Abstract
Cardiac magnetic resonance (CMR) has evolved into a major tool for the diagnosis and assessment of prognosis of patients suffering from heart failure. Anatomical and structural imaging, functional assessment, T1 and T2 mapping tissue characterization, and late gadolinium enhancement (LGE) have provided clinicians with tools to distinguish between non-ischemic and ischemic cardiomyopathies and to identify the etiology of non-ischemic cardiomyopathies. LGE is a useful tool to predict the likelihood of functional recovery after revascularization in patients with CAD and to guide the left ventricular (LV) lead placement in those who qualify for cardiac resynchronization (CRT) therapy. In addition, the presence of LGE and its extent in myocardial tissue relate to overall cardiovascular outcomes. Emerging roles for cardiac imaging in heart failure with preserved ejection fraction (HFpEF) are being studied, and CMR continues to be among the most promising noninvasive imaging alternatives in the diagnosis of this disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
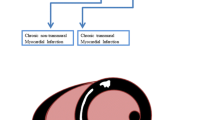
Despite great advances in the treatment of cardiovascular disease, heart failure remains as one of the most common causes of death and hospital readmissions in the USA, and the cost related with heart failure is near to 40 billion dollars per year [1]. Heart failure can be secondary to different etiologies, many of which are treatable and potentially reversible. Cardiac magnetic resonance (CMR) plays an integral role in the initial work-up of these conditions (Fig. 1). In the latest American College of Cardiology appropriate utilization guidelines for cardiovascular imaging in heart failure published in 2013, CMR is recommended as an appropriate imaging test in patients with newly suspected or diagnosed heart failure, also in those with heart failure associated with myocardial infarction (MI), in patients considered for revascularization, and in those who meet criteria for ICD and CRT implantation [2].
CMR role in diagnosis and prognosis of different cardiac pathologies in patients presenting with congestive heart failure symptoms. DCM dilated cardiomyopathy, CAD coronary artery disease, HCM hypertrophic cardiomyopathy, HTN hypertension, ARVC arrhythmogenic right ventricular cardiomyopathy, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction, CRT cardiac resynchronization therapy, CM cardiomyopathy
CMR Techniques Used in Heart Failure Evaluation
A typical CMR study takes 45 min, and scans are done with multiple 5- to 15-s breath holds. The first part of the protocol is typically an anatomic evaluation, including volume, mass, and functional assessment using steady-state free precession imaging (SSFP). CMR is considered the gold standard for measurement of left ventricular (LV) and right ventricular (RV) volumes and function and, importantly, is highly reproducible when compared to other modalities [3–5]. SSFP has become the standard technique for cardiac anatomic evaluation due to its higher contrast to noise ratio between the dark myocardium and bright blood pool and has replaced the older gradient echo sequence (GRE) routinely used until the end of the 1990s [6]. Another part of the standard CMR protocol involves the use of gadolinium-based contrast agents (GBCA) for evaluation of scar/fibrosis using late gadolinium enhancement (LGE). Inversion recovery pulse sequences are used with the inversion time set to null normal myocardium, therefore increasing the signal difference between normal and scarred/fibrotic segments. This allows the clinician to identify areas of fibrosis using CMR. LGE is quite reproducible. The clinical and histological changes occurring in the myocardium have been correlated and validated against histopathology [7, 8].
LGE can be used to differentiate between ischemic and non-ischemic cardiomyopathies and also among the non-ischemic pathologies by its distribution pattern. LGE aids in assessment of diagnosis and assessment of prognosis of patients with heart failure [9•]. Tissue characterization is another important tool used in CMR protocols. T1, T2, and T2* sequences can allow the imager to gain insight into the intrinsic characteristics of the myocardium [10]. Native T1 corresponds to the longitudinal relaxation time (T1) of a given tissue prior to the use of any GBCA. The most often used sequence is the modified look-locker inversion recovery (MOLLI) [11] sequence and its shorter version called ShMOLLI [12]. The T1 value is dependent on the magnetic field strength in which the images are acquired: 1.5 Tesla (T) or 3 T. T1 is commonly prolonged with fibrosis, edema, and infiltrative diseases and reduced in lipid, iron deposition, and in acute infarction.
