Abstract
Pulmonary hypertension (PH) leading to right ventricular failure (RVF) is a common complication of left heart failure irrespective of the left ventricular ejection fraction. PH due to left heart disease is the most common cause of PH. The prevalence of PH and RVF in left heart failure varies depending on the patient population studied, the method used to diagnose PH, and the hemodynamic criteria used to define PH. Elevated left-sided filling pressure and functional mitral regurgitation are the two major determinants of PH in left heart failure. PH is associated with markers of disease severity, advanced symptoms, and worse long-term outcomes including heart failure hospitalization and mortality in left heart failure. RVF has independent, incremental prognostic value over PH for adverse outcomes in left heart failure. PH and RVF may be potential therapeutic targets in patients with left heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a common complication of left heart failure. PH due to left heart disease is more prevalent than other World Health Organization (WHO) categories of PH [1]. Moreover, the prevalence of PH due to left heart disease will likely expand as the incidence of left heart failure continues to increase in a growing elderly population [2]. Elevated left ventricular filling pressure leading to reactive pulmonary arterial vasoconstriction and pulmonary vascular remodeling results in PH in left heart failure [3•]. The presence of PH increases the resistive and the pulsatile afterload of the right ventricle, ultimately leading to right ventricular failure (RVF) [4]. The coexistence of PH and RVF in left heart failure is associated with markers of disease severity, advanced symptoms, and worse long-term outcomes. In this article, we review the prevalence, determinants, and prognostic value of PH and RVF in left heart failure, specifically in heart failure with reduced ejection fraction (HFrEF), heart failure with preserved ejection fraction (HFpEF), and left-sided valvular heart disease.
Classification of PH Due to Left Heart Failure
PH due to left heart disease is defined as mean pulmonary artery pressure (PAP) ≥25 mmHg in the presence of pulmonary artery wedge pressure (PAWP) >15 mmHg [5]. It is further classified into subtypes depending on the presence of intrinsic pulmonary vascular disease (pre-capillary component) in addition to the elevated left-sided filling pressures (post-capillary component). Due to a lack of consensus, various classification systems have been used in the literature based on different hemodynamic criteria (Table 1).
Post-capillary PH
Elevated left-sided filling pressure leads to an obligatory, passive increase in PAP in order to maintain forward flow. Typically, this increase in PAP is proportionate to the elevated left-sided filling pressure and it normalizes with reduction in the left-sided filling pressure. At this stage, due to the absence of a pre-capillary component, both the transpulmonary gradient (TPG, the difference between mean PAP and PAWP) and the pulmonary vascular resistance (PVR) remain within normal limits (TPG < 12–15 mmHg and PVR < 2.5–3 Wood units (WU)). This stage of PH in left heart disease has been described in the literature using various nomenclatures including pulmonary venous hypertension, passive PH, or post-capillary PH [6]. For the purpose of this review, we will refer to this as post-capillary PH.
Mixed PH (Pre- and Post-capillary PH)
Over time, the elevated left-sided filling pressure triggers pulmonary arterial vasoconstriction. This is later followed by intrinsic pulmonary arteriolar remodeling due to decreased nitric oxide availability and increased endothelin expression [7, 8]. The superimposed pre-capillary component increases the PAP disproportionate to the left-sided filling pressure, resulting in an elevated TPG (>12–15 mmHg) and/or PVR (>2.5–3 WU). This is referred to as mixed PH (post- and pre-capillary component) or PH out of proportion to the left-sided filling pressures [6]. We will refer to this as mixed PH for the purpose of this review.
Isolated Post-capillary vs. Combined Post- and Pre-capillary PH
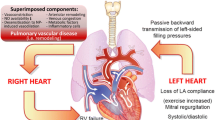
Traditionally, elevated TPG (>12–15 mmHg) or PVR (>2.5–3 WU) has been used to delineate the presence of a pre-capillary component in PH due to left heart disease [9•]. However, growing evidence suggests that both TPG and PVR are flow dependent and may not accurately reflect the presence of intrinsic pulmonary arteriolar remodeling [10••]. In contrast to TPG and PVR, diastolic pulmonary vascular pressure gradient (DPG, the difference between diastolic pulmonary artery pressure and pulmonary artery wedge pressure) is not flow dependent and more accurately identifies the presence of pre-capillary pulmonary arteriolar remodeling [11]. Hence, the most recent, fifth world symposium on PH proposed a new classification and hemodynamic definition for PH due to left heart disease based on DPG (Table 1): isolated post-capillary (DPG < 7 mmHg) and combined post-capillary and pre-capillary PH (DPG ≥ 7 mmHg) (Fig. 1) [12••].
