Abstract
Prediabetes, covering individuals with impaired fasting glycemia, impaired glucose tolerance, or high-risk HbA1c levels, is associated with a ∼20 % increased risk of developing cardiovascular disease (CVD) compared with normoglycemic individuals. It is well-known that lifestyle or pharmacologic interventions can prevent diabetes in prediabetic people; however, the evidence is less clear regarding prevention of CVD. Most diabetes prevention trials have failed to show beneficial effects on CVD morbidity and mortality despite significant improvements of CVD risk factors in individuals with prediabetes. Another challenge is how to estimate CVD risk in prediabetic people. In general, prediction models for CVD do not take glucose levels or prediabetes status into account, thereby underestimating CVD risk in these high-risk individuals. More evidence within risk stratification and management of CVD risk in prediabetes is needed in order to recommend useful and effective strategies for early prevention of CVD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Every year, more than 5 million patients with diabetes worldwide die from diabetes-related diseases [1], with cardiovascular disease (CVD) as the most prevalent cause of death [2]. The term prediabetes, representing a number of intermediate glycemic stages between normal glucose regulation and diabetes, is associated with a high risk of developing both diabetes and CVD [3, 4]. For that reason, it is currently debated whether individuals with prediabetes should be identified and targeted for interventions aimed at reducing CVD risk [5•]. In the following, we provide an update of the relationship between prediabetes and CVD risk as well as suggestions for how to identify, risk stratify and manage CVD risk in individuals with prediabetes.
Definition of Prediabetes
Although prediabetes is a high-risk state for developing type 2 diabetes, the term prediabetes has been criticized, mainly because approximately 30 % of people with prediabetes will not progress to type 2 diabetes [6–8]. Using an oral glucose tolerance test (OGTT) for the diagnosis of prediabetes, there is also a high day-to-day variation, especially for 2-hour plasma glucose concentration [9]. Furthermore, the fasting duration as well as the time of the OGTT also influence plasma glucose levels [10], altogether resulting in a high risk of misclassification. Other names for prediabetes have been suggested, (eg, “intermediate hyperglycemia” or “high risk state of developing diabetes” [11, 12]); however, for simplicity we will use the term prediabetes in this review.
Isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), combined IFG and IGT as well as high-risk HbA1c concentrations are all part of the term prediabetes. The diagnostic criteria for prediabetes differ between the American Diabetes Association (ADA) and the World Health Organization (WHO) [5•,13, 14] (Table 1), with lower cutpoints in the ADA criteria. As a result of the different cutpoints there will be more prediabetic individuals for a given population when using the ADA criteria compared with the WHO criteria. For example, a shift from the WHO to the ADA criterion for prediabetic fasting glucose will result in a 2–3 fold increase in the prevalence of IFG [15–17]. Moreover, it should be noted that individuals diagnosed with prediabetes based on the ADA criteria will, on average, be at lower risk of diabetes and CVD than individuals with WHO-defined prediabetes [15, 18••].
Cardiovascular Disease Risk in Prediabetes
Prediabetes by OGTT
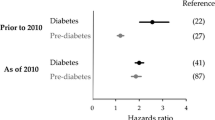
During the last decades it has been a general notion that people with IGT have higher risk of CVD morbidity and mortality than people with IFG. This thought is predominantly based on findings from the large European multicenter study DECODE, which found an independent association of future risk of CVD mortality with IGT, but not with IFG [19]. A Japanese study supported that IGT is more predictive than IFG in terms of CVD events and mortality [20]. In contrast, the AusDiab study found that, compared with NGT, the relative risk of developing CVD was 2.5 (95 % CI: 1.2–5.1) for people with IFG, but only 1.2 (0.7–2.2) for those with IGT using the WHO definition [21]. The Emerging Risk Factors Collaboration also reported 11 %–17 % higher risk of developing coronary heart disease (CHD) among people with IFG (5.6–7.0 mmol/L) compared with those with normal fasting glycemia [22]. In addition to ethnic differences and confounding factors, the diverse findings are likely to be explained by dissimilar control groups. For instance, the control group for IFG in the DECODE study [19] included participants with IGT or even diabetes based on 2-hour glucose concentration, making the “control” group at relatively higher risk in the DECODE study [19] compared with for instance the AusDiab study [21] and the Emerging Risk Factors Collaboration [22] using the more strict NGT criterion or normal fasting glucose as control groups.