Gadolinium does not cross cell membranes and therefore accumulates entirely in the extracellular compartment. Thus, T1 values can be obtained pre and post-contrast to calculate the extracellular volume (ECV). ECV is considered a marker of the extent of interstitial fibrosis in fibrotic diseases. Detecting changes in ECV may allow clinicians to quantify severity of disease, determine prognosis, and more importantly establish an early diagnosis, potentially allowing clinicians to change the course of a disease [13•, 14•]. T-weighted (W) images and more recently T2 mapping can identify myocardial edema and/or injury, differentiating between acute and chronic events [15]. T2*-W sequences have been traditionally used for the diagnosis of iron overload conditions including hemochromatosis or in patients who received multiple transfusions due to hematological diseases like aplastic anemia, thalassemia, and sickle cell anemia.
CMR Evaluation in Ischemic Cardiomyopathy
In the contest of patients with coronary artery disease and heart failure, CMR can be used for different reasons including (1) to rule out active ischemia and exclude ischemic heart disease as a possible reversible cause, (2) to assess myocardial viability prior to revascularization, (3) to assess prognosis, (4) to plan cardiac resynchronization therapy (CRT) implantation with biventricular pacing, or (5) to guide appropriate medical therapy. Stress CMR is performed routinely in many centers across Europe and the USA using adenosine or regadenoson infusion and is generally well tolerated. A negative study carries a good prognosis and is associated with very low risk of cardiovascular death and MI [16•].
The presence of LGE in a coronary distribution suggests CAD, but the absence of it does not automatically rules out CAD because extensive areas of hibernating myocardium may have no evidence of enhancement. However, CMR has shown to be helpful on reclassifying patients with initial diagnosis of non-ischemic cardiomyopathy after coronary angiogram. Soriano et al. [17] prospectively enrolled 71 patients with heart failure and LV dysfunction with no prior history of MI and performed CMR with LGE and coronary angiography (CA) to compare the results. Eighty-one percent of patients with obstructive CAD by CA and 9 % of patients with non-obstructive CAD showed subendocardial and/or transmural enhancement consistent with prior infarction, suggesting a role for CMR in reclassifying patients as ischemic in the absence of obstructive CAD. McCrohon et al. [18] found in a cohort of 90 patients with heart failure and LV dysfunction that even though the majority of patients had no evidence of LGE, a group of patients (13 %) have LGE in coronary distributions suggestive of CAD, patients who would have ended up misclassified as non-ischemic otherwise. These cases most likely were due to coronary recanalization post-infarction or embolic infarction.
LGE provides supplemental prognostic value when compared to traditional risk factors. In the setting of acute myocardial infarction (MI), microvascular obstruction (MO), microvascular hemorrhage, and tissue necrosis (infarct size) can be readily identified with CMR and they are associated with poor prognosis [19, 20]. Additionally, CMR has a role in predicting late myocardial recovery and in identifying areas at risk after MI [21, 22]. The excellent visualization of the myocardium allows an evaluation for LV thrombus, aneurysms, and pseudoaneurysms.
In patients with chronic CAD, determination of myocardial viability is an important role for CMR. Kim et al. [23] showed the ability of LGE to identify reversible and irreversible areas of myocardial injury prior to revascularization, which in turn is an important marker of likelihood of contractile recovery [24]. LGE and low-dose dobutamine (LDD) provide excellent accuracy in identifying segments that are targets for revascularization in patients with chronic CAD. LGE provides high sensitivity and negative predictive value (NPV) while LDD provides high specificity and positive predictive value (PPV) [25]. Both techniques can be integrated into one protocol if needed; nevertheless, LGE has become the standard test ordered in clinical practice due to practical and time issues regarding the use of dobutamine [26].