Mechanism of pulmonary hypertension in left heart failure. HFrEF heart failure with reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, LVEDP left ventricular end diastolic pressure, MR mitral regurgitation, LA left atrium, PCWP pulmonary capillary wedge pressure, PH pulmonary hypertension, mPAP mean pulmonary artery pressure, and DPG diastolic pulmonary gradient
PH and Right Ventricular Dysfunction in HFrEF
Prevalence of PH and Right Ventricular Dysfunction in HFrEF
The prevalence of PH and RVF failure in HFrEF varies depending on the patient population studied (ambulatory vs. hospitalized for decompensated heart failure), the method (echocardiography or right heart catheterization) used to diagnose PH, and the hemodynamic criteria used to define PH.
The prevalence of PH based on echocardiography varies from 35 to 47.5 % (Table 2). In a retrospective review of 1,541 stable ambulatory HFrEF patients with an ejection fraction <40 % from the Mayo Clinic, 35 % of the patients had a right ventricular systolic pressure (RVSP) >45 mmHg [13]. In a more recent retrospective, population-based longitudinal cohort study in Scotland, RVSP >45 mmHg was present in 47.5 % of HFrEF patients [14].
The prevalence of PH based on invasive hemodynamic assessment in HFrEF varies from 33 to 46 % (Table 2). In 450 ambulatory HFrEF patients referred for right heart catheterization, 33 % of the patients had a mean PAP >25 mmHg [15•]. Of the 1,134 patients evaluated for new onset cardiomyopathy at the Johns Hopkins Hospital, 46 % of patients had a mean PAP >25 mmHg [16]. In a different study of 320 ambulatory HFrEF patients who had right heart catheterization as a part of transplant evaluation, 36 % had mixed PH with a PVR >2.5 WU [17]. A recent retrospective review from the Mayo Clinic reported that 40 % of the HFrEF patients referred for invasive hemodynamic assessment had mixed PH with a PVR >3 WU [15•].
Determinants of PH and Right Ventricular Dysfunction in HFrEF
The two major determinants of PH in patients with HFrEF are elevated left-sided filling pressure and functional mitral regurgitation (Fig. 1). The severity of PH correlates directly with the severity of echo-derived indices of left-sided filling pressure (mitral valve E/E′ ratio and deceleration time) and the severity of functional mitral regurgitation based on the mitral regurgitant orifice area rather than the left ventricular ejection fraction [18••, 19, 20]. In a single center series of 388 patients (75 % with left ventricular ejection fraction <50 %), HFrEF patients with a left ventricular restrictive filling pattern had a higher systolic PAP compared to those with a non-restrictive filling pattern (41 vs. 37 mmHg, P < 0.02) [21]. Furthermore, age, female gender, left atrial enlargement, atrial fibrillation, and decreased pulmonary artery compliance have been correlated with the presence of PH in HFrEF [15•, 22]. Finally, polymorphism in the promoter region of the serotonin transporter gene has also been associated with the presence of PH in HFrEF patients. HFrEF patients with the homozygous long variant (LL) had a higher PAP compared to those with the homozygous short variant (SS) or heterozygous variant (SL) [23].
Prognostic Value of PH and Right Ventricular Dysfunction in HFrEF
Several studies have consistently associated echocardiography-derived indices of PH, estimated systolic PAP and tricuspid regurgitant velocity, with increased mortality in HFrEF. In a recent study of 419 ambulatory HFrEF patients followed for a median of 2.6 years, echocardiography-estimated systolic PAP ≥48 mmHg was associated with a threefold higher risk of death, cardiac transplantation, left ventricular assist device implantation, or heart failure hospitalization [24]. Similarly, a community-based study from Olmsted County, MN, reported a linear relationship between echocardiography-estimated systolic PAP and long-term all-cause mortality [25].
