Abstract
Purpose of Review
In this review, we discuss different endoscopic techniques in the eradication of Barrett’s esophagus (BE) as well as some controversies in the field of treatment.
Recent Findings
Patients with T1a esophageal adenocarcinoma and BE of high-grade dysplasia should undergo endoscopic ablative therapy. The most studied technique to date is radiofrequency ablation. It can be combined with endoscopic mucosal resection in cases containing nodular and flat lesions. Cryotherapy and APC have shown promise with good efficacy and safety profiles so far, but are not mainstream as more studies are needed. Surveillance is still required post-ablation since recurrence is common. Low-grade dysplasia can be treated with either endo-ablative therapy or surveillance. Non-dysplastic BE treatment is controversial and so far, only surveillance is recommended. Research is ongoing to better risk stratify these patients.
Summary
Our ability to diagnose and treat BE has come a long way in the past few years with the goal of preventing its progression into malignancy. The advent of endoscopic techniques in the eradication of BE has provided a less invasive and safer modality of treatment as compared to surgical esophagectomy. Data in the form of randomized trials and high-volume registries has provided good evidence to support the efficacy of these techniques and their long-term durability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barrett’s esophagus (BE), a premalignant condition where the normal squamous epithelium of the esophagus transforms into columnar epithelium with intestinal metaplasia (IM), is the most important risk factor for the development of esophageal adenocarcinoma. This happens through a sequential progression from no dysplasia to low-grade dysplasia (LGD) to high-grade dysplasia (HGD) eventually to invasive cancer. The risk of this progression from non-dysplastic BE is relatively low with annual risk of 0.3 to 0.5%; however, once adenocarcinoma develops, the 5-year survival is 15% [1, 2••]. For this reason, endoscopic surveillance and treatment of BE is paramount. The latest gastroenterology society guidelines recommend endoscopic eradication therapy rather than surveillance for treatment of patients with confirmed HGD and early adenocarcinoma with endoscopic therapy [3].
Previously, the gold standard of treatment used to be esophagectomy; however, due to high perioperative morbidity and mortality, it has been largely replaced by endoscopic techniques that are now the current mainstay of treatment. The goal of endoscopic therapy is complete eradication of Barrett’s esophagus defined as complete eradication of intestinal metaplasia (CE-IM) and complete eradication of dysplasia (CE-D).
Endoscopic therapies fall under the category of either mechanical removal of tissue such as with endoscopic mucosal resection (EMR) or ablation techniques currently utilized such as radiofrequency ablation (RFA), argon plasma coagulation (APC), or cryotherapy. The aim of this review is to provide an overview of these endoscopic modalities with a discussion on the technique, efficacy, long-term durability, and side effects of each treatment. We will also provide some insight into the management of LGD and non-dysplastic BE as well as current controversies and future perspectives on the treatment of BE.
Endoscopic Mucosal Resection
Technique
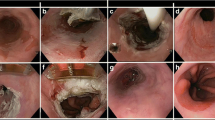
EMR is considered the treatment of choice for nodular disease in the esophagus. It can be used alone (complete EMR for resection of all BE) or, as we will discuss later, as an adjunct to RFA (focal EMR). The technique is usually performed in two different methods. The first is known as cap-assisted mucosectomy. This technique involves injecting saline into the submucosal space, applying a snare to the lifted area, and suctioning it into a specialized cap (Fig. 1). The second method is the ligation-and-snare technique or multiband ligation. A cap with multiple bands is initially used to suction the abnormal tissue. The bands are then wrapped around the area and a snare placed below the bands resects the tissue. These two techniques were compared in a randomized controlled trial by Pouw et al., who showed that no significant difference in the depth of resected tissue between both techniques; however, multiband ligation was more cost-efficient and had fewer complications [4].
