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803 The 2017 ACC/AHA heart failure guidelines is an important endorsement of the clinical utility of biomarkers in heart failure, especially in those at risk of developing heart failure. That being said, the level of evidence for inflammatory biomarkers have not changed from the 2013 recommendations (Level 2B) due to paucity of clinical evidence supporting incremental value of biomarker-guided therapeutic approaches with inflammatory biomarkers.
Bayes-Genis A, Nunez J, Lupon J. Soluble ST2 for Prognosis and Monitoring in Heart Failure: The New Gold Standard? J Am Coll Cardiol. 2017;70(19):2389–92. https://doi.org/10.1016/j.jacc.2017.09.031.
Yu Q, Watson RR, Marchalonis JJ, Larson DF. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol. 2005;289(2).
Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–47.
Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257(5068):387–9.
Villegas S, Villarreal FJ, Dillmann WH. Leukemia inhibitory factor and interleukin-6 downregulate sarcoplasmic reticulum Ca2+ ATPase (SERCA2) in cardiac myocytes. Basic Res Cardiol. 2000;95(1):47–54.
Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol. 2007;102(4):279–97.
Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK, et al. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2010;31(7):875–82.
Deng MC, Erren M, Lutgen A, Zimmermann P, Brisse B, Schmitz W, et al. Interleukin-6 correlates with hemodynamic impairment during dobutamine administration in chronic heart failure. Int J Cardiol. 1996;57(2):129–34.
Plenz G, Song ZF, Tjan TD, Koenig C, Baba HA, Erren M, et al. Activation of the cardiac interleukin-6 system in advanced heart failure. Eur J Heart Fail. 2001;3(4):415–21.
Gwechenberger M, Hulsmann M, Berger R, Graf S, Springer C, Stanek B, et al. Interleukin-6 and B-type natriuretic peptide are independent predictors for worsening of heart failure in patients with progressive congestive heart failure. J Heart Lung Transplant. 2004;23(7):839–44.
Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36(5):1587–93.
Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31(2):391–8.
• Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;14(10). The European BIOSTAT-CHF study is an important multicenter prospective cohort study to gain insights into inflammatory and other circulating biomarkers in chronic heart failure patients over time.
Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. 2017;19(5):627–34.
Chin BS, Conway DS, Chung NA, Blann AD, Gibbs CR, Lip GY. Interleukin-6, tissue factor and von Willebrand factor in acute decompensated heart failure: relationship to treatment and prognosis. Blood Coagul Fibrinolysis. 2003;14(6):515–21.
Miettinen KH, Lassus J, Harjola VP, Siirila-Waris K, Melin J, Punnonen KR, et al. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10(4):396–403.
Yokoyama T, Nakano M, Bednarczyk JL, McIntyre BW, Entman M, Mann DL. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation. 1997;95(5):1247–52.
Peng J, Gurantz D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-alpha-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res. 2002;91(12):1119–26.
Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, et al. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kgamma-dependent manner. Am J Physiol Cell Physiol. 2010;298(3):9.
Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–41.
Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55(19):2129–37.
Kapadia SR, Yakoob K, Nader S, Thomas JD, Mann DL, Griffin BP. Elevated circulating levels of serum tumor necrosis factor-alpha in patients with hemodynamically significant pressure and volume overload. J Am Coll Cardiol. 2000;36(1):208–12.
Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW, et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol. 1996;28(4):964–71.
Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103(16):2055–9.
Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118(6):625–31.
Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102(25):3060–7.
Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE RENEWAL and ATTACH. Int J Cardiol. 2002 Dec;86(2-3):123–30.
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109(13):1594–602.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107(25):3133–40.
Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94(21):11514–9.
Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98(3):351–60.
Frank D, Kuhn C, Brors B, Hanselmann C, Ludde M, Katus HA, et al. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51(2):309–18.
Dallmeier D, Brenner H, Mons U, Rottbauer W, Koenig W, Rothenbacher D. Growth differentiation factor 15, its 12-month relative change, and risk of cardiovascular events and total mortality in patients with stable coronary heart disease: 10-year follow-up of the KAROLA Study. Clinical Chemistry. 2016;62(7):982–92.
Eggers KM, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Jantzen F, et al. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non–ST-segment–elevation acute coronary syndrome. Circ Cardiovasc Genet. 2010;3(1):88–96.
Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(11):1054–60.
Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, et al. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122(14):1387–95.
Chan MMY, Santhanakrishnan R, Chong JPC, Chen Z, Tai BC, Liew OW, et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18(1):81–8.
