Abstract
Diabetes distress (DD) represents a significant clinical burden in which levels of DD are related to both glycated haemoglobin (HbA1c) and some self-management behaviours. DD is related to, but different from, depression. Differences in DD experienced in people with type 1 and type 2 diabetes have been observed. Commonly measured using the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS), rates of elevated DD in research study participants range from 20 to 30 %. Risk factors for elevated DD in type 1 diabetes are longer duration of diabetes, severe hypoglycaemia, younger age and being female. A systematic review of intervention studies assessing DD identified eight randomised controlled trials (RCTs) and nine pre-post design studies. Only three studies targeted DD with the intervention. Intervention types were diabetes self-management education (DSME), psychologically informed self-management and devices. DSME pre-post studies, namely the Dose Adjustment For Normal Eating (DAFNE) programme, produced more consistent improvements in DD and HbA1c at follow-up. Psychologically informed self-management was more heterogeneous, but several RCTs were effective in reducing DD. Group interventions offered the greatest benefits across intervention designs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes distress (DD) has been increasingly recognised in research practice for two decades but has only recently achieved a sharper focus in clinical practice. Much of the research attention has been in type 2 diabetes. This paper is the first to review the evidence for DD in type 1 diabetes. We present a definition of DD and consider its associations with the important diabetes endpoints of glycaemic control, self-management behaviours and depression. Rates of elevated DD in research populations and the relative merits of screening for DD are considered. We present a systematic review of interventions for managing DD in clinical environments. The paper concludes with a number of research priorities to further our understanding of DD in people with type 1 diabetes.
Definition of Diabetes Distress
DD reflects a range of different emotional response to patient’s perceptions of health threats balanced against an appraisal of available coping resources, and it is content related necessitating a focus on distinguishing amongst the different sources of distress in diabetes so that specific interventions can be initiated [1•]. Esbitt et al. [2] explain that DD is “predicated on a variety of medical, contextual and individual factors, not on the presence of a psychiatric condition” (p. 35).
Manifestation of Diabetes Distress in Type 1 and Type 2 Diabetes
Some studies suggest that DD does not discriminate by diabetes type [3, 4], but it cannot be presumed that emotional problems are similarly experienced and have the same consequences in type 1 and type 2 diabetes. Indeed, the most commonly reported emotional problems in type 1 diabetes relate to hypoglycaemia and complications, worry about the future and complications, feeling burnt out/overwhelmed and worrying about low blood sugar reactions [5–7], whereas in type 2 diabetes, emotional distress relates more to goal setting and food restrictions [7]. Qualitative work confirms stressors unique to type 1 diabetes: realisation of the possible consequences of previously poor self-management as adolescents, apprehension about pregnancy, anxiety about being perceived to have type 2 diabetes, frustration competing for resources with type 2 diabetes and many great concerns specific to insulin use [8••, 9]. Our own case note documentary analysis observed differences in the manifestation of DD in the two populations [10]. Type 1 diabetes case notes revealed core issues resulting in elevated DD being lack of diabetes control and fear of associated complications with common behavioural manifestations resulting in the maintenance of high blood glucose levels, low levels of blood glucose monitoring and medication non-concordance. In contrast, type 2 diabetes case notes indicated the following: isolation, work-related issues, family demands, obesity and lack of knowledge. We found some diabetes distress themes reported in both type 1 and type 2 diabetes: neuropathic pain, fear of complications, fear of hypoglycaemia, poor sleep, loss of medication or diet control, dietary control (calorie restriction in type 2 diabetes and carbohydrate counting in type 1 diabetes), loss of independence and lack of support. It is clear from this early understanding of differences in DD that different management foci may be required.
Diabetes Distress Related to Depression
Previous psychological research in diabetes has focussed on depression, but it is now apparent that there has been a lack of clarity and precision in the measurement of depression in diabetes [1•, 11•]. Depression and DD are strongly associated not only in type 2 diabetes [12] but also in type 1 diabetes [9, 13]. Prospective research in mixed diabetes samples suggests a bidirectional association [7, 14, 15], with emerging evidence in type 1 diabetes that DD exacerbates the risk of incident-depressive symptoms by twofold [16]. Depression assessment constitutes to symptom count irrespective of cause or context, whereas DD reflects an emotional response to the adversity associated with living with and managing diabetes [11•], and Fisher et al. [1•] explain that “exclusively symptom based depression scores most likely capture the affect component of DD” (p. 769). Qualitative studies suggest that where a person with type 1 diabetes has depression, it is often related to their experience of diabetes [9, 17]. Fisher et al. [1•] explain that emotional distress in diabetes should be considered “a single continuous dimension that has two primary characteristics: content and severity; that the primary content of emotional distress among these individuals include diabetes, it’s management, other life stresses and other contributors (e.g. personal characteristics, life history and genetics)” (p. 764). There is, therefore, a need to move beyond conceptualising distress in diabetes as diagnosable depression and recognise the impact of disease-related factors on emotional well-being [18].