Efficacy
Several studies have been conducted to determine the efficacy of EMR alone for the resection of all BE. In a large systematic review and meta-analysis by Tomizawa et al., which pooled 8 studies with 676 patients, data showed that CE-IM and CE-D following EMR were 85.0% (95% CI, 79.4–89.2%) and 96.6% (95% CI, 94.0–98.1%), respectively [5]. In another study that had similar results, Konda et al. evaluated 107 patients with BE who underwent complete EMR, and showed complete eradication was achieved in 80.4% of patients in the intent to treat analysis and in 98.8% in the per-protocol analysis [6]. Furthermore, in a study by Koutsoumpas et al., in which 91 patients underwent complete Barrett’s EMR, complete Barrett’s excision was achieved in 85.0% of the patients [7].
The above data show that remission post-EMR is high and can range between 80 and 98%, making it an appropriate tool to use in the eradication of nodular BE.
Long-Term Data
Despite short-term efficacy in the eradication of BE with EMR, long-term data on durability has shown that recurrences rates are not insignificant. In the same systematic review and meta-analysis by Tomizawa et al., the rates of recurrence of IM and neoplasia following EMR were found to be 15.7% (95% CI, 8.0–28.4%) and 5.8% (95% CI, 3.9–8.6%), respectively [5]. In another retrospective study by Anders et al. that followed 90 patients over a period of 3 years, 6.2% had recurrence of neoplastic BE and 39.5% of IM after a mean of 64.8 months following EMR [8]. These results indicate that short-term success does not necessarily translate into long-term durability, and regular surveillance and follow-up is required to ensure patients remain free of disease in the long run.
Adverse Events
As with any intervention, EMR is not free of complications, including stricture formation, bleeding, and perforation. Again in the above study by Tomizawa et al., stricture formation was shown to be the most prominent adverse event with an incidence of 37.4% (95% CI, 24.4–52.6%). Bleeding and perforation occurred in 7.9% (95% CI, 4.4–13.8%) and 2.3% (95% CI, 1.3–4.1%) of patients, respectively [5]. Similarly, in the study by Konda et al., strictures developed in 41.1%, symptomatic dysphagia in 37.3%, and two patients developed perforation [6]. However, in the study by Koutsompas et al. that used stepwise resection, 1.1% of patients developed stricture; one had delayed bleeding, but no perforations were reported [7].
In a retrospective study, Qumseya et al. attempted to identify the predictors of stricture formation. Of 136 patients, 27% had stricture formation. The two most important predictors identified were size of the lesion excised (OR 1.6, p = 0.01) and number of lesions removed (OR 2.3, p = 0.007). This meant that for each 1 cm of tissue removed, there was a 50% increased odds of developing stricture and for each lesion removed, there was a doubling of stricture formation [9].
In conclusion, EMR can be a highly effective technique in the complete eradication of Barrett’s esophagus especially for shorter BE extents, but if used extensively, can lead to high rates of stricture formation. It has also been shown that for longer segments of Barrett’s, the efficacy of the technique is lower and recurrence rates are higher. For these reasons, EMR is mostly recommended for patients with short, non-circumferential segments of Barrett’s and in longer BE, focal EMR is combined with mucosal ablation.
Mucosal Ablative Therapies
Ablative therapies, as a general principle, rely on neo-squamous re-epithelization whereby columnar epithelium is replaced with neo-squamous epithelium. Below, we shall discuss RFA, cryotherapy, and APC.
Radiofrequency Ablation
Technique
RFA uses radiofrequency energy to deliver heat to diseased tissue ultimately destroying it and replacing it with neo-squamous epithelium. RFA devices have come a long way since the technique was first introduced. One of the more recent devices that has been developed and is in use is the Express 360 RFA balloon catheter. It contains a 4-cm bipolar electrode wrapped around a self-adjusting balloon. The balloon inflates to the size of the esophageal inner diameter, and the electrodes provide radiofrequency energy to ablate the targeted tissue. This device simplified the procedure process by eliminating the need for a sizing process, thereby decreasing the procedure time by 20% as compared to its predecessors that also required a sizing catheter to be inserted beforehand [10]. There is also a focal ablation device which is used to treat shorter segments of BE. A recent study by Brown et al. that compared the effectiveness of focal vs balloon RFA devices found that treatment with the focal device resulted in a greater percentage reduction in BE length compared to the balloon system (73 vs 39%, p < 0.01) [11].