Sharma A, Stevens SR, Lucas J, Fiuzat M, Adams KF, Whellan DJ, et al. Utility of growth differentiation factor-15, a marker of oxidative stress and inflammation, in chronic heart failure: insights from the HF-ACTION study. JACC Heart Fail. 2017;5(10):724–34.
Cotter G, Voors AA, Prescott MF, Felker GM, Filippatos G, Greenberg BH, et al. Growth differentiation factor 15 (GDF-15) in patients admitted for acute heart failure: results from the RELAX-AHF study. Eur J Heart Fail. 2015;17(11):1133–43.
Boulogne M, Sadoune M, Launay JM, Baudet M, Cohen-Solal A, Logeart D. Inflammation versus mechanical stretch biomarkers over time in acutely decompensated heart failure with reduced ejection fraction. Int J Cardiol. 2017;226:53–9.
Kawanabe Y, Nauli SM. Endothelin. Cell Mol Life Sci. 2011;68(2):195–203.
Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–2.
Davenport AP, O'Reilly G, Molenaar P, Maguire JJ, Kuc RE, Sharkey A, et al. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: evidence for expression of ETB receptors in human vascular smooth muscle. J Cardiovasc Pharmacol. 1993;22(8):S22–5.
Hosoda K, Nakao K, Hiroshi A, Suga S, Ogawa Y, Mukoyama M, et al. Cloning and expression of human endothelin-1 receptor cDNA. FEBS Lett. 1991;287(1-2):23–6.
Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation. 1992;85(2):504–9.
Hülsmann M, Stanek B, Frey B, Sturm B, Putz D, Kos T, et al. Value of cardiopulmonary exercise testing and big endothelin plasma levels to predict short-term prognosis of patients with chronic heart failure. J Am Coll Cardiol. 1998;32(6):1695–700.
Pacher R, Stanek B, Hülsmann M, Koller-Strametz J, Berger R, Schuller M, et al. Prognostic impact of big endothelin-1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J Am Coll Cardiol. 1996;27(3):633–41.
Pousset F, Isnard R, Lechat P, Kalotka H, Carayon A, Maistre G, et al. Prognostic value of plasma endothelin-1 in patients with chronic heart failure. Eur Heart J. 1997;18(2):254–8.
Tsutamoto T, Hisanaga T, Fukai D, Wada A, Maeda Y, Maeda K, et al. Prognostic value of plasma soluble intercellular adhesion molecule-1 and endothelin-1 concentration in patients with chronic congestive heart failure. Am J Cardiol. 1995;76(11):803–8.
Masson S, Latini R, Anand IS, Barlera S, Judd D, Salio M, et al. The prognostic value of big endothelin-1 in more than 2,300 patients with heart failure enrolled in the Valsartan Heart Failure Trial (Val-HeFT). J Card Fail. 2006;12(5):375–80.
Tang WH, Shrestha K, Martin MG, Borowski AG, Jasper S, Yandle TG, et al. Clinical significance of endogenous vasoactive neurohormones in chronic systolic heart failure. J Card Fail. 2010;16(8):635–40.
Van Beneden R, Gurné O, Selvais PL, Ahn SA, Robert AR, J-m K, et al. Superiority of big endothelin-1 and endothelin-1 over natriuretic peptides in predicting survival in severe congestive heart failure: a 7-year follow-up study. J Card Fail. 2004;10(6):490–5.
Demissei BG, Postmus D, Cleland JG, O'Connor CM, Metra M, Ponikowski P, et al. Plasma biomarkers to predict or rule out early post-discharge events after hospitalization for acute heart failure. Eur J Heart Fail. 2017;19(6):728–38.
Metra M, Cotter G, El-Khorazaty J, Davison BA, Milo O, Carubelli V, et al. Acute heart failure in the elderly: differences in clinical characteristics, outcomes, and prognostic factors in the VERITAS study. J Card Fail. 2015;21(3):179–88.
Perez AL, Grodin JL, Wu Y, Hernandez AF, Butler J, Metra M, et al. Increased mortality with elevated plasma endothelin-1 in acute heart failure: an ASCEND-HF biomarker substudy. Eur J Heart Fail. 2016;18(3):290–7.
Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J. 2015;46:903–75 European Respiratory Journal. 2015;46(6):1855-6.
Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the Endothelin A Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):347–54.
