Abstract
Heart failure affects 5.1 million people in the USA annually. It accounts for a frequent cause of hospitalizations and disability. Patients with congestive heart failure have lower plasma levels of CoQ10, which is an independent predictor of mortality in this patient population. It has been hypothesized that a deficiency of CoQ10 can play a role in the development and worsening of heart failure, and that oral supplementation can possibly improve symptoms and survival in these patients. Based on previous small studies and meta-analyses, the use of CoQ10 in heart failure suggested an improvement ejection fraction, stroke volume, cardiac output, and cardiac index with CoQ10 supplementation, however most of these small studies appeared to be underpowered to result in any significant data. The results of the recent Q-SYMBIO trial demonstrated an improvement in heart failure symptoms with a significant reduction in major adverse cardiovascular events and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure affects 5.1 million people in the USA annually and it is associated with approximately 275,000 deaths per year [1]. Almost 50 % of people who develop heart failure die within the first 5 years of this diagnosis and it is a frequent cause of hospitalizations and disability amounting to total costs of an estimated US$32 billion annually [1].
In the last 30 years, there have been major improvements in the therapeutic management of heart failure; however, mortality rates continue to exceed 10 % per year [1]. Recently, long-term therapy with coenzyme Q10 (CoQ10) has been shown to improve heart failure symptoms, reduce major adverse cardiovascular events, and mortality and to be safe and well tolerated [2]. This review will summarize the literature related to CoQ10 in the treatment of heart failure.
Coenzyme Q10
Pathobiology
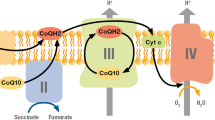
CoQ10 exists in two different forms: a reduced form, as ubiquinol (CoQ10H2), and as an oxidized form, ubiquinone (CoQ10) [3]. It is endogenously produced, and converts between the two forms, as the reduced or antioxidant form and as the oxidized form, as part of normal cellular enzyme functions [3]. It is a lipid-soluble molecule that uses lipoprotein-mediated transport for circulation, and is located in the lipid mitochondrial membranes, to protect the membranes from damage by free radicals [3]. It correlates with total and low density lipoprotein cholesterol levels and it acts as a free radical scavenger.
CoQ10 is found in the mitochondria of nearly every cell in the body; however it is most highly concentrated in the mitochondria of the heart, liver, and kidneys. Its main role is to participate in aerobic cellular respiration to create adenosine triphosphate (ATP) for the production of energy. It is an essential cofactor in oxidative phosphorylation in the mitochondria. It does this by mediating electron transfer in the electron transport chain, by shuttling them from complex I and II to complex III in the mitochondrial respiratory chain in order to generate ATP [3]. Ninety-five percent of the human body energy is generated in this way.
CoQ10 also works by having direct antioxidant effects and preventing membrane oxidation and lipid peroxidation, stabilizes LDL, and promotes recycling of alpha-tocopherol. This occurs when ubiquinone acts as a cofactor to produce ATP and is reduced to ubiquinol. Ubiquinol then interacts with free radicals and is oxidized back to ubiquinone [3].
Coenzyme Q10 levels are highest in the first 20 years of life and the levels decline as the subject ages. About 25 % can be supplied from the diet and it is present in certain foods especially meat, fish, and vegetable oils [3].
Coenzyme Q10 in Heart Failure
Coenzyme Q10 (CoQ10) or ubiquinone, is a coenzyme that is endogenously produced [2]. CoQ10 functions as an electron carrier in the mitochondria and is essential for the production of the majority of the cellular energy in the human body [2]. CoQ10 was first discovered and isolated, in 1957, in beef hearts at the University of Wisconsin Enzyme Institute [3]. In 1961, Peter Mitchel found it to be an important part of the electron transport chain and in the production of energy [4].
In the early 1970s, it was demonstrated by Folkers et al. that patients with congestive heart failure had lower levels of CoQ10 in their blood and cardiac tissue [5]. In a follow-up study, Folkers et al. demonstrated that myocardial CoQ10 levels declined as heart failure worsens, with the largest deficiency of CoQ10 levels in patients with NYHA class IV symptoms. [6]. It is also known that the low levels of CoQ10 in patients with heart failure are an independent predictor of mortality in these patients [7]. In addition, CoQ10 deficiency can play a role in the development and worsening of heart failure [2].