Efficacy
RFA has been studied extensively and a myriad of data exist that supports its use and efficacy making it the leading treatment of flat BE with HGD. The first randomized, multicenter controlled trial to evaluate RFA treatment of BE was the ablation of intestinal metaplasia (AIM) dysplasia trial wherein 127 patients with BE-related neoplasia (63 HGD and 64 LGD) were randomized to either RFA or a sham procedure. End points were assessed at 12 months after RFA treatment every 2 months. Results in the intention to treat analysis showed that 90.5and 81% of patients had neoplasia eradication in the LGD and HGD groups, respectively, compared to 22.7 and 19%, respectively, in the sham group. Also, 77.4% of patients had CE-IM vs 2.3% in the sham group [12••].
The AIM study was a landmark trial that paved the way for other large studies that also evaluated the role and efficacy of RFA as a treatment modality for BE. In a retrospective study by Bulsiewicz et al., 244 with BE-related neoplasia were treated with RFA; 80% achieved CE-IM and 87% achieved CR-D with 4 patients developing cancer despite RFA treatment [13]. In another large series from 13 European centers with 132 patients, Phoa et al. reported CE-D in 91% of patients and CE-IM in 88% of patients [14]. Corroborating these results even more, a meta- analysis by Orman et al., consisting of 18 studies with 3802 patients, showed CE-IM in 78% of patients and CE-D in 91% of the treated patients [15••]. Data to support the efficacy of RFA also exists in the real world setting outside of these controlled trials. Data from the UK RFA registry with 335 patients with BE-related neoplasia treated at 19 centers showed that 81% achieved CE-D and 62% with CE-IM at 1 year; however, 3% developed invasive cancer at 12 months and 5.1% had disease progression at 19 months [16••].
Long-Term Data
The next step was to evaluate the durability and long-term outcomes of the procedure. A 3-year follow-up of patients in the AIM dysplasia trial showed that of the patients available for follow-up, 98% had complete eradication of dysplasia and 91% had complete eradication of IM [12••]. Orman et al. reported data from 262 patients with 155 patient-years of observation, and during follow-up, the recurrence rate was 5.2%/year with a progression rate of 1.9%/year [15••]. In addition, Gupta et al. examined a series of 592 patients over an 8-year period which showed that 33% of successfully eradicated patients had recurrence after 2 years [17]. Finally, evaluating recurrence rates in the real world setting, data from the UK registry showed that at 19 months of follow-up, 5.1% of RFA-treated patients experienced recurrence of intestinal metaplasia [16••]. This collection of data shows that despite high short-term efficacy rates, there is still a risk of recurrence. For this reason, it is the accepted standard of practice that patients are followed up with periodic endoscopic surveillance following treatment.
Adverse Events
Even RFA, the preferred treatment for flat neoplastic BE, is not free of complications. In a systematic review and meta-analysis by Qumseya et al., pooled data from 37 studies with 9200 patients showed an adverse event rate of 8.8%. The most common side effect was stricture development at 5.6%, followed by bleeding at 1%, and a low rate of perforation at 0.6%. Adverse events were also associated with an increasing BE length and histology as well as if there was preceding EMR with the relative risk for adverse events being significantly higher for RFA with EMR [18•].