Cleland JG, Coletta AP, Freemantle N, Velavan P, Tin L, Clark AL. Clinical trials update from the American College of Cardiology meeting: CARE-HF and the remission of heart failure, Women's Health Study, TNT, COMPASS-HF, VERITAS, CANPAP PEECH and PREMIER. Eur J Heart Fail. 2005 Aug;7(5):931–6. https://doi.org/10.1016/j.ejheart.2005.04.002.
McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. Jama. 2007;298(17):2009–19.
Zile MR, Bourge RC, Redfield MM, Zhou D, Baicu CF, Little WC. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2(2):123–30.
Suthahar N, Meijers WC, Silljé HHW, de Boer RA. From inflammation to fibrosis—molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep. 2017;14(4):235–50.
de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11(9):811–7.
Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–56.
Jagodzinski A, Havulinna AS, Appelbaum S, Zeller T, Jousilahti P, Skytte-Johanssen S, et al. Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Int J Cardiol. 2015;192:33–9.
Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail. 2013;15(5):511–8.
de Boer RA, Lok DJA, Jaarsma T, van der Meer P, Voors AA, Hillege HL, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43(1):60–8.
Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, et al. The predictive value of galectin-3 for mortality and cardiovascular events in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Am Heart J. 2012;164(6):878–83.
Miller WL, Saenger AK, Grill DE, Slusser JP, Bayes-Genis A, Jaffe AS. Prognostic value of serial measurements of soluble suppression of tumorigenicity 2 and galectin-3 in ambulatory patients with chronic heart failure. J Card Fail. 2016;22(4):249–55.
Tang WHW, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011;108(3):385–90.
Meijers WC, de Boer RA, van Veldhuisen DJ, Jaarsma T, Hillege HL, Maisel AS, et al. Biomarkers and low risk in heart failure. Data from COACH and TRIUMPH. Eur J Heart Fail. 2015;17(12):1271–82.
Miro O, Gonzalez de la Presa B, Herrero-Puente P, Fernandez Bonifacio R, Mockel M, Mueller C, et al. The GALA study: relationship between galectin-3 serum levels and short- and long-term outcomes of patients with acute heart failure. Biomarkers. 2017;22(8):731–9.
Mueller T, Gegenhuber A, Leitner I, Poelz W, Haltmayer M, Dieplinger B. Diagnostic and prognostic accuracy of galectin-3 and soluble ST2 for acute heart failure. Clin Chim Acta. 2016;463:158–64.
Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RRJ, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12(8):826–32.
Meijers WC, Januzzi JL, deFilippi C, Adourian AS, Shah SJ, van Veldhuisen DJ, et al. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: a pooled analysis of 3 clinical trials. Am Heart J. 2014;167(6):853–60.e4.
Sudharshan S, Novak E, Hock K, Scott MG, Geltman EM. Use of biomarkers to predict readmission for congestive heart failure. Am J Cardiol. 2017;119(3):445–51.
Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur J Heart Fail. 2017;19(8):1001–10.
Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J. 2012;33(18):2290–6.
Imran TF, Shin HJ, Mathenge N, Wang F, Kim B, Joseph J, et al. Meta-analysis of the usefulness of plasma galectin-3 to predict the risk of mortality in patients with heart failure and in the general population. Am J Cardiol. 2017;119(1):57–64.
Sayed-Ahmed MM, Khattab MM, Gad MZ, Osman A-MM. Increased plasma endothelin-1 and cardiac nitric oxide during doxorubicin-induced cardiomyopathy. Pharmacol Toxicol. 2001;89(3):140–4.
Yamashita J, Ogawa M, Shirakusa T. Plasma endothelin-1 as a marker for doxorubicin cardiotoxicity. Int J Cancer. 1995;62(5):542–7.
Yamashita J-I, Ogawa M, Nomura K. Plasma endothelin-1 and doxorubicin cardiotoxicity. N Engl J Med. 1994;331(22):1528–9.
Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63(8):809–16.
Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. 2015;61(9):1164–72.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596–603.
van Boxtel W, Bulten BF, Mavinkurve-Groothuis AM, Bellersen L, Mandigers CM, Joosten LA, et al. New biomarkers for early detection of cardiotoxicity after treatment with docetaxel, doxorubicin and cyclophosphamide. Biomarkers. 2015;20(2):143–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomarkers of Heart Failure
Rights and permissions
About this article
Cite this article
Chaikijurajai, T., Tang, W.H.W. Reappraisal of Inflammatory Biomarkers in Heart Failure. Curr Heart Fail Rep 17, 9–19 (2020). https://doi.org/10.1007/s11897-019-00450-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-019-00450-1