CoQ10 as a therapeutic agent may have a beneficial effect in patients with heart failure by three different actions. First, it increases ATP generation and cellular energy by mediating electron transfer in the electron transport chain. Second, by reducing oxidative stress, a well-known marker of mortality in heart failure, and by preventing membrane oxidation and lipid peroxidation [8, 9] Third, by stabilizing calcium-dependent ion channels in the myocardium thus enhancing ATP synthesis [2, 10].
Coenzyme Q10
Clinical Trials (Table 1)
Randomized Controlled Studies
In 1980, Langsjoen and Folkers performed the first US trial of CoQ10 in the treatment of heart failure [11, 12]. Subsequently, they evaluated its long-term effects in 143 patients over a 6-year period. The data demonstrated that the overall mortality was one third of expected with CoQ10 and that there was an improvement in cardiac function on the active treatment [11, 13]. Other studies such as the trial by Oda et al. showed that there was a normalization of cardiac function in 40 patients with mitral valve prolapse who took 3.1–3.4 mg/kg day of CoQ10 [14]. Rossi et al. [15] followed 20 patients with ischemic cardiomyopathy for 3 months in a double-blinded fashion and demonstrated that patients on CoQ10 therapy had improvements in ejection fraction and exercise tolerance. In addition, Poggesi et al. confirm that patients on coenzyme Q10 with ischemic and dilated cardiomyopathy had an improvement in ejection fraction and exercise tolerance [16]. Other studies confirmed improvements in cardiac performance with the use of coenzyme Q10 in heart failure and also demonstrated an improvement in quality of life. Some of these studies demonstrated a minimal improvement in survival [17–25].
The largest randomized controlled study by Morisco et al. followed 641 patients with heart failure, NYHA class III or IV symptoms, for 1 year and demonstrated that patients taking supplemental CoQ10 had a 50 % reduction in hospitalizations although there was no improvement in mortality [26].
Conversely, there are three other studies, which did not show any benefit in cardiac function or exercise tolerance in patients treated with coenzyme Q10 [27–29]. These studies were found to have small sample sizes, the doses of coenzyme Q10 were lower, and the severity and duration of heart failure was not clear.
The majority of these trials confirmed that patients with heart failure had an improvement in hemodynamic parameters such as ejection fraction, and cardiac output with CoQ10 supplementation. In addition, CoQ10 improved functional capacity and quality of life. However the sample sizes in these trials were relatively small and most of these trials were underpowered to address major clinical endpoints.
Meta-analyses
In order to overcome these issues, three meta-analyses confirmed that there was an improvement in ejection fraction, stroke volume, cardiac output, and cardiac index in patients with heart failure on CoQ10 treatment. The first meta-analysis by Soja and Mortenson reviewed eight double-blinded placebo-controlled trials in patients with heart failure and demonstrated that compared to the placebo group, patients on coenzyme Q10, achieved a better ejection fraction, stroke volume, cardiac output, cardiac index, and end diastolic volume [30]. In another meta-analysis of 11 studies with coenzyme Q10 by Sander et al., they were able to show similar results with an improvement in ejection fraction (3.7 %), cardiac output (0.28 L/min), stroke volume, and cardiac index. [31] Moreover, the authors demonstrated that that ejection fraction improved by 6.74 % in patients without angiotensin-converting enzyme inhibitor (ACEi) therapy compared to 1.16 % change in patients with angiotensin-converting enzyme inhibitors [31].
Fotino et al. also showed similar results in a meta-analysis where CoQ10 supplementation improved ejection fraction (3.7 %) and cardiac output in patients with systolic heart failure [32]. A subgroup analysis revealed an improvement in ejection fraction by 4.8 % in patients with ejection fractions of greater than 30 % compared to no change in patients with an ejection fraction less than 30 % [32].
The data of these meta-analyses suggested that the utilization of coenzyme Q10 produces functional improvement and improved hemodynamic parameters in patients with heart failure. The effects of coenzyme Q10 on mortality were not clearly demonstrated.
Systematic Review
In 2014, a Cochrane systematic review evaluated seven randomized controlled trials [19, 20, 29, 33–36], with a total of 914 patients [10] that compared CoQ10 with placebo in patients with heart failure and demonstrated that there was no effect on total mortality.