EMR Combined with RFA
As discussed previously, RFA is used for the eradication of non-nodular BE while EMR is used for resection of nodular neoplastic BE. It happens to be, however, that the majority of patients with HGD and early cancer will need combination therapy with EMR plus RFA: removal of nodular lesions as well as eradication of non-nodular BE concomitantly. The dual use of these techniques allows the removal of focal, nodular areas with EMR and eradication of remaining flat intestinal metaplasia or dysplasia with RFA. In a study by Phoa et al. that included 13 European centers with 132 patients that underwent EMR followed by RFA, complete eradication of neoplasia was achieved in 92% of patients and complete eradication of intestinal metaplasia in 87% of patients. After a median of 27-month follow-up, only 4% had recurrence of neoplasia and 8% had recurrence of metaplasia [19]. The UK registry also showed that in patients with intramucosal cancer who had EMR, preceding RFA recurrence rates were lower as compared to RFA alone (4 vs 78%, respectively, p = 0.01). The above data demonstrates that when EMR is used prior to RFA, then efficacy rates and durability are improved as compared to RFA alone [20].
The other question is whether there is a difference between the safety and efficacy of focal EMR followed by RFA vs complete EMR alone. Desai et al. attempted to answer that question in a systematic review that included 9 studies (774 patients) of focal EMR + RFA and 11 studies (751 patients) using complete EMR. The authors demonstrated that both techniques had high BE eradication rates (CE-N 93.4%; CE-IM 73.1%) and (CE-N 94.9%; CE-IM 79.6%), respectively. However, complete EMR had higher rates of adverse events as compared to focal EMR + RFA with an OR of 4.73 for developing esophageal stricture (95% CI, 1.61–13.85; p = 0.005), OR of 7 for perforation (95% CI, 1.56–31.33; p = 0.01), and OR of 6.88 for bleeding (95% CI, 2.19–21.62; p = 0.001) [21••].
Cryotherapy
Technique
Cryotherapy involves delivery of a cryogen causing destruction of tissue due to extremely cold temperature. Two currently available devices have been evaluated for the treatment of BE patients: The true freeze system that uses low-pressure liquid nitrogen (CSA Medical, Maryland) and the C2 cryoballoon which uses carbon dioxide (C2 Therapeutics, California) delivered by spray catheter.
Efficacy
Data from a multicenter, prospective open-label registry using cryotherapy showed that in patients with LGD, rates of CE-D and CE-IM were 91 and 61% whereas in patients with HGD, CE-D and CE-IM rates were 81 and 65%, respectively. Furthermore, this study demonstrated that in patients with short-segment BE with any dysplasia, CE-D was achieved in 97% and CE-IM in 77% of patients [22]. In a recent retrospective, non-randomized study where cryotherapy was used as salvage therapy following failed RFA, the 1-year response rate was found to be 77% for cancer, 89% for dysplasia, and 94% for HGD [23].
Long-Term Data
In a single-center, retrospective study by Ramay et al., the long-term durability and recurrence rates of cryotherapy were assessed at 3 and 5 years. The incidence rates per person-year follow-up of intestinal metaplasia, dysplasia, and HGD were found to be 12.2, 4, and 1.4%, respectively. Progression to adenocarcinoma was not common and most recurrences were successfully treated. These data, again, do indicate however that long-term surveillance is required to monitor for possible disease recurrence even after successful cryotherapy [24].
Adverse Events
In general, cryotherapy has been shown to have a reasonable safety profile. Results from the national cryospray registry showed that none of the patients developed perforation or procedure-related death. Only 1 of 96 treated patients developed a stricture that did not require dilatation [21••]. In a recent study by Canto et al. evaluating the safety and efficacy of cryotherapy, the overall adverse event rate was 9%—these mainly included post-procedural pain. No stricture, bleeding, or perforation was noted [25].
Argon Plasma Coagulation
Technique
Argon plasma coagulation relies on non-contact thermal energy to ablate tissue. With the use of a probe passed through an endoscope, argon gas is ionized and then, an electric current is conducted through the jet of ionized argon resulting in coagulation of tissue. This technique, similar to other ablative techniques, can result in stricture formation. For this reason, hybrid APC has been introduced as a way to decrease rates of stricture formation. Hybrid APC consists of injecting saline in the submucosa, thereby protecting the deeper esophageal layers from injury.
