Abstract
Bile acids are synthesized in the liver from cholesterol and have traditionally been recognized for their role in absorption of lipids and in cholesterol homeostasis. In recent years, however, bile acids have emerged as metabolic signaling molecules that are involved in the regulation of lipid and glucose metabolism, and possibly energy homeostasis, through activation of the bile acid receptors farnesoid X receptor (FXR) and TGR5. Bile acid sequestrants (BASs) constitute a class of drugs that bind bile acids in the intestine to form a nonabsorbable complex resulting in interruption of the enterohepatic circulation. This increases bile acid synthesis and consequently reduces serum low-density lipoprotein cholesterol. Also, BASs improve glycemic control in patients with type 2 diabetes. Despite a growing understanding of the impact of BASs on glucose metabolism, the mechanisms behind their glucose-lowering effect in patients with type 2 diabetes remain unclear. This article offers a review of the mechanisms behind the glucose-lowering effect of BASs, and the efficacy of BASs in the treatment of type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bile acids are synthesized in the liver from cholesterol, and facilitate absorption of fatty acids and lipid-soluble vitamins. After secretion into the small intestine, bile acids are reabsorbed through passive and active mechanisms denoted as the enterohepatic circulation, leaving only a small fraction of the bile acids to be lost in the feces. However, fecal excretion of bile acids constitutes a major pathway of cholesterol clearance. In recent years, it has become clear that bile acids are not only simple fat solubilizers, but also metabolic signaling molecules. Bile acids activate the nuclear farnesoid X receptor (FXR), involved in the regulation of bile acid synthesis, excretion and transport, as well as lipid and glucose metabolism [1], and the membrane G protein-coupled TGR5 receptor [2] involved in incretin hormone secretion [3, 4] and possibly energy metabolism [5]. Bile acid sequestrants (BASs) are a class of drugs that bind bile acids in the intestine to form a nonabsorbable complex, which interrupt the enterohepatic recirculation of bile acids. The BASs remain unabsorbed in their passage through the intestine and, therefore, lack systemic toxicity. Originally, BASs were developed to reduce cholesterol levels in patients with hypercholesterolemia, and later they were shown to improve glycemic control in patients with type 2 diabetes. This article reviews the mechanisms behind the glucose-lowering effect of BASs and their efficacy in the treatment of type 2 diabetes. Extensive reviews of the effect of BASs on cholesterol and lipid metabolism [6], and of bile acid receptors FXR and TGR5 [1, 7], can be found elsewhere and is beyond the scope of this article.
Clinical Use and Efficacy of Bile Acid Sequestrants
BASs are orally administered, nonabsorbed, positively charged (at intestinal pH), polymers that bind the negatively charged bile acids in the intestine, and thereby prevent reabsorption and, consequently, increase fecal excretion [8, 9]. This in turn, increases bile acid synthesis, resulting in increased expression of LDL receptors in the liver and, subsequently, decreased circulating levels of LDL cholesterol. BASs have been known for decades [10-12] and the drug class constitutes first-generation cholestyramine and colestipol and second-generation colestimide (also known as colestilan, marketed only in Japan) and colesevelam hydrochloride (from now on referred to as colesevelam). In a clinical trial from 1984, cholestyramine treatment (24 g daily), with a mean follow-up of 7.4 years, was shown to reduce LDL cholesterol by 20 %, which was associated with a 19 % relative risk reduction in myocardial infarction and death by coronary heart disease in male patients with hypercholesterolemia [13].