When studying plasma glucose concentrations as continuous variables the results also differ between studies. The DECODE study and analyses from 5 different Finnish cohorts found that 2-hour plasma glucose concentration is a stronger predictor than fasting glucose (per standard deviation) for CVD morbidity and mortality among individuals with normal glucose tolerance, prediabetes or screen-detected diabetes [23•]. Moreover, the large American ARIC study did not find an association of fasting plasma glucose with either CHD or ischemic stroke [24]. However, in the AusDiab study [25], 1 standard deviation increase in fasting plasma glucose was associated with a ∼30 % increased risk of CVD mortality, which was similar to the excess risk (∼20 %) associated with 1 standard deviation increase in 2-hour plasma glucose concentration.
Meta-analyses of prospective observational studies examining CVD risk in people with IFG vs IGT as well as CVD risk in relation to changes in fasting vs post-OGTT plasma glucose concentrations as continuous variables have recently been conducted [18••,26••,27]. Ford et al. included 18 studies from 1997 through 2008, and concluded that people with prediabetes in general have ∼20 % increased risk of CVD, irrespective of the type of prediabetes (IFG vs IGT) or the criterion used to define IFG [18••]. Other meta-analyses including blood glucose in the nondiabetic range as a continuous variable have shown that both fasting and post-OGTT glucose concentrations are associated with incident CVD and CHD, but with slightly higher relative effects of 2-hour glucose than fasting glucose when taking the larger standard deviation of the 2-hour glucose measurement into account [26••,27].
Prediabetes by HbA1c
With the recent addition of HbA1c to the diagnostic criteria for prediabetes the association of prediabetic HbA1c levels with CVD outcomes is also of interest. In the ARIC study, HbA1c levels between 5.5 %–6.0 % were associated with 45 % increased risk of CHD, but not ischemic stroke, whereas HbA1c concentrations between 6.0 %–6.5 % (prediabetic range) were associated with 40 %–60 % increased risk of both CHD and stroke compared with people with HbA1c levels of 5.0 %–5.5 % [24]. Analyzed as a continuous variable in the AusDiab study, the excess relative risk of CVD mortality associated with 1 standard deviation increase in HbA1c was 20 %, whereas HbA1c was not associated with all-cause mortality [25]. A meta-analysis of 9 prospective studies, among them the ARIC and the AusDiab studies, concluded that a 1 %-point increase in HbA1c within the nondiabetic range was associated with 20 % increased risk of CHD [27].
To sum up, prediabetic levels of fasting glucose, 2-hour glucose, and HbA1c are all associated with increased CVD risk, suggesting that individuals with prediabetes defined by either criterion should be targeted for CVD prevention. There is no clear evidence as to whether people with IGT are at higher CVD risk than those with IFG or with prediabetes defined by HbA1c criteria. Inconsistency in study findings may be related to differences in sex and age as discussed below.
The Role of Sex and Age
In the nondiabetic population, CVD risk is lower in women than in men [28, 29]. The picture, however, is not that clear when studying people with diabetes [28, 30–33] or prediabetes [18••,34–36]. One meta-analysis showed no difference in the relative risk of CVD outcomes between men and women with IFG [18••]. Another meta-analysis found that the effect of post-OGTT glucose concentrations on CVD risk was less strong in men analyzed separately compared with men and women analyzed together [26••]. This finding suggests a stronger effect of post-OGTT glucose concentrations on CVD risk among women. The stronger associations found in women between glycemia and CVD risk is supported by a study of older American nondiabetic men and women, which showed that HbA1c was associated with a significantly increased risk for fatal CVD and ischemic heart disease among women, but not among men [37]. In contrast to these findings, results from a cross-sectional study recently suggested that the overall female advantage in CVD risk results from an attenuation of the relationship between insulin resistance and CVD risk [38].