Efficacy
The efficacy of APC has been studied in several trials; one of which is the APE study, a randomized study that compared APC with surveillance following endoscopic resection of the neoplastic BE lesions. Results in 63 patients showed significant decrease in secondary lesions in the APC-treated patients as compared to surveillance (3 vs 36.7%, respectively, p = 0.005) [26••]. Besides showing the efficacy of APC, this study also emphasized the need for the eradication of the entire BE segment even after the worst neoplastic areas are removed by EMR.
The efficacy of hybrid APC has also been shown to be on par with APC, but with lower rate of complications. In a pilot series by Manner et al., at a tertiary center that included 50 patients, 96% achieved macroscopic and 78% achieved histopathologic eradication [27].
Long-Term Data
Long-term data of APC in BE patients has been reported by some studies. One of the earlier studies was conducted by Kahaleh et al. with a median follow-up of 36 months. Of 39 patients with BE who underwent APC, over 50% of patients had endoscopic or histological relapse [28]. In another study by Sharma et al., 19 patients treated with APC, 70% of patients showed complete reversal of BE (endoscopic and histological) at 2 years of follow-up [29]. Finally, Bright et al. followed up patients for 5 years in a randomized trial and demonstrated that 70% of patients continued to have regression of their BE [30]. Long-term results after hybrid APC therapy are awaited.
Adverse Events
The most notable side effect of APC is stricture formation. In general, it has been reported that stricture formation occurs in 4–9% of the treated patients [26, 31]. However, with hybrid APC, Manner et al. reported only one treatment-related stricture (2%) with minor adverse events observed in 22% of patients (dysphagia, odynophagia, pain, fever with a duration of < 24 h, bleeding without transfusion need, or a decrease in the Hb level of < 2 g/dl) [26••]. An ex vivo head to head comparison of APC and hybrid APC demonstrated that the hybrid technique caused half of the coagulation depth as compared to APC confirming the notion that hybrid APC causes less injury to tissue [32].
Areas of Further Research
Predictors of Response to Endoscopic Therapy
Several predictors of successful response rate to BE endoscopic therapy have been studied. In a study by Pasricha et al. that used the US RFA registry with 5521 patients, it was found that BE recurred in 20% of patients after CE-IM (follow-up 2.4 years). Older age (OR 1.02 per year; 95% CI, 1.01–1.03), non-Caucasian race (OR 2.00; 95% CI, 1.2–3.34), and increasing BE length (OR 1.1; 95% CI, 1.06–1.15) were all reported as risk factors for recurrence [33].
In a study from the Netherlands by Van Vilesteren et al., four independent predictors of poor response were identified: active reflux esophagitis (OR 37.4; 95% CI, 3.2–433.2), endoscopic mucosal resection scar regeneration with new Barrett’s mucosa (OR 4.7; 95% CI, 1.1–20), esophageal lumen narrowing pre-RFA treatment (OR 3.9; 95% CI, 1–15.1), and years of neoplasia pre-RFA treatment (OR 1.2; 95% CI, 1–1.4) [34]. Finally, data from the UK registry demonstrated a 15% less likelihood of reversal of dysplasia for every 1-cm increase in BE length (OR 1.15; 95% CI, 1.07–1.26) [16••].
In summary, although a number of factors have been used to predict response or recurrence after BE endotherapy, besides the length of the BE segment, no other consistent factors have been determined.
Management of LGD
The role of endoscopic therapy in the management of LGD has shifted in the past few years. Until recently, the approach to LGD was a reserved one given lack of strong progression data, predictors of progression, and continued challenges with the pathological interpretation of LGD. However, some recent data has shifted this outlook.