Measurement of Diabetes Distress
The concept of DD emerged alongside the development of the Problem Areas in Diabetes Scale (PAID) [19], with later revisions resulting in the Diabetes Distress Scale (DDS) [20]. We recently completed the work to distinguish between measures of DD (unpublished data). The PAID was developed with a sample predominantly comprised of people with type 1 diabetes [19], and the psychometric properties of the DDS for adults with type 1 diabetes has recently been established [21]. These measures have been extensively psychometrically evaluated in type 1 and type 2 diabetes [22], parents [23], adolescents [24] and languages and cultures [21, 25, 26]. Qualitative work, however, suggests that additional aspects of DD important in type 1 diabetes are omitted from these measures: fear of hypoglycaemia, problems maintaining a normal work-life balance, fatigue [21] and guilt about social burden, for example the possibility of an emergency [9]. As a result of these concerns, the Type 1 Diabetes Distress Scale (DDS-T1) was recently developed [27]. The DDS and DDS-T1 are comprised of empirically established sub-scales such as the DDS emotional burden and regimen distress sub-scales [20, 27] and have been employed in research studies [28••]. Short forms and screeners such as the DDS-2 and PAID-5 are also available [3, 29–31].
Relationship to Endpoints in Type 1 Diabetes
Glycated Haemoglobin
Cross-sectional evidence has consistently shown that, any point in time, someone with elevated DD is likely also to have high glycated haemoglobin (HbA1c) in type 1 diabetes [5, 32]. However, DD is not prospectively related to HbA1c when baseline HbA1c is controlled for; someone experiencing higher DD is not apparently at risk of increasing their HbA1c, or indeed developing high HbA1c, as a result of this initial distress at follow-up [33••, 34]. However, some evidence in type 1 diabetes suggests that intervention-related changes in DD are associated with changes in HbA1c with a marginally significant trend, suggesting that these concurrent changes are related, although causality cannot be inferred [35]. This mirrors findings in type 2 diabetes [36]. Furthermore, Weinger and Jacobson found that high baseline DD hampers improvement in HbA1c, suggesting that interventions must address existing DD to evidence improvement in clinical outcomes [35]. Some unpublished studies have failed to support an association between DD and HbA1c in type 1 diabetes though [37, 38], suggesting a complex relationship between these variables that requires further exploration. DD has been shown to explain the relationship between depressive symptoms and HbA1c [3, 39]. This mirrors evidence in mixed and type 2 diabetes samples [7, 40–42].
Self-Management Behaviours
Cross-sectional evidence suggests that DD impacts self-management behaviours in type 1 diabetes, namely less physical activity, poorer diet [21] and eating styles that are associated with overeating and high HbA1c [6] and insulin restriction [43, 44]. Other studies suggest that DD is not associated with self-monitoring of blood glucose, smoking and alcohol consumption, and that its association with physical activity may be explained by more general emotional distress [13]. This evidence base is very much underdeveloped at present, though. Martyn-Nemeth et al. identified that there may be a level at which DD becomes immobilising, resulting in fewer behaviours to avoid hypoglycaemia at very high levels of DD [6]. Sturt et al. found that in type 1 and type 2 diabetes, people with elevated diabetes distress alongside psychological morbidity, including low mood, were unable to convert strongly desired self-care intentions into actions [10]. Conversely, individuals with diabetes distress only were more successful at initiating self-care behaviours and developing self-efficacy, indicating that DD alone is easier to target [10]. Other mixed type and type 2 diabetes-only studies have found that it is the co-morbidity of DD and depression that is associated with the highest levels of HbA1c [45–47]. This suggests that when you have both DD and depression, it impacts the most on self-management behaviours that aim to control glycaemia and becomes most difficult to resolve.
Regimen Distress and Diabetes Endpoints
Research in type 1 diabetes has explicitly demonstrated that the element of DD that appears to drive the aforementioned associations with HbA1c, and self-management behaviour, is regimen distress [6, 21, 33••, 35, 39, 48]. The smallest change in regimen distress which can be subjectively realised by individuals, 0.5 standard deviation (SD) change, is associated with a difference of 7 mmol/mol (0.6 %) in HbA1c [39].
Thresholds for, and Rates of, Clinically Relevant DD
No epidemiological studies have assessed for DD; therefore, all data on degrees of DD amongst people with diabetes and proportions of people with diabetes experiencing elevated DD are derived from interventional or cross-sectional research studies which, in itself, results in a likely population bias. Investigators have used a range of thresholds across type 1 and type 2 diabetes populations to define elevated DD from PAID scores in the population aged below 30 years [49, 50] to 45–50 years [51, 52]. Studies in type 1 diabetes have endeavoured to establish the curvilinear relationship which has been observed in type 2 diabetes between DD and HbA1c, diet and physical activity [53]. In type 2 diabetes, the shape of these relationships indicated thresholds for low (a DDS mean score of 1–2), moderate (a DDS mean score of 2–3) and high (a DDS mean score over 3) clinically relevant DD; each successive increase in DD is associated with a 0.5 SD increase in HbA1c or decrease in self-management behaviour. However, the studies in type 1 diabetes found no evidence of these relationships [21, 39], suggesting that emotional problems have different implications in type 1 and type 2 diabetes and that, in type 1 diabetes, interventions can be applied, and will be effective, at any non-zero level of DD [21].
The majority of empirical studies to date have used thresholds of PAID ≥40 and a DDS score >3 to indicate elevated DD [28••]. In scoping the literature, we identified 11 studies which have reported proportions of type 1 diabetes populations with elevated DD [2, 21, 38, 47, 54–60]. These proportions range from 8 % [57] to 65 % [2]. Nearly half of these studies (combined population of 875 participants) reported proportions of participants with elevated DD between 17 and 31 % [38, 47, 58, 60, 61]. The mean ages of participants in these studies were between 37 and 52 years with the largest study reporting on 466 participants, mean age 37 years, finding 28 % to have elevated DD [47]. A large international study with 8500 participants (DAWN2 study), of which 16 % of them have type 1 diabetes, found that 44.6 % of the study population has elevated DD on the PAID-5 [62]. The evidence suggest that 20–30 % of people with type 1 diabetes will be experiencing elevated diabetes distress that will be affecting their self-management behaviours and their glycaemic control. Given that the majority of the evidence we have observed did not have psychological morbidity inclusion criteria and that those with psychological morbidity would be regarded as hard to reach and unlikely to volunteer for research participation, it is likely that this is an underestimation of the true picture.