Thus, although CoQ10 appeared to be safe based on the previous small studies and meta-analyses that appeared underpowered, the ACC/AHA did not recommend CoQ10 in the 2013 guidelines [37].
Q-SYMBIO Trial
In order to clarify the effects of coenzyme Q10 on mortality and other endpoints, the Q-SYMBIO trial was designed. The Q-SYMBIO trial is a prospective, randomized, double-blinded, placebo-controlled, multicenter trial of CoQ10 as an adjunctive treatment for chronic heart failure. It included 420 patients with moderate to severe CHF on standard therapy, who were assigned to receive, for a 2-year period, CoQ10 100 mg three times daily (n = 202) or placebo (n = 218). Changes in symptoms, biomarkers, long-term outcomes, and major adverse cardiovascular events (MACE) were recorded [38, 39].
No significant differences were observed for secondary end points (NYHA functional class, visual analogue scale score for dyspnea, fatigue, and improvement in symptoms, N-terminal pro-brain natriuretic polypeptide (NT-proBNP) and 6-min walk test) between two groups at 16 weeks. However, at week 106 (intention to treat), there was a significant reduction in MACE (defined as a hospitalization due to worsening heart failure, death from a cardiovascular cause, urgent heart transplantation, or artificial mechanical support) in the CoQ10 group. In addition, NYHA class improved by one class and serum NT-proBNP decreased from 1137 to 881 pg/mL. There was also a decrease in the rates of cardiovascular mortality (9 vs 16 %), all-cause mortality (10 vs 18 %), and hospitalizations. The number of adverse events tended to be less in the CoQ10 group. In summary, Q-SYMBIO demonstrated that the long-term supplementation of CoQ10 in patients with heart failure seems safe and not only improves symptoms but reduces MACEs and decreases mortality [38, 39].
Coenzyme Q10
Supplements and Interaction With Medical Therapy
The difficulty in achieving therapeutic levels of coenzyme Q10 is that this compound is fat soluble therefore intestinal absorption can be difficult, with as much as 50 % being excreted. The absorption of coenzyme Q10 in patients with heart failure is more difficult because of intestinal edema and congestion [40, 41]. Therefore, having a formulation that allows for better bioavailability is important. Bioavailability is the lowest in the powder form, increases as an oil emulsion, and is best as solubilized compounds and nanoparticles [2]. Higher doses of CoQ10 are associated with higher serum levels, however absorption is non-linear, so higher doses are more effective if they are divided into multiple doses [3].
The majority of supplements of coenzyme Q10 are in the oxidized form, ubiquinone, which has been in the majority of the clinical trials. Although the more expensive formulation ubiquinol is now available in pill form, it should not make a difference to prescribe either one, since ubiquinone is converted to ubiquinol after absorption. However, one study demonstrated that ubiquinol was better absorbed into the bloodstream and patients have higher serum levels [42, 43].
A serum blood levels of 3.5 μg/mL or greater is needed for improvement of cardiac function according to the clinical trials [42] There are no serious adverse effects with coenzyme Q10 except for mild gastrointestinal discomfort [43].
Recommended doses of CoQ10 are the following:
-
Congestive heart failure (CHF): 300 mg/day in 2–3 divided doses [8, 44]
-
Coronary artery disease: 300 mg daily in 2–3 divided doses [45]
-
Statin user: 100–200 mg/day although not definitive evidence [48–50]
Statins and beta-blockers are frequent agents utilized in patients with heart failure that have been shown to decrease CoQ10 levels by inhibiting the mevalonate pathway. Statins can reduce blood serum levels of CoQ10 by almost much as 40 % [50]. It has been suggested that fatigue, muscle pain, and weakness with statin use are related to a deficiency in coenzyme Q10. Interestingly, in patients with normal cardiac function, who received 20 mg of atorvastatin, 70 % developed diastolic dysfunction that was reversible with CoQ10 supplementation [51].
Conclusion
Currently, Europe, Russia, the USA, and Japan make up 85 % of the total consumption of CoQ10 supplementation. In Japan, CoQ10 was approved as treatment for heart failure in 1974 [29]. In 1982, it became one of the top five medications used in Japan [52]. The use of coenzyme Q10 is not advocated in the American College of Cardiology/American Heart Association HF guidelines from 2013.