The presence of mixed PH is associated with a higher risk of adverse outcomes compared to post-capillary PH. Miller et al. studied 463 stable ambulatory HFrEF patients referred for invasive hemodynamic assessment [15•]. Over a median follow-up of 2.1 years, patients with mean PAP >25 mmHg had a twofold higher risk of death compared to those without PH. Among those with PH, patients with mixed PH demonstrated a 1.5-fold higher risk of death compared to those with post-capillary PH. In this study, PVR >4 WU and pulmonary artery compliance <2.0 ml/mmHg were the strongest independent predictors of death [15•]. Likewise, in a different study from the Johns Hopkins Hospital, the risk of death increased significantly in patients with HFrEF when PVR increased beyond three WU [16].
Change in PAP over time provides additional prognostic information on HFrEF. An increase in mean PAP over time in serial hemodynamic assessments was independently associated with an increased risk of death in patients with HFrEF [26]. Interestingly, in corollary, a reduction in estimated systolic PAP with cardiac resynchronization therapy in HFrEF patients was associated with a reduction in death, cardiac transplantation, or heart failure-related hospitalizations [27].
The prognostic importance of PH in HFrEF varies depending on the underlying etiology of the left ventricular systolic dysfunction. The risk of death associated with the presence of PH is much higher in patients with myocarditis compared to those with ischemic or non-ischemic cardiomyopathy [16]. In a study of 1,134 patients with cardiomyopathies of varying etiologies, over a median follow-up of 4.4 years, the hazard ratio of death increased by 25 % for each 5 mmHg increase in mean PAP in the total cohort. However, in patients with myocarditis, for the same 5 mmHg increase in mean PAP, the hazard ratio for death increased remarkably by 85 % [16].
The presence of RVF has incremental prognostic value over PH for adverse outcomes in HFrEF [28, 29]. Right ventricular fractional area change (RVFAC) <35 % was associated with a twofold increased risk of death, cardiac transplantation, left ventricular assist device implantation, or heart failure hospitalization independent of elevated systolic PAP [24].
Epidemiology of PH and Right Ventricular Dysfunction in HFpEF
Prevalence of PH and Right Ventricular Dysfunction in HFpEF
PH is more prevalent in HFpEF than in HFrEF. The prevalence of PH in HFpEF varies from 18 to 83 % depending on the patient population studied and the cutoff value of estimated systolic PAP used to define PH (Table 2). In a population-based study of 244 HFpEF patients in Olmsted County, MN, 83 % of the patients had estimated systolic PAP >35 mmHg [30]. The median estimated systolic PAP in this cohort was 48 mmHg (IQR 37–56). However, in a UK-based study of 354 consecutive HFpEF patients referred to a heart failure clinic, only 18 % had a RVSP >35 mmHg, which is equivalent to an estimated systolic PAP of >45 mmHg [18••]. In HFpEF patients hospitalized for heart failure exacerbation in New York, the mean estimated systolic PAP was 47 ± 14 mmHg [31]. In the echocardiographic substudy of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial (TOPCAT), 450 patients had a measurable tricuspid regurgitant jet velocity [32]. Of these 450 patients, 36 % had a tricuspid regurgitant jet velocity >2.9 m/s (equivalent to an estimated systolic PAP of at least 35 mmHg + right atrial pressure) and 11 % had a PVR >2.5 WU.
The data on the prevalence of RVF in HFpEF is limited. In the echocardiographic substudy of the TOPCAT trial, 31 % of the patients had a RVFAC <45 %, 11 % had a RVFAC <40 %, and 4 % had a RVFAC <35 % [32]. In a recent study of 96 HFpEF patients referred to the Mayo Clinic Cardiac Catheterization Laboratory, 33 % of the patients had RVFAC <35 % [33•]. Puwanant et al. reported a similar prevalence of 33 % right ventricular dysfunction in patients with HFpEF [34]. In this study, HFpEF patients had a better right ventricular function based on RVFAC, tricuspid annular plane systolic excursion (TAPSE), and peak systolic tricuspid annular tissue velocity (S′) compared to HFrEF patients. In a prospective study of 419 HFpEF patients referred to a single tertiary medical center, 34 % had right ventricular hypertrophy, 28 % had TAPSE <18 mm, and 15 % had RVFAC < 35 % [35].