Specifically in type 1 diabetes, risk factors for DD include a longer duration of diabetes [21, 63] and episodes of severe hypoglycaemia [5]. Age is also negatively correlated with DD [5, 21] with adolescents and younger adults endorsing feeling scared when thinking about living with diabetes, guilty about getting off track with diabetes management, unsatisfied with their diabetes physician, discouraged with their diabetes routine and experiencing uncomfortable interactions about diabetes with family/friends as more serious concerns than do older adults with type 1 diabetes [5]. DD is greater for women than men [21, 49, 63, 64] with women also exhibiting higher prevalence of subcutaneous insulin infusion, greater self-monitoring of blood glucose and a higher level of motivation, yet no difference in HbA1c level, perhaps suggesting that greater effort in maintaining HbA1c is at the cost of higher DD [49]. Interestingly, women, but not men, with type 1 diabetes have been shown to experience greater DD when they live without a partner, an effect that is partly explained by social support albeit the precise mechanisms of this association have yet to be established [65].
The Pros and Cons of Screening
Routine psychosocial screening for DD and depression has been recommended at key time points in the care pathway, including diagnosis, annual reviews, inpatient episodes, new complications and when issues of glycaemic control, self-management and quality of life arise [66]. The incorporation of psychosocial assessment and treatment into routine care, through a collaborative team approach, is recommended [11•, 66, 67]; however, no screening studies involving only the assessment for DD have been conducted solely in type 1 diabetes populations. These recommendations assume, firstly, that the screening process is effective in detecting vulnerable people; secondly, that psychosocial care pathways are routinely available; and thirdly, that these services are acceptable to patients. Fleer et al. found in a mixed type 1/type 2 diabetes sample that only 36 of the 104 participants found to have elevated DD accepted further referral to psychological services [57]. With limited resources, Byrne et al. argue that individuals likely to benefit the most should be targeted for intervention, including those with higher DD at baseline [50]. Ironically, the hard-to-reach group that does not respond to screening has the most to benefit from it [57]. Conflicting evidence exists concerning diabetes health-care professionals’ capacity to clinically detect DD. Pouwer et al. report under detection of DD by diabetes nurse specialists in 75 % of patients with established distress [68]. Conversely, Sturt et al. found that clinicians were able to detect elevated DD during their routine consultations [10]. Clinicians may not seek to uncover DD if local psychological care services and care pathways do not exist for the management of elevated DD. Fleer et al. suggested that, where integrated systems are not available, we should not screen [57]. Undertaking service audit to identify local prevalence of elevated DD may have greater merit enabling business cases to be developed for the provision of psychological care pathways in diabetes.
The Treatment and Management of Diabetes Distress: a Systematic Review
Methods
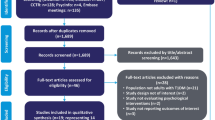
Given the relative novelty of clinical detection and management of DD in clinical practice outside of research studies, we sought to undertake a systematic approach to the identification and appraisal of effective treatment and management strategies. We updated a previous review of effective interventions for reducing DD in type 1 and type 2 diabetes populations [28••]. We searched Medline, PsycINFO and Embase from March 2013 to March 2015, for additional research studies reporting DD outcomes using the full PAID or DDS. From our previous review [28••], 14 type 1 diabetes studies were included. Figure 1 illustrates our combined flow diagram of included studies. The updated search identified 293 citations, and two people assessed each. Mixed diabetes population studies were included where the type 1 diabetes population data could be reported separately. One further unique experimental study was included. Two papers, published post March 2015, were identified through personal contacts. Due to heterogeneity in both intervention and research design, we undertook a narrative synthesis.
Managing Diabetes Distress
Seventeen studies reporting DD-related intervention outcomes specifically for adults with T1DM were identified; of these, eight were randomised controlled trials (RCTs) and nine were pre-post studies. The PAID was used in 15 studies and the DDS in 2 studies. These studies are described in Table 1. Reduction of DD was the sole or co-primary outcome in two studies [56, 69] and a secondary outcome in the remainder. This indicates that the majority of studies are not targeting DD but rather determining whether an intervention targeting another outcome worsens or improves DD. Reduction of DD was reported in all studies, however not always significantly. Our synthesis has categorised interventions into three groups: diabetes self-management education (DSME), self-management with a psychological component and devices.
Six studies investigated DSME interventions which aimed to reduce HbA1c by providing knowledge about diabetes and the technical skills needed to manage the condition and may include goal setting and problem-solving. All DSME intervention studies but one [50] (who did not report significance levels) reported significant reductions in DD [35, 70–73] and in HbA1c when reported [35, 71, 72]. The DSME intervention studies were pre-post design, and five of the six evaluated the Dose Adjustment For Normal Eating (DAFNE) programme and thus are generally more homogeneous which may account for the consistent positive impact on DD across these DSME interventions. The RCT studies of DAFNE [74] did not report DD as an outcome, and so, it is not possible to be definitively convinced that DSME, and DAFNE in particular, improved DD compared to controls. A study to evaluate the impact of the DAFNE programme on people with elevated DD would be an important next step in the evaluation of both DAFNE and elevated DD.