CMR continues to evolve as a promising technique in guiding LV lead placement for CRT. Several studies have shown lower rates of CRT responders when the lead is placed in scarred myocardium on LGE [27–29, 30•].
Predicting therapeutic response with beta-blockers of patients with heart failure and ischemia is possible with CMR. Bello et al. [31] demonstrated an inverse relationship between the scar extent at baseline and likelihood of contractile recovery 6 months later, therefore, predicting the recovery and remodeling of a myocardial segment based on LGE.
CMR Evaluation in Non-Ischemic Cardiomyopathy
CMR can help differentiate among the multiple etiologies of non-ischemic cardiomyopathy. Tissue characterization and LGE are the pillars of the diagnostic approach to patients with heart failure and provide additional prognostic information to the clinician in the early phase of the disease [32•].
The presence and extent of LGE in non-ischemic cardiomyopathies can identify patients who are at risk of future heart failure re-admissions. These would allow hospitals and providers to target patients at risk and start aggressive monitoring protocols in the outpatient setting to those who exhibited significant evidence of LGE in an attempt to reduce repetitive heart failure hospital admissions, a major cost burden to society [33].
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease with prevalence of at least 1 in 500 patients in the general population, representing the most common genetic cause of heart disease and most frequent cause of sudden cardiac death (SCD) among young athletes [34]. CMR plays a key role in the diagnostic work-up of this disease. High-resolution SSFP permits accurate assessment of wall thickness. It is particularly useful in those patients with difficult echocardiographic windows and unusual locations of hypertrophy such as the basal lateral wall and apex. T1 relaxation times are prolonged in patients with HCM and allow detection of underlying disease beyond the areas identified with LGE [35, 36]. LGE can detect areas of fibrosis in patients with HCM. Traditional risk factors for SCD in patients with HCM such as family history of SCD, personal history of ventricular fibrillation (VF) or ventricular tachycardia (VT), frequent non-sustained VT on 24 ambulatory holter, LV wall thickness greater than 30 mm sometimes can be inconclusive in determining a patient’s risk of SCD. Recent data suggest that the extent of LGE (> 15 % of the myocardial mass) can be added to the list and potentially play a major role in SCD stratification in this subset of patients [37•]. A recent meta-analysis [38] that included over a thousand individuals (n = 1063) with HCM, showed that LGE was present in 60 % of patients and highlighted the relationship between LGE and cardiovascular mortality in HCM. In addition, the presence of LGE showed a significant trend in predicting sudden death in these patients. To further evaluate this prospectively in a large scale, the National Institute of Health is currently funding a study to determine if these associations of LGE and other novel markers that include serum biomarkers of collagen metabolism will be able to predict SCD and other MACE in patients with HCM [39].
Myocarditis is caused by inflammation of the myocardium as a result of a viral infection; CMR plays an important role in diagnosis and prognosis of this disease. Some of the sequences used for the diagnosis are T2-W dark blood and T2-W bright blood, T1 and T2 mapping (which are now replacing the pure imaging sequences), and LGE [40, 41•]. The typical patient with myocarditis presents to the emergency room with chest pain, troponin elevation and non-obstructive CAD or with normal coronary arteries on invasive coronary angiography. The LGE patterns are classically described as mid-wall and/or subepicardial enhancement often in the basal inferolateral walls, findings that have been validated by histopathological studies [42]. However, the LGE pattern not only constitutes a non-invasive diagnostic tool but also can add prognostic information as well. Grun et al. [43] showed that the presence of LGE is the best single predictor of all-cause mortality in a population of 222 patients with biopsy-proven viral myocarditis, independent of clinical symptoms at presentation.