In addition to sex, age seems to be an important contributor to the diversity of study findings. Whereas differences between men and women are more pronounced among younger people [38], differences between fasting and 2-hour glucose concentrations on CVD risk is more noticeable in older individuals. In a stratified meta-analysis of 13 studies, the relative risk of post-OGTT glucose concentrations on CVD events was significantly higher than that of fasting plasma glucose in individuals with a mean age ≥55 years of age, but not among those <55 years [26••]. However, the relative risk of both fasting and post-OGTT glucose in general tended to be higher among the younger study participants [26••]. Furthermore, an international case–control study, the INTERHEART study, found that the association between glycemia (HbA1c) and incident myocardial infarction was stronger among younger individuals compared with older individuals [39]. Together, these findings indicate that the relative importance of plasma glucose levels on CVD risk decreases with age, probably as a result of development of other cardiometabolic risk factors.
Does Prevention of Diabetes Reduce CVD Risk in People with Prediabetes?
Both lifestyle and pharmacologic interventions have proven effective in terms of lowering diabetes risk in individuals with prediabetes [6, 7, 40–42]. However, despite the elevated CVD risk among people with prediabetes, it has been difficult to document CVD risk reduction as a result of diabetes prevention strategies (Table 2).
Lifestyle Interventions
The Chinese Da Qing IGT and diabetes study was the first large randomized controlled trial to show beneficial effects of lifestyle intervention on prevention of diabetes in individuals with IGT [40]. Despite the beneficial effects on glucose outcomes, the 20-year follow-up of the Da Qing study showed no effect of lifestyle intervention on CVD events and mortality [43]. Likewise, the Finnish Diabetes Prevention Study also failed to show beneficial effects of lifestyle intervention on CVD outcomes even though a significant reduction in diabetes risk was found [44]. In the 10-year follow-up of the Finnish Diabetes Prevention Study, total mortality and CVD incidence were not different between the intervention and control groups, but the study participants who had IGT at baseline had lower all-cause mortality and CVD incidence, compared with a Finnish population-based cohort of people with IGT [44], suggesting that regular follow-up of individuals at high risk may improve long-term outcomes. Despite the lack of significant effects on major CVD events, lifestyle intervention can reduce CVD risk factors in people with prediabetes. The Diabetes Prevention Program showed improvements in both lipid levels and blood pressure in prediabetic individuals who regressed to NGT during the study period, and the largest improvements were seen in the intensive lifestyle intervention group [45, 46]. However, the Diabetes Prevention Program has not yet reported long-term effects of the different prevention strategies on CVD events.
Pharmacologic Interventions
In addition to lifestyle interventions, several pharmacologic trials have shown beneficial effects on diabetes risk reduction in individuals with IGT [7, 41, 42, 47]. However, in terms of CVD prevention, the pharmacologic studies have been as disappointing as the trials focusing on intensive lifestyle modification. The NAVIGATOR trial did not find any effect of treatment with nateglinide (insulin secretagogue) or valsartan (a blocker of the renin-angiotensin system) on CVD outcomes in individuals with IGT [47, 48]. Nor did the DREAM trial show any benefit of treatment with rosiglitazone (insulin sensitizer) or ramipril (a blocker of the renin-angiotensin system) on incident CVD events in people with IFG or IGT [49]. The ORIGIN study examined the effect of insulin glargine on CVD outcomes, but also showed no difference compared with placebo treatment in people with IFG and/or IGT [50]. The most promising study so far has been the diabetes prevention trial STOP-NIDDM [41], in which a significant 49 % reduction in major CVD events were found in participants with IGT who were randomized to the α-glucosidase inhibitor acarbose and followed for 3–4 years [51••]. Also the risk of developing hypertension was significantly decreased with acarbose treatment in the STOP-NIDDM trial [51••]. The authors speculate that the beneficial effect was related to concomitant reductions in body weight, waist circumference, blood pressure, and plasma triglyceride levels [51••], but also changes in the gut incretin hormone glucagon-like peptide 1 [52] could have played a role. It should be noted that the STOP-NIDMM trial was powered for incident diabetes, not for CVD, and more studies are needed before acarbose should be recommended for primary prevention of CVD.