Nine studies comprised our second category, self-management interventions with a psychological component. In addition to some degree of self-management education, these studies have a psychological component focusing on the multidimensional aspects and perceptions of living with diabetes. These interventions aim to develop strategies to cope with the emotional stress of managing the disease and modify unhelpful cognitions. In addition, knowledge provided by the self-management components is utilised by focusing on experiential learning that influence behaviours, psychological adaptation and glycaemic control [56, 69, 75–77, 78•, 79, 80, 81••]. The results of these were less clear regarding significant changes in DD. Four studies showed significant reductions of DD in addition to significant reductions in HbA1c [76, 77, 80, 81••]. Zoffmann et al. showed significant reductions of both DD and HbA1c in women but not in men [78•]. In two pilot studies, Snoek et al. showed significant reductions in HbA1c and marginally significant reduction in DD (p = 0.06) and Esbit et al. reported an effect size of 0.34 relating to DD but no effect on HbA1c [69, 79]. Likewise, Due-Christensen et al. reported an effect size of 0.55 relating to reduction in DD (p ≤ 0.001) but no change in HbA1c was seen [56]. Interventions were primarily delivered by diabetes educators. Psychologists or psychiatrists were part of the intervention in six studies [56, 69, 75, 76, 79, 81••]. The psychological intervention components were empowerment and supportive counselling, use of self-determination approaches and cognitive behaviour therapy (CBT).
Two studies tested the efficacy of devices: continuous glucose monitoring [61] and sensor-augmented pump therapy [82]. Pump initiation with three individual sessions focusing on blood glucose control reduced DD as compared to multiple daily injection (MDI) treatment [82]. DD was not affected negatively by use of continuous glucose monitoring (CGM) with either real-time or retrospective bio-feedback [61].
The most common feature of effective interventions across the 17 studies was the group format which likely taps into natural social support, social learning theory and social comparison theory enabling people to establish a sense of normalcy and acquire positive vicarious learning experiences which successfully aid in breaking isolation and feelings of loneliness in living with type 1 diabetes [83]. Groups aimed to share how participants addressed emotional or cognitive problems in relation to performing diabetes-specific behaviours and challenges in coping with the demands of diabetes. Problem-solving, goal setting, focus on motivational barriers and facilitators were also utilised. Homework sheets were used to develop person-specific knowledge of illness perception and to enhance reflection on beliefs and attitudes towards diabetes that might need to be changed or reinforced. Studies using a group format and goal setting, problem-solving, reflection, written homework, motivational focus, supportive listening, cognitive restructuring and addressing emotional challenges seem to offer greater reductions in DD and HbA1c.
The populations under study were predominantly mid-40s with diabetes duration of more than 13 years displaying levels of DD ranging from 20 to 44.4 on PAID with the majority scoring >30. The review has identified a lack of interventions targeting elevated DD, aiming at emerging adults and also older adults. As it seems DD is present throughout the lifespan, it would be important to address this during the early years of adulthood and also in the early stages of diabetes to prevent long-standing DD. In addition, interventions targeting older adults with DD relating to a more severe disease because of complications might be beneficial.
To summarise, the management of DD in type 1 diabetes is in its infancy, in relation to both research evidence and clinical practice. DSME appears to reduce DD in type 1 diabetes. Psychologically enhanced self-management interventions reviewed were more heterogeneous than the DSME, predominantly DAFNE, studies that we have reviewed. Nonetheless, these theory-based interventions may have the potential to address elevated DD. Group-based interventions appear to have merit.
Conclusions
Summary of Evidence
This comprehensive review of the topic has identified that elevated DD is experienced by 20–30 % of people with type 1 diabetes and that there are well-validated scales for assessing DD, and whilst many intervention studies have assessed for it, few have targeted elevated DD. There is a rising imperative to clinically consider the role of elevated DD when providing routine care for type 1 diabetes populations. There is growing, albeit currently underdeveloped, evidence of a relationship between DD, self-management behaviours and glycaemic control. There is enough evidence though to warrant the further exploration of the role of elevated DD in influencing HbA1c and self-management behaviours crucial to good diabetes health such as blood glucose monitoring and insulin administration or restriction.
Controversial Issues
Cross-sectional evidence developed in type 2 diabetes is contradictory and ambiguous; investigators have found DD to be independently associated with some self-management behaviours, and to explain some, albeit not all, of the associations of depressive symptoms with these outcomes [42, 84], others have shown that it is depressive symptoms, not DD, that exhibit an independent association with self-management behaviours [40, 85]. Whilst prospective research has found self-management behaviours specifically related to diabetes, and which directly influence HbA1c (i.e. medication adherence), are influenced by DD, only depressive symptoms impact other more lifestyle-oriented behaviours including those that are recommended in diabetes [40]. This evidence is not available in type 1 diabetes.
Recommendations for Further Research
As the focus of DD research has shifted to type 1 diabetes only very recently, many important questions remain. Much of the extant research is in younger adults, for example those aged 18–35 years. Most of the research in type 1 diabetes has been done in Scandinavian countries, namely Norway and Denmark potentially limiting the generalisability of the findings. It remains unclear whether diabetes specialists are able to detect DD clinically within their routine consultations and, more so, what is the impact on detection rates when a clear care pathway for elevated DD exists? Diabetes population screening, using the available validated tools, is not appropriate in the absence of effectiveness and cost-effectiveness evidence related to caseness. Research in these areas has not yet commenced.