Amyloidosis is an infiltrative systemic disease that produces classic LGE patterns as well. The amyloid protein increases the GBCA uptake in the myocardium and prolongs T1 relaxation time particularly as high as the blood pool T1 values. The LGE pattern and abnormal T1 values help in the diagnosis and prognosis of the disease based on the extent of the amyloid infiltration [44•]. SSFP imaging can demonstrate biventricular hypertrophy and dysfunction and bi-atrial enlargement commonly found in patients with cardiac amyloidosis. Usually two kinds of amyloid infiltrate the myocardium. Immunoglobulin light-chain or AL, also known as primary systemic and transthyretin or ATTR, also known as senile. CMR can help in the diagnosis of both types and increasingly being used to discriminate between the two types of amyloid. Fontana et al. [45] determined the native T1 values in 85 patients with ATTR amyloidosis, 79 patients with AL amyloidosis, 46 patients with HCM, and 52 healthy volunteers. AL had the highest values for T1, followed by ATTR. Furthermore, ATTR was associated with more extensive LGE with a proportion close to 2 to 1 compared to AL. The investigators developed a diagnostic scoring system to help differentiate between the two amyloid conditions with 87 % sensitivity and 96 % specificity.
Sarcoidosis is a systemic multi-organ disease of unknown etiology that is characterized by granuloma formation. Cardiac sarcoid can be present in up to one fourth of patients with the disease and mortality in those who present with symptoms can be as high as 25 %, an important target for early diagnosis and potential therapeutic intervention [46]. Sarcoidosis can be identified with CMR. Patients with cardiac sarcoid involvement commonly present with mid-wall or epicardial pattern of LGE that do not correspond to any coronary distribution. Nagai et al. [47] detect LGE in 13 % of patients with sarcoidosis in a cohort of 61 asymptomatic patients who carried the diagnosis of sarcoidosis but had not yet been diagnosed with cardiac sarcoid. Furthermore, Patel et al. [48] showed an increased risk for ventricular tachyarrhythmia in a group of 52 asymptomatic individuals with biopsy-proven extra-cardiac sarcoidosis and preserved LV function. These findings suggest that CMR can potentially be used in asymptomatic sarcoidosis patients without cardiac manifestation to potentially alter their disease progression by instating early and aggressive therapies. In addition, Greulich et al. [49•] studied 155 symptomatic patients with systemic sarcoidosis who underwent CMR to rule out cardiac involvement. LGE was found in 26 % of patients and was found to be the best independent predictor of death and adverse events when compared to LV ejection fraction (EF), LV end-diastolic volume (LVEDV), and/or symptoms of heart failure. Figure 2 shows different examples of the use of CMR in patients with non-ischemic and ischemic cardiomyopathies.
Clinical examples of the use of CMR in different cardiac pathologies. a Evidence of transmural anteroapical LGE consistent with MI in a patient with chest pain. b Extensive microvascular obstruction (MO) in patient with late-presenting STEMI. c Transmural LGE and evidence of apical thrombus in patient with CAD and prior apical MI. d Asymmetric septal hypertrophy in a patient with HCM. e Case of non-compaction cardiomyopathy. f Mid-epicardial LGE in a 38-year-old male presenting with NSTEMI and no evidence of CAD on coronary angiography, consistent with myocarditis
Other Cardiomyopathies
CMR can be used in the diagnosis and prognosis determination by using T2* protocols allowing the quantification of cardiac iron levels. High iron levels are commonly seen in conditions like thalassemia due to frequent blood transfusions and in hemochromatosis. This technique enables use of therapies aiming at reducing the levels of iron and therefore decreasing mortality rate in this subset of patients [50].
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is characterized by fibrofatty infiltration of the right or both ventricles and presenting usually before the fourth decade of life with palpitations, syncope, or SCD. Multiple criteria have been described [51] because of the challenging task of imaging the right ventricle. However, CMR is a diagnostic tool that not only allows high-resolution images of both the RV and LV but allows the identification of LGE patterns seen in subset of patients with ARVC [52].