Determinant of PH and Right Ventricular Dysfunction in HFpEF
Similar to HFrEF, elevated left-sided filling pressure due to left ventricular diastolic dysfunction is the predominant determinant of PH in patients with HFpEF (Fig. 1). Lam et al. reported a linear correlation between estimated systolic PAP and PAWP estimated based on the mitral E/E′ ratio (r = 0.21; P < 0.007) [30]. Another echocardiography-based study of 69 patients with HFpEF also reported a strong correlation between estimated systolic PAP and PAWP (r = 0.73) [36]. In this study, HFpEF patients with a restrictive physiology of the left ventricle had a higher systolic PAP compared to those with a non-restrictive pattern. PH has also been associated with the presence of functional mitral regurgitation in HFpEF [37].
The risk factors that predispose to the development of mixed PH in HFpEF are unclear. Several possible factors have been proposed. First, age is considered a risk factor for the development of mixed PH in HFpEF, as older persons are more prone to develop arterial stiffening in the systemic as well as the pulmonary circulation [38]. Systolic PAP has been shown to increase with increasing age [39]. Also, HFpEF patients with PH are more likely to be older compared to those without PH [30]. Second, female gender is another potential risk factor. In 676 consecutive HFpEF patients referred to a single center, female gender was the only independent predictor of mixed PH (odds ratio 2.12, 95 % confidence interval (CI) 1.05–4.30, P = 0.03) [22]. The exact reason behind this association is unclear. Systemic arterial stiffness increases with age more commonly in women; hence, older women are more likely to have increased pulmonary artery stiffness [40–42]. Finally, obesity, atrial arrhythmias, and chronic obstructive lung disease have been associated with increased risk of developing PH in patients with HFpEF [43].
Melenovsky et al. reported correlates of right ventricular dysfunction in HFpEF in 96 patients with HFpEF who had simultaneous right heart catheterization and echocardiogram within 48 h. In this study, right ventricular dysfunction was more common in men than in women despite similar severity of PH. In addition, patients in atrial fibrillation were more likely to have right ventricular dysfunction compared to those in sinus rhythm. Other factors independently associated with right ventricular dysfunction were systolic PAP, presence of coronary artery disease, lower systemic arterial pressures, and lower left ventricular ejection fraction.
Prognostic Value of PH and RVF in HFpEF
The presence of PH is associated with increased mortality in HFpEF. In the study by Lam et al., over a median follow-up of 2.8 years, every 10 mmHg increase in estimated systolic PAP was associated with a 1.2-fold increased risk of death independent of age [30]. In the same study, HFpEF patients with an estimated systolic PAP >48 mmHg had a higher mortality compared to those with an estimated systolic PAP <48 mmHg. Likewise, in the Danish prospective placebo-controlled multicenter Echocardiography and Heart Outcome Study (ECHOS), of the 97 patients with HFpEF who had a good-quality tricuspid regurgitation jet velocity, patients with estimated systolic PAP >39 mmHg had an increased mortality compared to those with estimated systolic PAP <39 mmHg [21]. In a prospective study of 334 patients with PH due to left heart disease, of which 90 % had HFpEF, 1-year survival was 82 % and 2-year survival was only 74 % [44].
Similar to HFrEF, the presence of right ventricular dysfunction is associated with independent incremental risk for adverse outcomes over PH in HFpEF. HFpEF patients with RVFAC <35 % have an increased risk of death compared to patients without right ventricular dysfunction (RVFAC ≥ 35 %) regardless of systolic PAP [33•]. In a recent study of 419 HFpEF patients prospectively followed for a median follow-up of 18 months, the presence of right ventricular remodeling was a strong predictor of mortality. In this study, every 1 standard deviation increase in right ventricular wall thickness was associated with a 1.4-fold increased risk of death [35].
PH and Right Ventricular Dysfunction in Left-Sided Valvular Heart Disease
Mitral Valve Disease
PH frequently complicates both mitral stenosis and mitral regurgitation (Table 2). In a recent series of 314 patients with isolated mitral stenosis undergoing percutaneous mitral balloon commissurotomy, 73 % had a mean PAP >25 mmHg and 19 % had a TPG >15 mmHg [45]. In a different, single center series of 559 patients who underwent percutaneous mitral balloon valvotomy, 41 % had invasively measured systolic PAP >50 mmHg [46]. In an early series of 58 patients with isolated mitral stenosis who underwent mitral valvotomy, 26 % of patients had a PVR >6 WU [47].