People with DD and without co-morbid depression may be more responsive to intervention which presents a case for research to detect and manage DD. The prevalence and natural history of DD and DD with co-morbid depression is unknown at the diabetes population level. DSME appears to reduce DD in type 1 diabetes, and many national diabetes policies recommend the routine provision of DSME. People experiencing elevated DD are likely to need greater support to achieve DSME participation, but the benefits to them may well out way the additional resource required to engage them in DSME. Research to evaluate the impact of DSME in patients with elevated DD is warranted. Evidence of one to one or ehealth/mhealth interventions, and research in older-age participants, is lacking.
Work delineating the prospective, time-varying, associations between DD and HbA1c and self-management behaviours, whilst accounting for depressive symptoms, is required in type 1 diabetes. Should these relationships be confirmed, it is critical to then establish the causal linkages between these variables; the pace of the associations; the complex interactive biological, behavioural and affective mechanisms/third variables involved; and the contextual and individual difference variables that determine these associations and their causal pathways (e.g. stage of disease, age, gender, burden of disease, presence of co-morbidities) [86••]. Once this evidence base has been established, there is a need to then develop and test interventions targeting DD, the mechanisms that underpin the association of DD and HbA1c and specific sub-groups at risk of high DD and for whom the DD/HbA1c association is particularly strong, in order to maximise outcomes in type 1 diabetes. Such studies should also elicit the mechanisms, mediators and moderators of any improvement in DD and other endpoints.
A few of these questions have been explored in type 2 diabetes, and there is a need to continue to understand the similarities and differences in the causes and consequences of, and treatment options for, DD as they relate to type 1 and type 2 diabetes populations.
Abbreviations
- DD:
-
Diabetes distress
- RCT:
-
Randomised controlled trial
- HbA1c:
-
Glycated haemoglobin
- DAFNE:
-
Dose Adjustment For Normal Eating
- DSME:
-
Diabetes self-management education
- PAID:
-
Problem Areas in Diabetes Scale
- DDS:
-
Diabetes Distress Scale
- SD:
-
Standard deviation
- CGM:
-
Continuous glucose monitoring
- CBT:
-
Cognitive behaviour therapy
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31(7):764–72. This paper offers perspectives on the depression-DD distinction.
Esbitt SA, Tanenbaum ML, Gonzalez JS. Disentangling clinical depression from diabetes-specific distress: making sense of the mess we’ve made. 2013. In: Screening for depression and other psychological problems in diabetes [Internet]. London: Springer. Available from: http://shibboleth.ovid.com/secure/?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=psyc10&AN=2012-23566-002. http://sfx.kcl.ac.uk/kings?sid=OVID:psycdb&id=pmid:&id=doi:10.1007%2F978-0-85729-751-8_2&genre=article&atitle=Disentangling+clinical+depression+from+diabetes-specific+distress%3A+Making+sense+of+the+mess+we%27ve+made.&title=&issn=&date=2013&volume=&issue=&spage=27&aulast=Esbitt%2C+Sabrina+A&isbn=978-0-85729-750-1&__char_set=utf8.
Friis AM, Johnson MH, Cutfield RG, Consedine NS. Does kindness matter? Self-compassion buffers the negative impact of diabetes-distress on HbA. Diabet Med: J Br Diabet Assoc. 2015 Apr 2.
Sturt J, Dennick K, Hessler D, Hunter B, Oliver J, Purrsell E, et al. High rates of elevated diabetes distress in research populations: a systematic review and meta-analysis. Diabetes UK Professional Conference; London, UK, 2015.
Lerman-Garber I, Barron-Uribe C, Calzada-Leon R, Mercado-Atri M, Vidal-Tamayo R, Quintana S, et al. Emotional dysfunction associated with diabetes in Mexican adolescents and young adults with type-1 diabetes. Salud Publica Mex. 2003;45(1):13–8.
Martyn-Nemeth P, Quinn L, Hacker E, Park H, Kujath AS. Diabetes distress may adversely affect the eating styles of women with type 1 diabetes. Acta Diabetol. 2014;51(4):683–6.
Reddy J, Wilhelm K, Campbell L. Putting PAID to diabetes-related distress: the potential utility of the problem areas in diabetes (PAID) scale in patients with diabetes. Psychosomatics. 2013;54(1):44–51.
Balfe M, Doyle F, Smith D et al. What’s distressing about having type 1 diabetes? A qualitative study of young adults’ perspectives. BMC Endocrine Disorders. 2013 Jul 2013;13(25). First and only qualitative study eliciting the experience of DD in general but also specifically in type 1 diabetes.
Tanenbaum ML, Gonzalez JS. The influence of diabetes on a clinician-rated assessment of depression in adults with type 1 diabetes. Diabet Educ. 2012;38(5):695–704.
Sturt J MK, Dennick K, Narasimha M, Sankar S & Kumar S. What characterises diabetes distress and its resolution? A documentary analysis. International Diabetes Nursing. 2015a.
Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabet Endocrinol. 2015;3(6):450–60. This paper offers perspectives on the depression-DD distinction.
Pouwer F, Skinner TC, Pibernik-Okanovic M, Beekman ATF, Cradock S, Szabo S, et al. Serious diabetes-specific emotional problems and depression in a Croatian-Dutch-English survey from the European Depression in Diabetes [EDID] Research Consortium. Diabet Res Clin Pract. 2005;70(2):166–73.
Lloyd CE, Pambianco G, Orchard TJ. Does diabetes-related distress explain the presence of depressive symptoms and/or poor self-care in individuals with type 1 diabetes? Diabet Med. 2010;27(2):234–7.