The results of the recent Q-SYMBIO trial demonstrate that the utilization of coenzyme Q10 in patients with heart failure improves symptoms and is associated with a significant reduction in major adverse cardiovascular events and mortality without any major side effects [38, 39]. In summary, the evidence suggests that supplemental CoQ10 may be a useful option for the management of patients with heart failure.
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245.
DiNicolantonio J, Bhutani J, McCarty M, O’Keefe J. Coenzyme Q10 for the treatment of heart failure: a review of the literature. Open Heart. 2015;2(1):e000326.
Mantle D. Coenzyme Q10 and cardiovascular disease: an overview. October 2015. British J. Cardiology. 2015;22:160.
Kapoor P, Kapoor AK. Coenzyme Q10—a novel molecule. J Indian Acad Clin Med. 2013;14:1.
Folkers K, Littarru GP, Ho L, et al. Evidence for a deficiency of coenzyme Q10 in human heart disease. Int Z Vitaminforsch. 1970;40(3):380–90.
Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q 10. Proc Natl Acad Sci U S A. 1985;82:901–4.
Molyneux SL, Florkowski CM, George PM, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52:1435–4. Langsjoen, P. Alleviating Congestive Heart Failure with Coenzyme Q10. LE Magazine Febuary 2008.
Rauchova H, Lenaz G, Drahota Z. Function of coenzyme Q in the Cekk: some biochemical and physiological properties. Physiol Res. 1995;44:209–16.
Tsutsui T, Tsutamoto T, Wada A, et al. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;39(6):957–62.
Madmani ME, Yusuf Solaiman A, Tamr Agha K, et al. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. 2014;6:CD008684.
Langsjoen P. Alleviating congestive heart failure with coenzyme Q10. LE magazine. 2008.
Langsjoen PH, Vadhanavikit S, Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proc Natl Acad Sci U S A. 1985;82(12):4240–4.
Langsjoen PH, Folkers K. A six-year clinical study of therapy of cardiomyopathy with coenzyme Q10. Int J Tissue React. 1990;12(3):169–71.
Oda T. Effect of coenzyme Q10 on load-induced cardiac dysfunction: double-blind study and investigation of dose-response relationship. In: Lenaz G, Barnabei O, Rabbi A, editors. Highlights in ubiquinone research. Taylor and francis: London; 1990. p. 232–7.
Rossi E, Lombardo A, Testa M, Lippa S, Oradei A, Littarru GP, et al. Coenzyme Q10 in ischaemic cardiopathy. In: Folkers K, Yamagami T, Littarru GP, editors. Biomedical and clinical aspects of coenzyme Q, vol. 6. Amsterdam: Elsevier; 1991. p. 321–6.
Poggesi L, Galanti G, Comeglio M, et al. Effect of coenzyme Q10 on left ventricular function in patients with dilative cardiomyopathy: a medium-term randomized double-blind study versus placebo. Curr Ther Res. 1991;49:878–86.
Rengo F, Abete P, Landino P, et al. Role of metabolic therapy in cardiovascular disease. Clin Investig. 1993;71:S124–S8.
Hofman-Bang C, Rehnqvist N, Swedberg K, et al. Coenzyme Q 10 as an adjunctive in the treatment of chronic congestive heart failure. J Card Fail. 1995;1:101–7.
Munkholm H, Hansen HH, Rasmussen K. Coenzyme Q10 treatment in serious heart failure. Biofactors. 1999;9(2-4):285–9.
Morisco C, Nappi A, Argenziano L, Sarno D, Fonatana D, Imbriaco M, et al. Noninvasive evaluation of cardiac hemodynamics during exercise in patients with chronic heart failure: effects of short-term coenzyme Q10 treatment. Mol Asp Med. 1994;15:s155–163.
Judy W, Hall J, Toth P, et al. Improved long-term survival in coenzyme Q10 treated chronic heart failure patients compared to conventionally treated patients. In: Folkers K, Littarru G, Yamagami T, editors. Biomedical and clinical aspects of coenzyme Q, vol. 6. Amsterdam: Elsevier, Publishers; 1991. p. 291–8.
Langsjoen PH, Langsjoen PH, Folkers K. Long-term efficacy and safety of coenzyme Q10 therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1990;65(7):521–3.