Takotsubo or stress cardiomyopathy is a reversible condition with a unique pattern of regional LV dysfunction which is typically apical but can include the mid ventricle or rarely the base alone. It is typically seen in later decades of life with female predominance and usually triggered by external stressors. Patients usually present with chest pain, heart failure, typical pattern of LV dysfunction, elevation of troponins, and non-obstructive CAD. CMR has evolved as a useful technique to establish a definitive diagnosis and differentiate the disease from other forms of non-ischemic cardiomyopathies such as myocarditis. LGE usually is not found, and T2-W images and/or T2 mapping suggests diffuse myocardial edema [53].
CMR is also helpful in the diagnosis of Chagas disease [54], LV non-compaction [55, 56], and infiltrative disorders like Anderson-Fabry disease [57]. Maceira et al. [58] found significant cardiac changes in a cohort of 94 asymptomatic individuals who were active cocaine abusers when compared to a group of healthy volunteers. LV end-systolic, LV mass index, RV end-systolic, and LV and RV ejection fractions were all abnormal. These changes could represent early myocardial damage from the repetitive use of the sympathomimetic drug. Thirty percent of patients had myocardial LGE and up to 71 % of patients had abnormal findings in their CMR evaluation.
Habibi et al. [59] found an important relationship between CMR-measured LA size and function in patients who participated in the Multi-Ethnic Study of Atherosclerosis (MESA) Study. In these patients, LA changes preceded the development of heart failure. Neilan et al. [60•] were able to identify individuals at increased risk of future MACE after surviving a SCD episode and showed that CMR was able to reclassify a significant proportion of those patients and provide a correct diagnosis. Of the 137 patients that underwent CMR, 71 % had LGE and over 75 % had an arrhythmogenic substrate identified. Ten percent of patients had myocarditis, 2 % were diagnosed with HCM, 2 % were diagnosed with sarcoidosis, and another 2 % had ARVC.
More recently, the medical community is paying closer attention to patients with heart failure with preserved ejection fraction (HFpEF) who account for up to 50 % of cases of heart failure. CMR is currently being developed as a potential diagnostic tool, involving diastolic assessment, myocardial perfusion reserve analysis, and quantification to help in the diagnosis of microvascular disease and T1 mapping characterization. The future looks promising for these techniques in this special subset of patients with heart failure [61].
Conclusions
CMR has evolved over the last decade to become a key player in the diagnosis and prognosis of numerous types of cardiomyopathies encountered every day in clinical practice. CMR is the gold standard for measuring cardiac chamber size, volume, and function including the RV. The high-quality images obtained by SSFP enable accurate wall motion analysis and anatomic/functional valve evaluation. T1 and T2 mapping for tissue characterization can identify the underlying etiology of heart failure. In addition, LGE provides a unique non-invasive assessment of scar/fibrosis within the myocardium that no other technique offers. A CMR-focused evaluation can aid in the early diagnosis of multiple cardiac conditions, potentially changing therapeutic steps along the way. The extent of LGE in different cardiac diseases continues to highlight the importance of fibrosis as a key independent risk factor associated with high MACE and poor response to medical and revascularization therapies. CMR will continue to play a key role in the diagnosis and prognosis of patients with heart failure in the years ahead.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Readmissions Reduction Program from The Centers for Medicare & Medicaid Services. Retrieved from http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html.
Patel MR, White RD, Abbara S, Bluemke DA, Herfkens RJ, Picard M, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: a joint report of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Foundation Appropriate Use Criteria Task Force. J Am Coll Cardiol. 2013;61:2207–31.
Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–96.
Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34.
Marcu CB, Beek AM, Van Rossum AC. Cardiovascular magnetic resonance imaging for the assessment of right heart involvement in cardiac and pulmonary disease. Heart Lung Circ. 2006;15:362–70.