Ehrmann D, Hermanns N, Kulzer B, Bergis N, Maier B, Haak T. Depression in diabetes: the role of diabetes-related distress on incidence and recovery. Diabetologia. 2013; Conference: 49th Annual Meeting of the European Association for the Study of Diabetes, EASD 2013, Barcelona, Spain. Conference start: 20130923. Conference end: 7. Conference Publication: (var.pagings). 56 (p. S463).
Snoek FJ, Kersch NY, Eldrup E, Harman-Boehm I, Hermanns N, Kokoszka A, et al. Monitoring of Individual Needs in Diabetes (MIND)-2: follow-up data from the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) MIND study. Diabetes Care. 2012;35(11):2128–32.
Ehrmann D, Hermanns N, Haak T, Kulzer B. The influence of diabetes-related distress on depression. Diabetes. 2014; Conference: 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, USA. Conference start: 20140613. Conference end: 7. Conference Publication: (var.pagings). 63 (p. A201).
Gask L, Macdonald W, Bower P. What is the relationship between diabetes and depression? A qualitative meta-synthesis of patient experience of co-morbidity. Chronic Illn. 2011;7(3):239–52.
Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care. 2011;34(1):236–9.
Polonsky W, Anderson B, Lohrer P, Welch G, Jacobson A, Aponte J, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–60.
Polonsky W, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–31.
Joensen LE, Tapager I, Willaing I. Diabetes distress in type 1 diabetes—a new measurement fit for purpose. Diabet Med: J Br Diabet Assoc. 2013;30(9):1132–9.
Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69–72.
Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio J, Anderson BJ, Laffel LM. Re-examining a measure of diabetes-related burden in parents of young people with type 1 diabetes: the Problem Areas in Diabetes Survey-Parent Revised version (PAID-PR). Diabet Med. 2012;29(4):526–30.
Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes—teen version. Pediatr Diabetes. 2011;12(4 Pt 1):341–4.
Snoek FJ, Pouwer F, Welch GW, Polonsky WH. Diabetes-related emotional distress in Dutch and U.S. diabetic patients: cross-cultural validity of the problem areas in diabetes scale. Diabetes Care. 2000;23(9):1305–9.
Amsberg S, Wredling R, Lins PE, Adamson U, Johansson UB. The psychometric properties of the Swedish version of the Problem Areas in Diabetes Scale (Swe-PAID-20): scale development. Int J Nurs Stud. 2008;45(9):1319–28.
Fisher L, Polonsky W, Hessler D, Stryker L, Masharani U, Blumer I, et al. A new validated measure of diabetes distress for adults with type 1 diabetes. 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, USA; 2014.
Sturt J, Dennick K, Hessler D, Hunter B, Oliver J, Fisher L. Effective interventions for reducing diabetes distress: systematic review and meta-analysis. International Diabetes Nursing. 2015b. The first meta-analysis of effective interventions for managing DD in type 1 and type 2 diabetes.
Jones A, Olsen MZ, Perrild HJ, Willaing I. The psychological impact of living with diabetes: descriptive findings from the DAWN2 study in Denmark. Primary Care Diabetes. 2015 Apr 15.
Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6(3):246–52.
McGuire BE, Morrison TG, Hermanns N, Skovlund S, Eldrup E, Gagliardino J, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53(1):66–9.
Law GU, Walsh J, Queralt V, Nouwen A. Adolescent and parent diabetes distress in type 1 diabetes: the role of self-efficacy, perceived consequences, family responsibility and adolescent-parent discrepancies. J Psychosom Res. 2013;74(4):334–9.
Strandberg RB, Graue M, Wentzel-Larsen T, Peyrot M, Thordarson HB, Rokne B. Longitudinal relationship between diabetes-specific emotional distress and follow-up HbA1c concentration in adults with type 1 diabetes. Diabet Med. 2015. The first prospective study of the association between DD and HbA1c in type 1 diabetes.
Peyrot MMJJ, Kruger DF. A biopsychosocial model of glycaemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav. 1999;40(2):141–58.
Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42(2):123–31.
Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med. 2012;35(3):299–304.
Ali Z, Patel NH. Glycaemic control, emotional attitudes and quality of life in patients living with type 1 diabetes. Diabetic Medicine. 2013; Conference: Diabetes UK Professional Conference 2013, Manchester, UK. Conference start: 20130313. Conference end: 5. Conference Publication: (var.pagings). 30 (pp. 104–105).
Sheils E, Knott J, Cavan D, Shaban C. Fear of hypoglycaemia: is there an association with glycaemic control, hypoglycaemic symptoms and diabetes emotional distress in people with type 1 diabetes? Diabetic Medicine. 2012; Conference: Diabetes UK Professional Conference 2012, Glasgow, UK. Conference start: 20120307. Conference end: 9. Conference Publication: (var.pagings). 29 (p. 157).
Strandberg RB, Graue M, Wentzel-Larsen T, Peyrot M, Rokne B. Relationships of diabetes-specific emotional distress, depression, anxiety, and overall well-being with HbA1c in adult persons with type 1 diabetes. J Psychosom Res. 2014;77(3):174–9.
Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472–8.
Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23–8.
Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33(5):1034–6.
Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jaconson AM, Cole CF. Insulin omission in women with IDDM. Diabetes Care. 1994;10:1178–85.