Glucose Reduction vs Reversal of Underlying Pathophysiology
The lack of beneficial effects of lifestyle and pharmacologic interventions on CVD outcomes in individuals with prediabetes raises the question of whether glucose lowering per se should be the main focus of type 2 diabetes treatment or its prevention. It may be more beneficial to focus on changing the processes that underlie hyperglycemia or reversal of the vascular damage that may already have occurred. Insulin resistance often clusters with other cardiometabolic risk factors, such as visceral adiposity, hypertension, and dyslipidemia, making insulin resistance likely to be responsible for the higher CVD risk in hyperglycemic individuals [53–56]. By using gold standard measures of insulin sensitivity, an observational study showed that insulin resistance is largely responsible for the relationship between plasma glucose levels and CVD risk as estimated by the Framingham CVD risk score [54]. Because of this close association between insulin resistance and CVD risk, treatment modalities for improvement of insulin resistance, rather than glucose levels per se, may be more efficient in terms of reducing CVD risk. For instance, a slowing of the progression of carotid intima-media thickening of 30 %–70 % was found in people with prediabetes treated with pioglitazone (insulin sensitizer) for <3 years [57, 58]. However, so far there is no evidence of beneficial effects of insulin-sensitizing agents on long-term CVD outcomes in individuals with prediabetes.
Management of CVD Risk in Prediabetes
Strategies for CVD Risk Management in Prediabetes are Needed
The high risk of CVD among people with prediabetes warrants increased awareness of systematic CVD risk management in clinical practice. In the light of the disappointing results of prevention of diabetes on incident CVD, the elevated CVD risk in prediabetes cannot be tackled by focusing solely on hyperglycemia, but must be addressed directly. Both EASD [59•] and ADA [5•] recommend that individuals with prediabetes should be informed of their increased risk for CVD and counseled about effective strategies to lower their risk. However, no studies have assessed the benefit of primary prevention of CVD in individuals with prediabetes, and only few studies have had the power to perform sub-group analysis according to glucose tolerance status in the prediabetic range. In the Jupiter study, people without previous CVD or diabetes were randomized to statin therapy (rosuvastatin) or placebo and followed for up to 5 years [60]. Individuals with prediabetes by fasting glucose or HbA1c who received rosuvastatin had a significant reduction in CVD endpoints compared with those receiving placebo. Importantly, however, rosuvastatin increased the risk of developing diabetes, but it was concluded that the benefits on CVD and mortality exceeded the diabetes hazard [60]. More randomized controlled trials focusing on management of lipids and hypertension in individuals with prediabetes are needed. In the meantime, individuals with prediabetes are in need of early risk assessment to identify comorbidities and factors that increase cardiovascular risk. This includes evaluation of: (1) risk factors (eg, lifestyle factors including smoking, hypertension, and dyslipidemia); (2) early microvascular complications and cardiac autonomic dysfunction; and (3) comorbidities (eg, heart failure and arrhythmias). Accordingly, successful risk prevention depends on a comprehensive detection and management of all modifiable risk factors, as can be visualized by the use of risk engines.