Thiele H, Nagel E, Paetsch I, Schnackenburg B, Bornstedt A, Kouwenhoven M, et al. Functional cardiac MR imaging with steady-state free precession (SSFP) significantly improves endocardial border delineation without contrast agents. J Magn Reson Imaging. 2001;14:362–7.
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002.
Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23.
Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250–8. LGE in patients with NICM is associated with increased risk of all-cause mortality, heart failure hospitalization, and SCD.
Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–22.
Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–6.
Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, et al. Shortened modified look-locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69.
Yi CJ, Wu CO, Tee M, Liu CY, Volpe GJ, Prince MR, et al. The association between cardiovascular risk and cardiovascular magnetic resonance measures of fibrosis: the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Magn Reson. 2015;17:15. Patients with greater CVD risks had greater T1 mapping indices by CMR, indicative of greater myocardial fibrosis.
Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. Consensus Group document in the use of T1 mapping and ECV in CMR.
Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, et al. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–78.
Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:826–38. Patients with a negative stress CMR study have very low risk of CVD death and MI, therefore highlighting the role of CMR in the prognosis of patients with known or suspected CAD.
Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–8.
McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9.
Larose E, Rodes-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, et al. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol. 2010;55:2459–69.
De Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Stiermaier T, et al. Prognosis after ST-elevation myocardial infarction: a study on cardiac magnetic resonance imaging versus clinical routine. Trials. 2014;15:249.
Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–76.
Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43.
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53.
Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, et al. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110:1535–41.
Bove CM, DiMaria JM, Voros S, Conaway MR, Kramer CM. Dobutamine response and myocardial infarct transmurality: functional improvement after coronary artery bypass grafting—initial experience. Radiology. 2006;240:835–41.
Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5:494–508.
Parker KM, Bunting E, Malhotra R, Clarke SA, Mason P, Darby AE, et al. Postprocedure mapping of cardiac resynchronization lead position using standard fluoroscopy systems: implications for the nonresponder with scar. Pacing Clin Electrophysiol. 2014;37:757–67.
White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–60.
Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–76.
Bilchick KC, Kuruvilla S, Hamirani YS, Ramachandran R, Clarke SA, Parker KM, et al. Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol. 2014;63:1657–66. CMR might play a role in decreasing the number of non-CRT responders by evaluating the influence of mechanical, electrical and scar properties of the left ventricle and guiding lead placement.
Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–53.
Poyhonen P, Kivisto S, Holmstrom M, Hanninen H. Quantifying late gadolinium enhancement on CMR provides additional prognostic information in early risk-stratification of nonischemic cardiomyopathy: a cohort study. BMC Cardiovasc Disord. 2014;14:110. LGE extent gives additional prognostic information compared to traditional risk factors in patients with NICM.
Wong TC, Piehler KM, Zareba KM, Lin K, Phrampus A, Patel A, et al. Myocardial damage detected by late gadolinium enhancement cardiovascular magnetic resonance is associated with subsequent hospitalization for heart failure. J Am Heart Assoc. 2013;2:e000416.
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA Guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–60.
Salerno M, Kramer CM. Prognosis in hypertrophic cardiomyopathy with contrast-enhanced cardiac magnetic resonance: the future looks bright. J Am Coll Cardiol. 2010;56:888–90.
Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:726–33.
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–95. LGE extent quantification by CMR provides additional information for assessing SCD risk among patients with HCM.
Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–7.
Kramer CM, Appelbaum E, Desai MY, Desvigne-Nickens P, DiMarco JP, Friedrich MF, et al. Hypertrophic Cardiomyopathy Registry (HCMR): The rationale and design of an international, observational study of hypertrophic cardiomyopathy. Am Heart J. 2015.in press.
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–87.
Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6:1048–58. T1 mapping showed excellent and superior diagnostic performance in the detection of acute myocarditis than traditional T2-W CMR.
Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–9.
Grun S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–15.