Goebel-Fabbri AEAB, Fikkan J, Franko DL, Pearson K, Weinger K. Improvement and emergence of insulin restriction in women with type 1 diabetes. Diabetes Care. 2011;34(3):545–50.
van Bastelaar KM, Pouwer F, Geelhoed-Duijvestijn PH, Tack CJ, Bazelmans E, Beekman AT, et al. Diabetes-specific emotional distress mediates the association between depressive symptoms and glycaemic control in type 1 and type 2 diabetes. Diabet Med. 2010;27(7):798–803.
Pibernik-Okanovic M, Grgurevic M, Begic D, Szabo S, Metelko Z. Interaction of depressive symptoms and diabetes-related distress with glycaemic control in type 2 diabetic patients. Diabet Med. 2008;25(10):1252–4.
Schmitt A, Reimer A, Kulzer B, Haak T, Gahr A, Hermanns N. Negative association between depression and diabetes control only when accompanied by diabetes-specific distress. J Behav Med. 2014;38(3):556–64.
Graue M, Haugstvedt A, Wentzel-Larsen T, Iversen MM, Karlsen B, Rokne B. Diabetes-related emotional distress in adults: reliability and validity of the Norwegian versions of the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS). Int J Nurs Stud. 2012;49(2):174–82.
Zoffmann V, Vistisen D, Due-Christensen M. A cross-sectional study of glycaemic control, complications and psychosocial functioning among 18- to 35-year-old adults with type 1 diabetes. Diabet Med. 2014;31(4):493–9.
Byrne M, Newell J, Coffey N, MC OH, Cooke D, Dinneen SF. Predictors of quality of life gains among people with type 1 diabetes participating in the Dose Adjustment for Normal Eating (DAFNE) structured education programme. Diabetes Res Clin Pract. 2012;98(2):243–8.
Ting RZ, Nan H, Mandy WM, Kong AP, Ma RC, Wong RY, et al. Diabetes-related distress and physical and psychological health in Chinese type 2 diabetic patients. Diabetes Care. 2011;34(5):1094–6.
West C, McDowell J. The distress experienced by people with type 2 diabetes. Br J Commun Nurs. 2002;7(12):606–13.
Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259–64.
Rosenbek Minet LK, Wagner L, Lonvig EM, Hjelmborg J, Henriksen JE. The effect of motivational interviewing on glycaemic control and perceived competence of diabetes self-management in patients with type 1 and type 2 diabetes mellitus after attending a group education programme: a randomised controlled trial. Diabetologia. 2011;54(7):1620–9.
Psychological effects of continuous glucose monitoring in type 1-diabetes. Verhaltenstherapie & Verhaltensmedizin. 2009;30(4):458–69. Psychologische effekte der kontinuierlichen glukosemessung bei typ-1-diabetes.
Due-Christensen M, Zoffmann V, Hommel E, Lau M. Can sharing experiences in groups reduce the burden of living with diabetes, regardless of glycaemic control? Diabet Med. 2012;29(2):251–6.
Fleer J, Tovote KA, Keers JC, Links TP, Sanderman R, Coyne JC, et al. Screening for depression and diabetes-related distress in a diabetes outpatient clinic. Diabet Med. 2013;30(1):88–94.
Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. How to screen for depression and emotional problems in patients with diabetes: comparison of screening characteristics of depression questionnaires, measurement of diabetes-specific emotional problems and standard clinical assessment. Diabetologia. 2006;49(3):469–77.
Duda-Sobczak A, Zozulinska-Ziolkiewicz D, Pisarczyk-Wiza D, Wierusz-Wysocka B. The assessment of factors related to depressive symptoms in adult patients with type 1 diabetes. Diabetologia. 2014; Conference: 50th Annual Meeting of the European Association for the Study of Diabetes, EASD 2014, Vienna, Austria. Conference start: 20140915. Conference end: 9. Conference Publication: (var.pagings). 57 (1 SUPPL. 1) (pp. S420-S421).
Kibbey KJ, Speight J, Wong JLA, Smith LA, Teede HJ. Diabetes care provision: barriers, enablers and service needs of young adults with type 1 diabetes from a region of social disadvantage. Diabet Med. 2013;30(7):878–84.
Hermanns N, Kulzer B, Gulde C, Eberle H, Pradler E, Patzelt-Bath A, et al. Short-term effects on patient satisfaction of continuous glucose monitoring with the GlucoDay with real-time and retrospective access to glucose values: a crossover study. Diabetes Technol Ther. 2009;11(5):275–81.
Nicolucci A, Kovacs Burns K, Holt R, Comaschi M, Hermanns N, Ishii H, et al. Diabetes attitudes, wishes and needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30(7):767–77.
Luyckx K, Rassart J, Weets I. Illness self-concept in type 1 diabetes: a cross-sectional view on clinical, demographic, and psychosocial correlates. Psychol Health Med. 2015;20(1):77–86.
Brierley S, Johnson B, Young V, Eiser C, Heller S. The importance of measuring diabetes distress in young people with type 1 diabetes. Diabetic Medicine. 2012; Conference: Diabetes UK Professional Conference 2012, Glasgow, UK. Conference start: 20120307. Conference end: 9. Conference Publication: (var.pagings). 29 (p. 159).
Joensen LE, Almdal TP, Willaing I. Type 1 diabetes and living without a partner: psychological and social aspects, self-management behaviour, and glycaemic control. Diabet Res Clin Pract. 2013;101(3):278–85.
Association AD. Standards of medical care in diabetes. Diabetes Care. 2015;38(Supplement 1):S1–94.
Hajos T, Pouwer F, Skovlund S, Den Oudsten B, Geelhoed‐Duijvestijn P, Tack C, et al. Psychometric and screening properties of the WHO‐5 well‐being index in adult outpatients with type 1 or type 2 diabetes mellitus. Diabet Med. 2013;30(2):e63–9.