Evaluation of CVD Risk in Prediabetes
Prediction models of CVD risk have been developed both for the general population and for persons with diabetes. Risk models for the general population include age, male sex, high blood pressure, anti-hypertensive treatment, smoking, dyslipidemia, and diabetes [61–66]. The need for diabetes-specific CVD risk models [67–69] arose from studies showing an impact of age at diabetes diagnosis as well as diabetes duration on the prognosis for developing diabetes-related complications [70]. Also, there has been some evidence for a stronger association between triglycerides and HDL-cholesterol with CHD in individuals with type 2 diabetes compared with individuals from the general population [71]. Although it is evident that prediabetes increases the risk of CVD by approximately 20 % [18••,72], it is less clear whether prediabetes directly causes CVD or whether its effect is mediated through other known risk factors for CVD. Most risk models for CVD in the general population merely include presence of diabetes and not glucose [61, 64–66, 73–75]. Only two risk models for fatal CVD have included glucose as a risk factor [76, 77], and so far no risk prediction models for nonfatal CVD events have included glucose or prediabetes. One argument for developing separate models for prediabetic vs normoglycemic individuals is if certain known risk factors for CVD operate differently in the different states, ie, if the relative risk is different in people with prediabetes than in those with normal glucose regulation. Another argument would be the existence of markers posing an increased CVD risk only in the prediabetic state. However, there are no studies showing a modifying effect of glucose (or prediabetes) on any of the known risk factors for CVD. Also, no CVD markers specific to the prediabetic state have been reported. Accordingly, there is currently no evidence supporting the need for separate CVD risk models for people with prediabetes. While there may be no basis for prediabetes-specific CVD models, being in the prediabetic state seems to pose an excess risk for developing CVD independent of other risk factors [21, 22, 24]. Hence, risk scores developed for normal glucose tolerance may systematically underestimate CVD risk in prediabetic individuals. However, because the relationship between CVD and glucose seems to be linear with no evidence of a threshold effect [19, 25, 78, 79], it seems more reasonable to include glucose as a linear term in future CVD risk models [80] rather than including a binary variable based on somewhat arbitrarily defined glucose ranges (prediabetes) [81].
Conclusions
Individuals with IFG, IGT, or prediabetic HbA1c levels have ∼20 % increased risk of developing CVD compared with individuals with normal glucose regulation. Several studies have shown beneficial effects of lifestyle modification or pharmacologic treatment on prevention of diabetes in individuals with prediabetes (mainly IGT), but the evidence regarding CVD prevention is weak. While interventions with lifestyle modification or pharmacologic agents seem to improve CVD risk factors, only acarbose have proven effective in terms of reducing CVD events among people with IGT. More studies examining effects of lipid- and blood pressure-lowering treatments in prediabetes are needed.
Another important concern is how people in need for CVD prevention should be identified. Several risk prediction models for CVD have been developed; however, none of them include presence of prediabetes as a risk factor. Future risk prediction models should take into account blood glucose as a continuous variable and preferably with different impact of blood glucose in different age groups, because the relative importance of blood glucose on CVD risk seems to decrease with age.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
International Diabetes Federation. Diabetes Atlas, 5th Edition Update for 2012. 2012.
The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–90.
Perreault L, Færch K. Approaching pre-diabetes. J Diabetes Complications. 2013; [Epub ahead of print].
American Diabetes Association Standards of Medical Care in Diabetes - 2014. Diabetes Care. 2014;7:S14–80. These guidelines represent the most up-to-date American recommendations on management of diabetes and prediabetes.
Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50.
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
De Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn study. JAMA. 2001;285:2109–13.
Mooy JM, Grootenhuis PA, de Vries H, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39:298–305.
Hulmán A, Færch K, Vistisen D, et al. Effect of time of day and fasting duration on measures of glycaemia: analysis from the Whitehall II Study. Diabetologia. 2013;56:294–7.
World-Health, Organization and International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva: World Health Organization; 2006.
The International Expert Committee International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32:1327–34.
Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Report of a WHO consultation. Diabetic Med. 1998;15:539–53.
World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation. Geneva, Switzerland: WHO Press; 2011. p. 1–25.
Borch-Johnsen K, Colagiuri S, Balkau B, et al. Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycaemia. Diabetologia. 2004;47:1396–402.