A multicenter randomized controlled trial by Phoa et al. evaluated the risk of progression to HGD/cancer in two groups of LGD patients, one treated with RFA and the other underwent surveillance without ablation. The diagnosis of LGD was confirmed by two to three expert GI pathologists. From an initial LGD population of 511, only 247 met the “actual” diagnosis of LGD. The risk of progression, at 1 year, was 1.5% in the RFA-treated patients and 26.5% in the surveillance group [10]. In a retrospective study by Small et al., the risk of progression of LGD to HGD and to esophageal adenocarcinoma was significantly lower in patients treated with RFA vs surveillance with a hazard ratio of 0.06 (95% CI, 0.008–0.48) [35].
With that said, one of the biggest hurdles in the management of patients with LGD is the ambiguity of diagnosis. There is an extremely high intra/inter-observer variability in establishing the presence of LGD. Montgomery et al. showed that even amongst experienced academic pathologists, the kappa value for diagnosis of LGD was 0.32 [36••]. In a recent European study that included 147 LGD patients, 85% were downgraded to a diagnosis of no dysplasia after slide review by two expert pathologists [37]. Finally, in a retrospective review of LGD patients being treated endoscopically, of 255 patients who had LGD that was persistent and confirmed by three pathologists, there was a high risk of progression to HGD or EAC reaching 18%. The risk of progression correlated positively with the number of pathologists that confirmed the diagnosis of LGD with OR of 47.14 [38].
Since the decision to treat LGD is based upon histopathological findings, it is important to confirm the diagnosis with a second pathologist who is experienced in the diagnosis of BE, to ensure that there are no synchronous visible lesions and that LGD is persistent on the subsequent endoscopy. Recently, published guidelines support this concept in patients with LGD [3].
Non-Dysplastic BE
There has long been a controversy regarding the management of NDBE with respect to endoscopic therapy. Given that more recent studies have shown that the risk of progression of NDBE is as low as 0.16–0.4% per year [1, 2], the recommendations have now leaned toward surveillance rather than treatment of such lesions. Studies evaluating the cost effectiveness of treatment vs surveillance have concluded that ablation therapy is not cost effective for NDBE and is thus not recommended [39]. There have been attempts to risk stratify patients into high- and low-risk groups with the aim of offering the high-risk group treatment rather than just surveillance. This has been an active area of research with several investigators studying the role of biomarkers while others attempting to determine clinical and epidemiological characteristics that can be predictive of risk.
Conclusions
Endoscopic therapy of BE is an ever-changing and evolving field. To date, several methods have shown promise in their ability to induce neo-squamous epithelization and eradicate lesions that have a potential for cancerous progression. EMR is the go-to method for the eradication of visible, nodular lesions. It has shown high efficacy ranging between 80 and 98%, but wide spread/complete EMR can be associated with increased stricture formation. For flat BE mucosa that needs to be treated after focal EMR, the most studied and most widely used technique thus far is RFA with high efficacy data that has been reproduced by several large studies. Its use with EMR has proven to be useful for the eradication of lesions that have both flat and nodular components. Cryotherapy appears to be a promising technique so far with relatively safe side effect profile. However, interpreting the published data thus far is tricky, given that most patients reported have been chosen after failing other ablative techniques. APC seems to be an easy to use and safe technique especially the novel hybrid APC; however, long-term data are awaited. All techniques have been associated with some risk of persistent disease and variable rates of recurrences. Thus, in all patients post endoscopic therapy, surveillance is still required to ensure there is no recurrence of lesions. Guidelines have also recently changed to reflect the growing evidence of the role of ablation in LGD. A critical issue, though, is the confirmation of the diagnosis by two expert pathologists and to show the persistence of LGD before proceeding with definitive treatment. The future of BE eradication is a promising one with several advances looming at the horizon as more cutting-edge research pushes the envelope of therapy and surveillance one step closer to complete eradication.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance •• Of major importance
Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103(13):1049–57.
•• Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365(15):1375–83. Established the significant risk of BE progression to adenocarcinoma.
Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG clinical guideline: diagnosis and Management of Barrett’s esophagus. Am J Gastroenterol. 2016;111(1):30–50. quiz 1
Pouw RE, van Vilsteren FG, Peters FP, Alvarez Herrero L, Ten Kate FJ, Visser M, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc. 2011;74(1):35–43.
Tomizawa Y, Iyer PG, Wong Kee Song LM, Buttar NS, Lutzke LS, Wang KK. Safety of endoscopic mucosal resection for Barrett’s esophagus. Am J Gastroenterol. 2013;108(9):1440–7. quiz 8
Konda VJ, Gonzalez Haba Ruiz M, Koons A, Hart J, Xiao SY, Siddiqui UD, et al. Complete endoscopic mucosal resection is effective and durable treatment for Barrett’s-associated neoplasia. Clin Gastroenterol Hepatol. 2014;12(12):2002–10.e1-2.
Koutsoumpas A, Wang LM, Bailey AA, Gillies R, Marshall R, Booth M, et al. Non-radical, stepwise complete endoscopic resection of Barrett’s epithelium in short segment Barrett’s esophagus has a low stricture rate. Endosc Int Open. 2016;04(12):E1292–E7.
Anders M, Bahr C, El-Masry MA, Marx AH, Koch M, Seewald S, et al. Long-term recurrence of neoplasia and Barrett’s epithelium after complete endoscopic resection. Gut. 2014;63(10):1535–43.
Qumseya B, Panossian AM, Rizk C, Cangemi D, Wolfsen C, Raimondo M, et al. Predictors of esophageal stricture formation post endoscopic mucosal resection. Clinical endoscopy. 2014;47(2):155–61.
Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311(12):1209–17.
Brown J, Alsop B, Gupta N, Buckles DC, Olyaee MS, Vennalaganti P, et al. Effectiveness of focal vs. balloon radiofrequency ablation devices in the treatment of Barrett’s esophagus. United European Gastroenterol J. 2016;4(2):236–41.
•• Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360(22):2277–88. Landmark randomized control trial on RFA in Barrett’s esophagus, first of its kind.
Bulsiewicz WJ, Kim HP, Dellon ES, Cotton CC, Pasricha S, Madanick RD, et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11(6):636–42.
Phoa KN, Pouw RE, van Vilsteren FG, Sondermeijer CM, Ten Kate FJ, Visser M, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013;145(1):96–104.
•• Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(10):1245–55. Provided large volume data from USA, Europe, and the UK on the durability of RFA in BE.
•• Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145(1):87–95. Provided large volume data from USA, Europe, and the UK on the durability of RFA and EMR in BE.
Gupta M, Iyer PG, Lutzke L, Gorospe EC, Abrams JA, Falk GW, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology. 2013;145(1):79–86.e1.
• Qumseya BJ, Wani S, Desai M, Qumseya A, Bain P, Sharma P, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14(8):1086–95.e6. Studied risk factors associated with increased adverse events in RFA in BE.
Phoa KN, Pouw RE, Bisschops R, Pech O, Ragunath K, Weusten BL, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut. 2016;65(4):555–62.
Haidry RJ, Lipman G, Banks MR, Butt MA, Sehgal V, Graham D, et al. Comparing outcome of radiofrequency ablation in Barrett’s with high grade dysplasia and intramucosal carcinoma: a prospective multicenter UK registry. Endoscopy. 2015;47(11):980–7.
•• Desai M, Saligram S, Gupta N, Vennalaganti P, Bansal A, Choudhary A, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc. 2017;85(3):482–95.e4. Provided large volume data on the efficacy and safety of combination of EMR and RFA.
Ghorbani S, Tsai FC, Greenwald BD, Jang S, Dumot JA, McKinley MJ, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s dysplasia: results of the National Cryospray Registry. Dis Esophagus. 2016;29(3):241–7.