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488–97. Non-contrast T1 mapping has high diagnostic accuracy for detecting cardiac AL amyloidosis and potentially more sensitive for detecting early disease than LGE imaging.
Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–65.
Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92:282–8.
Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest. 2014;146:1064–72.
Patel AR, Klein MR, Chandra S, Spencer KT, Decara JM, Lang RM, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail. 2011;13:1231–7.
Greulich S, Deluigi CC, Gloekler S, Wahl A, Zurn C, Kramer U, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–11. The presence of LGE in patients with systemic sarcoidosis is the best independent predictor of potentially fatal events.
Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–8.
Romero J, Mejia-Lopez E, Manrique C, Lucariello R. Arrhythmogenic right ventricular cardiomyopathy (ARVC/D): a systematic literature review. Clin Med Insights Cardiol. 2013;7:97–114.
Te Riele AS, Tandri H, Bluemke DA. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. J Cardiovasc Magn Reson. 2014;16:50.
Dastidar AG, Frontera A, Palazzuoli A, Bucciarelli-Ducci C. TakoTsubo cardiomyopathy: unravelling the malignant consequences of a benign disease with cardiac magnetic resonance. Heart Fail Rev. 2015;015-9489-4.
Rochitte CE, Nacif MS, de Oliveira Junior AC, Siqueira-Batista R, Marchiori E, Uellendahl M, et al. Cardiac magnetic resonance in Chagas’ disease. Artif Organs. 2007;31:259–67.
Grothoff M, Pachowsky M, Hoffmann J, Posch M, Klaassen S, Lehmkuhl L, et al. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol. 2012;22:2699–709.
Choudhary P, Hsu CJ, Grieve S, Smillie C, Singarayar S, Semsarian C, et al. Improving the diagnosis of LV non-compaction with cardiac magnetic resonance imaging. Int J Cardiol. 2015;181:430–6.
Parsai C, O'Hanlon R, Prasad SK, Mohiaddin RH. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson. 2012;14:54,429X-14-54.
Maceira AM, Ripoll C, Cosin-Sales J, Igual B, Gavilan M, Salazar J, et al. Long term effects of cocaine on the heart assessed by cardiovascular magnetic resonance at 3T. J Cardiovasc Magn Reson. 2014;16:26.
Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, et al. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging. 2014;7:570–9.
Neilan TG, Farhad H, Mayrhofer T, Shah RV, Dodson JA, Abbasi SA, et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging. 2015;8:414–23. LGE extent identified patients with markedly increased risk of future adverse events among individuals with aborted sudden cardiac arrest.
Leong DP, De Pasquale CG, Selvanayagam JB. Heart failure with normal ejection fraction: the complementary roles of echocardiography and CMR imaging. JACC Cardiovasc Imaging. 2010;3:409–20.
Compliance with Ethics Guidelines
Conflict of Interest
Jorge A. Gonzalez declares that he has no conflict of interest.
Christopher M. Kramer has received research equipment support from Slemens Healthcare and consultant work for Merck, Myokardia, and St. Jude Medical.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pathophysiology of Myocardial Failure
Rights and permissions
About this article
Cite this article
Gonzalez, J.A., Kramer, C.M. Role of Imaging Techniques for Diagnosis, Prognosis and Management of Heart Failure Patients: Cardiac Magnetic Resonance. Curr Heart Fail Rep 12, 276–283 (2015). https://doi.org/10.1007/s11897-015-0261-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-015-0261-9
Keywords
- Cardiac magnetic resonance (CMR)
- Heart failure (HF)
- Heart failure with preserved ejection fraction (HFpEF)
- Heart failure with reduced ejection fraction (HFrEF)
- Left ventricular dysfunction (LV dysfunction)
- Cardiomyopathies
- Infiltrative cardiomyopathies
- Late gadolinium enhancement (LGE)
- T1 mapping
- T2 mapping
- T2 weighted images
- T2* imaging