Xu Y, Wang L, He J. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59.
Færch K, Vistisen D. Response to Xu et al. “Prevalence and Control of Diabetes in Chinese Adults”. JAMA. 2014;311(2):200.
Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–7. This review and meta-analysis shows that both impaired fasting glucose and impaired glucose tolerance are associated with ∼20 % increased risk of developing cardiovascular disease.
DECODE Study Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405.
Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–4.
Barr ELM, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151–7.
Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
Qiao Q, Pyorala K, Pyorala M, et al. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J. 2002;23:1267–75. This study shows that 2-hour glucose independent of fasting glucose is a significant predictor of cardiovascular disease, whereas fasting glucose does not have an independent effect over 2-hour glucose.
Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–11.
Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia. 2009;52:415–24.
Kodama S, Saito K, Tanaka S, et al. Fasting and post-challenge glucose as quantitative cardiovascular risk factors: a meta-analysis. J Atheroscler Thromb. 2012; [Epub ahead of print]. This review and meta-analysis shows that both fasting and postload glucose is associated with cardiovascular disease, but the effect of postload glucose is larger than that of fasting glucose.
Sarwar N, Aspelund T, Eiriksdottir G, et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 2010;7:e1000278.
Hyvarinen M, Tuomilehto J, Laatikainen T, et al. The impact of diabetes on coronary heart disease differs from that on ischaemic stroke with regard to the gender. Cardiovasc Diabetology. 2009;8:17.
Stranges S, Guallar E. Cardiovascular disease prevention in women: a rapidly evolving scenario. Nutr Metabol Cardiovasc Dis. 2012;22:1013–8.
Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo study. JAMA. 1991;265:627–31.
Penno G, Solini A, Bonora E, et al. Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: the RIACE Italian multicentre study. J Intern Med. 2013;274:176–91.
Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8.
Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23:962–8.
Donahue RP, Dorn JM, Stranges S, Swanson M, Hovey K, Trevisan M. Impaired fasting glucose and recurrent cardiovascular disease among survivors of a first acute myocardial infarction: evidence of a sex difference? The Western New York experience. Nutr Metabol Cardiovasc Dis. 2011;21:504–11.
Leosdottir M, Willenheimer R, Persson M, Nilsson P. The association between glucometabolic disturbances, traditional cardiovascular risk factors and self-rated health by age and gender: a cross-sectional analysis within the Malmo Preventive Project. Cardiovasc Diabetol. 2011;10:118.
Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–55.
Park S, Barrett-Connor E, Wingard DL, Shan J, Edelstein S. GHb is a better predictor of cardiovascular disease than fasting or postchallenge plasma glucose in women without diabetes. The Rancho Bernardo study. Diabetes Care. 1996;19:450–6.
Kim SH, Reaven G. Sex differences in insulin resistance and cardiovascular disease risk. J Clin Endocrinol Metab. 2013;98:E1716–21.
Gerstein HC, Islam S, Anand S, et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia. 2010;53:2509–17.
Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44.
Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7.
Gerstein HC, Yusuf S, Bosch J, et al. The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–105.
Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–9.
Uusitupa M, Peltonen M, Lindström J, et al. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study - secondary analysis of the randomized trial. PLoS One. 2009;4:e5656.
Goldberg RB, Temprosa M, Haffner S, et al. Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care. 2009;32:726–32.
The Diabetes Prevention Program Outcomes Study Research Group, prepared on behalf of the DPPOS Research Group, Orchard TJ, et al. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med. 2013;30:46–55.
The NAVIGATOR study group. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–90.
The NAVIGATOR study group. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–76.
The DREAM Trial effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. 2008;31:1007–14.
The ORIGIN, Investigators T. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–94. This is the only diabetes prevention trial showing beneficial effects on CVD outcomes in individuals with prediabetes.