In a more recent multicenter international registry of 459 patients with isolated mitral regurgitation due to filial mitral leaflets, 23 % of patients had Doppler-estimated systolic PAP >50 mmHg [48]. Alexopoulos et al. studied 41 patients with isolated mitral regurgitation who had invasive right heart hemodynamics [49]. In this series, 76 % of the patients had a mean PAP >30 mmHg and 44 % had a mean PAP >50 mmHg.
The presence of PH depicts poor prognosis in patients with mitral valve disease. In the multicenter international mitral regurgitation registry, over a median follow-up of 4 years, systolic PAP >50 mmHg was associated with increased risk of all-cause mortality, cardiovascular mortality, and heart failure [48]. In addition, PH was associated with increased risk of early 30-day mortality and heart failure after surgical mitral valve replacement. In a large single center series of 2,316 patients who underwent mitral valve replacement for either mitral stenosis or regurgitation (2:1 ratio), patients with systolic PAP >50 mmHg had a higher 30-day mortality compared to those with systolic PAP <50 mmHg (10.6 vs. 3.6 %) [50]. However, in this study, there was no difference in long-term outcomes between those with and without PH.
Aortic Valve Disease
PH is common in isolated aortic stenosis. The prevalence of PH defined as invasively measured systolic PAP >50 mmHg varies from 15 to 29 % (Table 2) [51–53]. The severity of PH in aortic stenosis correlates with the severity of diastolic dysfunction and the elevation in left ventricular end diastolic pressure as opposed to the aortic valve area [54, 55]. PH is less common in aortic regurgitation when compared to other valvular heart diseases. In a series of 506 patients with aortic regurgitation, Doppler-estimated systolic PAP >60 mmHg was noted in approximately 16 % of patients with severe aortic regurgitation and it was associated with left ventricular enlargement and functional mitral regurgitation [56].
In patients with severe aortic stenosis, elevated systolic PAP is associated with increased mortality [57]. Elevated systolic PAP also increases the early 30-day and long-term mortality after surgical aortic valve replacement [58–60]. Systolic PAP >60 mmHg was also associated with increased early and long-term mortality after transcatheter aortic valve replacement [61, 62]. However, in one study, transcatheter aortic valve replacement was associated only with an increased 1-year mortality, but not with increased 30-day mortality [63].
Conclusion
PH and RVD are common in left heart failure; however, the exact prevalence is not known due to the heterogeneity in the diagnostic criteria used and the patient population studied. Regardless of the underlying etiology, the presence of PH depicts poor prognosis in patients with left heart failure.
References
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245.
Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–90. This is a state of the art review of pulmonary hypertension in left heart failure.
Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–33.
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50.
Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, et al. World Health Organization pulmonary hypertension group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant: Off Publ Int Soc Heart Transplant. 2012;31:913–33.
Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation. 2000;102:1718–23.
Givertz M, Colucci W, LeJemtel T, Gottlieb S, Hare J, Slawsky M, et al. Acute endothlin A receptor blockade causes selective pulmonary vasodilation in patients with chronic heart failure. Circulation. 2000;101:2922–7.
Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–65. In this article, the epidemiology and clinical characteristics of PH due to HFpEF is described in a well characterized cohort of HFpEF patients.
Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J. 2013;41:217–23. This article describes the limitation sof transpulmonary gradient and the role of diastolic pressure gradient in diagnosing pulmonary hypertension in left heart failure.
Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143:758–66.
Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–108. This paper provides new hemodynamic definitions, terminology, and treatment insights of PH due to left heart disease.
Miller WL, Mahoney DW, Michelena HI, Pislaru SV, Topilsky Y, Enriquez-Sarano M. Contribution of ventricular diastolic dysfunction to pulmonary hypertension complicating chronic systolic heart failure. J Am Coll Cardiol Img. 2011;4:946–54.
Szwejkowski BR, Elder DH, Shearer F, Jack D, Choy AM, Pringle SD, et al. Pulmonary hypertension predicts all-cause mortality in patients with heart failure: a retrospective cohort study. Eur J Heart Fail. 2012;14:162–7.
Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail. 2013;1:290–9. In this article, the authors describes the epidemiology, clinical characteristics, risk factors, and long-term outcomes of PH due to HFrEF.