Judy W, Hall J, Toth P, et al. Double blind-double crossover study of coenzyme Q10 in heart failure. Biomed Clin Aspects Coenzyme Q. 1986;5:315–23.
VanFraechem JPH, Picalausa C, Folkers K. Ef—fects of CoQ10 on physical performance and recovery in myo- cardial failure. In: Folkers K, Yamamura Y, editors. Biomedical and clinical aspects of coenzyme Q, vol. 5. Amsterdam: Elsevier Publishers; 1986. p. 377.
Schneeberger W, Muller-Steinwachs J, Anda L, et al. A clinical double blind and crossover trial with coenzyme Q10 on patients with cardiac disease. In: Folkers K, Yamamura Y, editors. Biomedical and clinical aspects of coenzyme Q, vol. 5. Amsterdam: Elsevier; 1986. p. 325–33.
Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multi-center randomized study. Clin Investig. 1993;71:S134–6.
Permanetter B, Rössy W, Klein G, Weingartner F, Seidl KF, Blömer H. Ubiquinone (coenzyme Q10) in the long-term treatment of idiopathic dilated cardiomyopathy. Eur Heart J. 1992;13(11):1528–33.
Watson PS, Scalia GM, Gaibraith AJ, Burstow DJ, Aroney CN, Bett JH. Is coenzyme Q10 helpful for patients with idiopathic cardiomyopathy? Med J Aust. 2001;175(8):447. author reply 447-8.
Khatta M, Alexander BS, Krichten CM, et al. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med. 2000;132(8):636–40.
Soja AM, Mortenson SA. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Mol Aspects Med. 1997;18:159–68.
Sander CCI, Patel AA, et al. The impact of coenzyme q10 on systolic function in patients with chronic heart failure. J Card Failure. 2006;12:464–72.
Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q10 supplementation on heart failure: a meta-analysis. Am J Clin Nutr. 2013;97:268–75.
Kocharian A, Shabanian R, Rafiei-Khorgami M, Kiani A, Heidari-Bateni G, Kocharian A, et al. Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiology in the Young. 2009;19(5):501–6.
Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors. 2008;32(1-4):145–9.
Berman M, Erman A, Ben-Gal T, Dvir D, Georghiou GP, Stamler A, et al. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clin Cardiol. 2004;27(5):295–9.
Keogh A, Fenton S, Leslie C, Aboyoun C, Macdonald P, Zhao YC, et al. Randomised double-blind, placebo-controlled trial of coenzyme Q10 therapy in class II and III systolic heart failure. Heart, Lung and Circulation. 2003;12(3):135–41.
2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239
Mortensen S, Kumar A, Filipiak K, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure. Results from the Q-SYMBIO study. Eur J Heart Fail. 2013;15(S1):S20.
Mortensen SA, Rosenfeldt F, Kumar A, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641–9.
Langsjoen PHm Langsjoen AM. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors. 2008;32(1-4):119–28.
Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH (trade mark)) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 2007;47(1):19–28.
Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9(2-4):273–84.
Mortensen SA. Coenzyme Q10: will this natural substance become a guideline-directed adjunctive therapy in heart failure? JCHF. 2015;3(3):270–1.
Belardinelli R, Mucaj A, Lacalaprice F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27(22):2675–81.
Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, Littarru GP. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J. 2007;28(18):2249–55.
Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J. 2001;94(11):1112–7.
Ho MJ, Bellusci A, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst Rev. 2009;4, CD007435.
Caso G et al. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99(10):1409–12.
Marcoff L, Thompson P. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. JACC. 2007;49(23):2231–7.
Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, et al. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol. 1993;33(3):226–9.
Silver MA, Langsjoen PH, Szabo S, Patil H, Zelinger A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. Am J Cardiol. 2004;94(10):1306–10.
Our History Timeline. www.Kanekanutrients.com
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sylvia Oleck and Hector O Ventura have no disclosures.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Therapy
Rights and permissions
About this article
Cite this article
Oleck, S., Ventura, H.O. Coenzyme Q10 and Utility in Heart Failure: Just Another Supplement?. Curr Heart Fail Rep 13, 190–195 (2016). https://doi.org/10.1007/s11897-016-0296-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-016-0296-6