Seifarth C, Bergmann J, Holst JJ, Ritzel R, Schmiegel W, Nauck MA. Prolonged and enhanced secretion of glucagon-like peptide 1 (7–36 amide) after oral sucrose due to alpha-glucosidase inhibition (acarbose) in Type 2 diabetic patients. Diabet Med. 1998;15:485–91.
DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1998;14:173–94.
Færch K, Bergman B, Perreault L. Does insulin resistance drive the association between hyperglycemia and cardiovascular risk? PLoS One. 2012;7:e39260.
Haffner SM, Mykkanen L, Festa A, Burke JP, Stern MP. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000;101:975–80.
Zhang Y, Lee ET, Howard BV, et al. Insulin resistance, incident cardiovascular diseases, and decreased kidney function among nondiabetic American Indians: the Strong Heart Study. Diabetes Care. 2013; [Epub ahead of print].
DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–15.
Xiang AH, Hodis HN, Kawakubo M, et al. Effect of pioglitazone on progression of subclinical atherosclerosis in non-diabetic premenopausal Hispanic women with prior gestational diabetes. Atherosclerosis. 2008;199:207–14.
Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013; doi:10.1093/eurheartj/eht108. These guidelines represent the most up-to-date European recommendations on management of diabetes and prediabetes.
Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2011;380:565–71.
D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743–53.
Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003.
Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8.
Pencina MJ, D’Agostino Sr RB, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart study. Circulation. 2009;119:3078–84.
Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart. 2007;93:172–6.
Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–82.
Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101:671–9.
Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47:1747–59.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–33.
Davis TM, Stratton IM, Fox CJ, Holman RR, Turner RC. U.K. Prospective Diabetes Study 22. Effect of age at diagnosis on diabetic tissue damage during the first 6 years of NIDDM. Diabetes Care. 1997;20:1435–41.
Feher MD, Elkeles RS. Lipid modification and coronary heart disease in type 2 diabetes: different from the general population? Heart. 1999;81:10–1.
Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43.
Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105:310–5.
Ferrario M, Chiodini P, Chambless LE, et al. Prediction of coronary events in a low incidence population. Assessing accuracy of the CUORE Cohort Study prediction equation. Int J Epidemiol. 2005;34:413–21.
Wu Y, Liu X, Li X, et al. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation. 2006;114:2217–25.
Braun J, Bopp M, Faeh D. Blood glucose may be an alternative to cholesterol in CVD risk prediction charts. Cardiovasc Diabetology. 2013;12. doi:10.1186/1475-2840-12-24.
The DECODE Study Group. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia. 2004;47(12);2118–28.
The DECODE Study Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26:688–96.
Bartnik M, Ryden L, Ferrari R, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004;25:1880–90.
Schöttker B, Müller H, Rothenbacher D, Brenner H. Fasting plasma glucose and HbA1c in cardiovascular risk prediction: a sex-specific comparison in individuals without diabetes mellitus. Diabetologia. 2013;56:92–100.
Streiner DL. Breaking up is hard to do: the heartbreak of dichotomizing continuous data. Can J Psychiatry. 2002;47:262–6.
Compliance with Ethics Guidelines
Conflict of Interest
Kristine Færch has a board membership with and is employed by Steno Diabetes Center A/S. She has stock/stock options with Novo Nordisk A/S. Dorte Vistisen is employed by Steno Diabetes Center A/S. She has stock/stock options with Novo Nordisk A/S. Nanna Borup Johansen is employed by Steno Diabetes Center A/S. She has stock/stock options with Novo Nordisk A/S. Marit Eika Jørgensen is employed by Steno Diabetes Center A/S. She has stock/stock options in Novo Nordisk A/S.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Macrovascular Complications in Diabetes
Rights and permissions
About this article
Cite this article
Færch, K., Vistisen, D., Johansen, N.B. et al. Cardiovascular Risk Stratification and Management in Pre-Diabetes. Curr Diab Rep 14, 493 (2014). https://doi.org/10.1007/s11892-014-0493-1
Published:
DOI: https://doi.org/10.1007/s11892-014-0493-1