Cappola TP, Felker GM, Kao WH, Hare JM, Baughman KL, Kasper EK. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation. 2002;105:1663–8.
Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–6.
Damy T, Goode KM, Kallvikbacka-Bennett A, Lewinter C, Hobkirk J, Nikitin NP, et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J. 2010;31:2280–90. This study demonstrates the key role of elevated left sided filling pressure in the pathogenesis of PH due to left heart failure.
Capomolla S, Febo O, Guazzotti G, Gnemmi M, Mortara A, Riccardi G, et al. Invasive and non-invasive determinants of pulmonary hypertension in patients with chronic heart failure. J Heart Lung Transplant: Off Publ Int Soc Heart Transplant. 2000;19:426–38.
Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–91.
Kjaergaard J, Akkan D, Iversen KK, Kjoller E, Kober L, Torp-Pedersen C, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99:1146–50.
Tatebe S, Fukumoto Y, Sugimura K, Miyamichi-Yamamoto S, Aoki T, Miura Y, et al. Clinical significance of reactive post-capillary pulmonary hypertension in patients with left heart disease. Circ J: Off J Japan Circ Soc. 2012;76:1235–44.
Olson TP, Snyder EM, Frantz RP, Turner ST, Johnson BD. Repeat length polymorphism of the serotonin transporter gene influences pulmonary artery pressure in heart failure. Am Heart J. 2007;153:426–32.
Kalogeropoulos AP, Siwamogsatham S, Hayek S, Li S, Deka A, Marti CN, et al. Echocardiographic assessment of pulmonary artery systolic pressure and outcomes in ambulatory heart failure patients. J Am Heart Assoc. 2014;3:e000363.
Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–31.
Grigioni F, Potena L, Galie N, Fallani F, Bigliardi M, Coccolo F, et al. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant: Off Publ Int Soc Heart Transplant. 2006;25:1241–6.
Shalaby A, Voigt A, El-Saed A, Saba S. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol. 2008;101:238–41.
Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8.
Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation. 2013;128:1623–33.
Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26.
Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, et al. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: Results of the New York heart failure registry. J Am Coll Cardiol. 2004;43:1432–8.
Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2014;7:104–15.
Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur heart J. 2014. This paper reports the prevalence, determinants, and prognostic value of right ventricular dysfunction in patients with heart failure with preserved ejection fraction.
Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, Chandrasekaran K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr: J Work Group Echocardiogr Eur Soc Cardiol. 2009;10:733–7.
Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–99.
Bouchard JL, Aurigemma GP, Hill JC, Ennis CA, Tighe DA. Usefulness of the pulmonary arterial systolic pressure to predict pulmonary arterial wedge pressure in patients with normal left ventricular systolic function. Am J Cardiol. 2008;101:1673–6.
Marechaux S, Neicu DV, Braun S, Richardson M, Delsart P, Bouabdallaoui N, et al. Functional mitral regurgitation: a link to pulmonary hypertension in heart failure with preserved ejection fraction. J Card Fail. 2011;17:806–12.
Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45.
McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–802.
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62.
Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–71.
Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37:1374–80.
Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol. 2010;106:284–6.
Agarwal R, Shah SJ, Foreman AJ, Glassner C, Bartolome SD, Safdar Z, et al. Risk assessment in pulmonary hypertension associated with heart failure and preserved ejection fraction. J Heart Lung Transplant. 31:467–477
Hart SA, Krasuski RA, Wang A, Kisslo K, Harrison JK, Bashore TM. Pulmonary hypertension and elevated transpulmonary gradient in patients with mitral stenosis. J Heart Valve Dis. 2010;19:708–15.
Fawzy ME, Hassan W, Stefadouros M, Moursi M, El Shaer F, Chaudhary MA. Prevalence and fate of severe pulmonary hypertension in 559 consecutive patients with severe rheumatic mitral stenosis undergoing mitral balloon valvotomy. J Heart Valve Dis. 2004;13:942–7. discussion 947–948.
Semler HJ, Shepherd JT, Wood EH. The role of vessel tone in maintaining pulmonary vascular resistance in patients with mitral stenosis. Circulation. 1959;19:386–94.
Barbieri A, Bursi F, Grigioni F, Tribouilloy C, Avierinos JF, Michelena HI, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J. 2011;32:751–9.