Pouwer F, Beekman AT, Lubach C, Snoek FJ. Nurses’ recognition and registration of depression, anxiety and diabetes-specific emotional problems in outpatients with diabetes mellitus. Patient Educ Couns. 2006;60(2):235–40.
Snoek FJ, Van Der Ven NCW, Lubach CHC, Chatrou M, Ader HJ, Heine RJ, et al. Effects of cognitive behavioural group training (CBGT) in adult patients with poorly controlled insulin-dependent (type 1) diabetes: a pilot study. Patient Educ Couns. 2001;45(2):143–8.
Engel L, Cummins R. Impact of dose adjustment for normal eating in Australia (OzDAFNE) on subjective wellbeing, coping resources and negative affects in adults with type 1 diabetes: a prospective comparison study. Diabet Res Clin Pract. 2011;91(3):271–9.
Hopkins D, Lawrence I, Mansell P, Thompson G, Amiel S, Campbell M, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care. 2012;35(8):1638–42.
Keen AJA, Duncan E, McKillop-Smith A, Evans ND, Gold AE. Dose Adjustment for Normal Eating (DAFNE) in routine clinical practice: who benefits? Diabet Med. 2011;29(5):670–6.
McIntyre HD, Knight BA, Harvey DM, Noud MN, Hagger VL, Gilshenan KS. Dose adjustment for normal eating (DAFNE)—an audit of outcomes in Australia. Med J Aust. 2010;192(11):637–40.
DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. British Medical Journal. 2002;325(7367):746.
Snoek FJ, van der Ven NCW, Twisk JWR, Hogenelst MHE, Tromp-Wever AME, van der Ploeg HM, et al. Cognitive behavioural therapy (CBT) compared with blood glucose awareness training (BGAT) in poorly controlled type 1 diabetic patients: long-term effects on HbA moderated by depression. A randomized controlled trial. Diabet Med. 2008;25(11):1337–42.
Amsberg S, Anderbro T, Wredling R, Lisspers J, Lins PE, Adamson U, et al. A cognitive behavior therapy-based intervention among poorly controlled adult type 1 diabetes patients—a randomized controlled trial. Patient Educ Couns. 2009;77(1):72–80.
Zoffmann V, Lauritzen T. Guided self-determination improves life skills with type 1 diabetes and A1C in randomized controlled trial. Patient Educ Couns. 2006;64(1–3):78–86.
Zoffmann V, Vistisen D, Due-Christensen M. Flexible guided self-determination intervention for younger adults with poorly controlled type 1 diabetes, decreased HbA and psychosocial distress in women but not in men: a real-life RCT. Diabetic Medicine. 2015 Jan 19. This study provides information on gender differences in DD.
Esbitt SA, Batchelder AW, Tanenbaum ML, Shreck E, Gonzalez JS. “Knowing that you’re not the only one”: perspectives on group-based cognitive-behavioral therapy for adherence and depression (CBT-AD) in adults with type 1 diabetes. Cognitive and Behavioral Practice. 2014 (0).
Hermanns N, Kulzer B, Ehrmann D, Bergis-Jurgan N, Haak T. The effect of a diabetes education programme (PRIMAS) for people with type 1 diabetes: results of a randomized trial. Diabetes Res Clin Pract. 2013;102(3):149–57.
Hermanns N, Schmitt A, Gahr A, Herder C, Nowotny B, Roden M, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38(4):551–60. This study specifically targets diabetes distress in patients with co-morbid subclinical depression. DIAMOS intervention was efficacious, compared to controls, in treating sub-threshold depression and elevated diabetes distress more effectively than education alone. Additionally, it prevented deterioration from sub to major depression.
Hermanides J, Norgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled type 1 diabetes: a randomized controlled trial. Diabet Med. 2011;28(10):1158–67.
Solomon P. Peer support/peer provided services underlying processes, benefits, and critical ingredients. Psychiatr Rehabil J. 2004;27(4):392–401.
Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30(3):542–8.
Gonzalez JS, Delahanty LM, Safren SA, Meigs JB, Grant RW. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51(10):1822–5.
Hessler D, Fisher L, Glasgow RE, Strycker LA, Dickinson LM, Arean PA, et al. Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care. 2014;37(3):617–24. One of only two prospective studies of the association between DD and HbA1c that has established the nature of the longitudinal relationship between these variables.
Compliance with Ethics Guidelines
Conflict of Interest
Jackie Sturt, Kathryn Dennick and Kate McCarthy declare that they have no conflict of interest.
Mette Due-Christensen reports salary and research funding from Foundation of European Nurses in Diabetes (FEND) and salary from Steno Diabetes Centre. Dr. Due-Christensen is presently employed at Steno Diabetes Centre A/S. Steno Diabetes Centre is a research hospital and an integrated part of the public Danish National Health Service that is owned by Novo Nordisk A/S. Steno Diabetes Centre receives part of its core funding from unrestricted grants from the Novo Nordisk Foundation and Novo Nordisk A/S. Dr. Due-Christensen owns shares in Novo Nordisk. No potential conflicts of interest relevant to this article exist.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Treatment of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Sturt, J., Dennick, K., Due-Christensen, M. et al. The Detection and Management of Diabetes Distress in People With Type 1 Diabetes. Curr Diab Rep 15, 101 (2015). https://doi.org/10.1007/s11892-015-0660-z
Published:
DOI: https://doi.org/10.1007/s11892-015-0660-z