Sengupta N, Ketwaroo GA, Bak DM, Kedar V, Chuttani R, Berzin TM, et al. Salvage cryotherapy after failed radiofrequency ablation for Barrett’s esophagus-related dysplasia is safe and effective. Gastrointest Endosc. 2015;82(3):443–8.
Ramay FH, Cui Q, Greenwald BD. Outcomes after liquid nitrogen spray cryotherapy in Barrett’s esophagus-associated high-grade dysplasia and intramucosal adenocarcinoma: 5-year follow-up. Gastrointest Endosc. 2017. doi:10.1016/j.gie.2017.02.006.
Canto MI, Shin EJ, Khashab MA, Molena D, Okolo P, Montgomery E, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy. 2015;47(7):591.
•• Manner H, Rabenstein T, Pech O, Braun K, May A, Pohl J, et al. Ablation of residual Barrett’s epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy. 2014;46(1):6–12. A randomized trial that looked at long term outcomes following APC of BE.
Manner H, May A, Kouti I, Pech O, Vieth M, Ell C. Efficacy and safety of Hybrid-APC for the ablation of Barrett’s esophagus. Surg Endosc. 2016;30(4):1364–70.
Kahaleh M, Van Laethem JL, Nagy N, Cremer M, Deviere J. Long-term follow-up and factors predictive of recurrence in Barrett’s esophagus treated by argon plasma coagulation and acid suppression. Endoscopy. 2002;34(12):950–5.
Sharma P, Wani S, Weston AP, Bansal A, Hall M, Mathur S, et al. A randomised controlled trial of ablation of Barrett’s oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut. 2006;55(9):1233–9.
Bright T, Watson DI, Tam W, Game PA, Astill D, Ackroyd R, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg. 2007;246(6):1016–20.
Schulz H, Miehlke S, Antos D, Schentke KU, Vieth M, Stolte M, et al. Ablation of Barrett’s epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc. 2000;51(6):659–63.
Manner H, Neugebauer A, Scharpf M, Braun K, May A, Ell C, et al. The tissue effect of argon-plasma coagulation with prior submucosal injection (Hybrid-APC) versus standard APC: a randomized ex-vivo study. United European Gastroenterol J. 2014;2(5):383–90.
Pasricha S, Bulsiewicz WJ, Hathorn KE, Komanduri S, Muthusamy VR, Rothstein RI, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12(11):1840–7.e1.
van Vilsteren FG, Alvarez Herrero L, Pouw RE, Schrijnders D, Sondermeijer CM, Bisschops R, et al. Predictive factors for initial treatment response after circumferential radiofrequency ablation for Barrett’s esophagus with early neoplasia: a prospective multicenter study. Endoscopy. 2013;45(7):516–25.
Small AJ, Araujo JL, Leggett CL, Mendelson AH, Agarwalla A, Abrams JA, et al. Radiofrequency ablation is associated with decreased neoplastic progression in patients with Barrett’s esophagus and confirmed low-grade dysplasia. Gastroenterology. 2015;149(3):567–76.e3. quiz e13-4
•• Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32(4):368–78. Highlighted the importance of histopathological confirmation of LGD.
Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105(7):1523–30.
Duits LC, van der Wel MJ, Cotton CC, Phoa KN, Ten Kate FJ, Seldenrijk CA, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology. 2017;152(5):993–1001.e1.
Hur C, Choi SE, Rubenstein JH, Kong CY, Nishioka NS, Provenzale DT, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology. 2012;143(3):567–75.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on GI Oncology
Rights and permissions
About this article
Cite this article
Hamade, N., Sharma, P. Ablation Therapy for Barrett’s Esophagus: New Rules for Changing Times. Curr Gastroenterol Rep 19, 48 (2017). https://doi.org/10.1007/s11894-017-0589-2
Published:
DOI: https://doi.org/10.1007/s11894-017-0589-2