Alexopoulos D, Lazzam C, Borrico S, Fiedler L, Ambrose JA. Isolated chronic mitral regurgitation with preserved systolic left ventricular function and severe pulmonary hypertension. J Am Coll Cardiol. 1989;14:319–22.
Cesnjevar RA, Feyrer R, Walther F, Mahmoud FO, Lindemann Y, von der Emde J. High-risk mitral valve replacement in severe pulmonary hypertension—30 years experience. Eur J Cardiothorac Surg: Off J EurAssoc Cardiothorac Surg. 1998;13:344–51. discussion 351–342.
Faggiano P, Antonini-Canterin F, Ribichini F, D’Aloia A, Ferrero V, Cervesato E, et al. Pulmonary artery hypertension in adult patients with symptomatic valvular aortic stenosis. Am J Cardiol. 2000;85:204–8.
Johnson LW, Hapanowicz MB, Buonanno C, Bowser MA, Marvasti MA, Parker Jr FB. Pulmonary hypertension in isolated aortic stenosis. Hemodynamic correlations and follow-up. J Thorac Cardiovasc Surg. 1988;95:603–7.
Silver K, Aurigemma G, Krendel S, Barry N, Ockene I, Alpert J. Pulmonary artery hypertension in severe aortic stenosis: incidence and mechanism. Am Heart J. 1993;125:146–50.
Aragam JR, Folland ED, Lapsley D, Sharma S, Khuri SF, Sharma GV. Cause and impact of pulmonary hypertension in isolated aortic stenosis on operative mortality for aortic valve replacement in men. Am J Cardiol. 1992;69:1365–7.
Casaclang-Verzosa G, Nkomo VT, Sarano ME, Malouf JF, Miller Jr FA, Oh JK. E/Ea is the major determinant of pulmonary artery pressure in moderate to severe aortic stenosis. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2008;21:824–7.
Khandhar S, Varadarajan P, Turk R, Sampat U, Patel R, Kamath A, et al. Survival benefit of aortic valve replacement in patients with severe aortic regurgitation and pulmonary hypertension. Ann Thorac Surg. 2009;88:752–6.
Ben-Dor I, Goldstein SA, Pichard AD, Satler LF, Maluenda G, Li Y, et al. Clinical profile, prognostic implication, and response to treatment of pulmonary hypertension in patients with severe aortic stenosis. Am J Cardiol. 2011;107:1046–51.
Cam A, Goel SS, Agarwal S, Menon V, Svensson LG, Tuzcu EM, et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2011;142:800–8.
Roselli EE, Abdel Azim A, Houghtaling PL, Jaber WA, Blackstone EH. Pulmonary hypertension is associated with worse early and late outcomes after aortic valve replacement: implications for transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2012;144:1067–74. e1062.
Miceli A, Varone E, Gilmanov D, Murzi M, Simeoni S, Concistre G, et al. Impact of pulmonary hypertension on mortality after operation for isolated aortic valve stenosis. Int J Cardiol. 2013;168:3556–9.
Iung B, Laouenan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau-Gouge P, et al. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100:1016–23.
Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–90.
Lucon A, Oger E, Bedossa M, Boulmier D, Verhoye JP, Eltchaninoff H, et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: study from the FRANCE 2 registry. Circ Cardiovasc Interv. 2014;7:240–7.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Thenappan Thenappan has received compensation from Medscape for assisting in the development of an online educational program in PAH.
Mardi Gomberg-Maitland has served as a consultant for Actelion, Bayer, Gilead, Medtronic, Merck, Bellerophon (formerly known as Ikaria), and United Therapeutics as a member of steering committees and DSMB/event committees; has received honoraria for CME from Medscape and AB Comm; is a member of the PCORI Advisory Panel on Rare Diseases; and is a Special Government Employee for the Food and Drug Administration (FDA) Cardio-Renal Division; Actelion, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, and Ventripoint have provided funding to the University of Chicago during the past year to support Dr. Gomberg-Maitland’s conducting of clinical trials.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thenappan, T., Gomberg-Maitland, M. Epidemiology of Pulmonary Hypertension and Right Ventricular Failure in Left Heart Failure. Curr Heart Fail Rep 11, 428–435 (2014). https://doi.org/10.1007/s11897-014-0216-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-014-0216-6