In 1994, Garg and Grundy reported that 6-week treatment with cholestyramine (8 g daily), in a placebo-controlled, crossover design, reduced mean plasma fasting plasma glucose (FPG) by 13 % in patients with type 2 diabetes, and showed a tendency toward reduction in HbA1c [14]. Three double-blinded, placebo-controlled randomized clinical trials evaluated the efficacy of colesevelam in patients with type 2 diabetes. Bays et al evaluated colesevelam vs placebo as add on to metformin, in a 26-week study, and reported a mean treatment difference in HbA1c of 0.54 % and a reduction in FPG of 13.9 mg/dL [15]; Fonseca et al evaluated colesevelam vs placebo as add-on to sulfonylurea treatment, in a 26-week study, and reported a mean treatment difference in HbA1c of 0.54 % and a reduction in FPG of 13.5 mg/dL [16]; and finally, Goldberg et al evaluated colesevelam vs placebo as add-on to insulin, in a 16-week study, and reported a mean treatment difference in HbA1c of 0.50 % and a reduction in FPG of 14.6 mg/dL [17]. The 3 studies reported reductions in LDL cholesterol between 6.7 % and 15.9 % and increases in serum triglyceride levels between 4.7 % and 21.5 % [15-17]. Similarly, a 24-week clinical trial evaluating colestimide treatment (vs placebo) in patients with type 2 diabetes reported reductions in mean HbA1c (from 7.71 % to 6.97 %) and FPG (from 147.4 to 127.0 mg/dL) [18].
In 2008, the US Food and Drug Administration approved colesevelam for the treatment of hyperglycemia in type 2 diabetes, and recently colesevelam was included in the diabetes management algorithm of the American Association of Clinical Endocrinologist, as add-on to metformin or other first-line therapies [19].
Safety and Tolerability
As aforementioned, BASs are not absorbed in the intestine and therefore lack systemic toxicity, and are not dependent on liver and kidney function [9]. However, BASs are associated with gastro-intestinal adverse events, primarily constipation [15-17]. Whereas first-generation BASs are only charge specific, second-generation BASs act through charge and hydrophobic binding. This results in a higher affinity for bile acids and second-generation BASs are thought to be 4–6 times more potent than first-generation BASs [20], allowing for lower dosing and better tolerability [21]. BASs may increase serum triglycerides, but the clinical implication of this is unknown. A prospective study including data on more than 300,000 people did not find a correlation between serum triglycerides and risk of coronary heart disease after controlling for standard risk factors [22]. However, the use of BASs is contraindicated in patients with serum triglycerides in >500 mg/dL and should be used with caution in patients with serum triglycerides between 300–500 mg/dL. BASs have not been associated with serious adverse events in clinical trials [15-18].
Drug interactions with first-generation BASs have been reported with a wide range of commonly used drugs, such as levothyroxine, warfarin, and digoxin. These interactions are primarily due to reduced absorption, caused by nonspecific binding in the intestine. Colesevelam, however, appears to have no clinically significant effect on the pharmacokinetics of concurrently administered drugs such as statins, digoxin, warfarin, and metoprolol [23].
The Bile Acid Receptors
In recent years, it has become clear that bile acids are signaling molecules with classical endocrine properties and work as complex metabolic modulator of lipid and glucose metabolism, through activation of the bile acid receptors FXR and TGR5.
The Farnesoid X Receptor
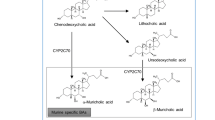
As mentioned above, bile acids regulate their own synthesis by feedback signaling through the nuclear receptor FXR, a ligand-activated transcription factor, in the liver, with the primary bile acid chenodeoxycholic acid being the most potent natural ligand [1, 24-26]. Activation of FXR in the liver increases the excretion of bile acids into the intestine [1, 27], and promote transcription of the inhibitory small heterodimer partner (SHP), which in turn reduce the activity of CYP7A1, the rate-limiting enzyme of the so-called “classic” pathway of bile acid synthesis. Via SHP, FXR activation also inhibits the apical sodium-dependent bile acid transporter in the intestine, resulting in an increased fecal loss of bile acids [27]. Bile acids also activate FXR in the intestine, inducing expression and secretion of fibroblast growth factor 19 (FGF-19). FGF-19 binds to the hepatic receptor complex Fgfr4/β-Klotho, which down regulates CYP7A1 and, thus, bile acid synthesis [28]. Bile acids may also directly suppress CYP7A1 via an FGF-19-independent mechanism [29]. Thus, FXR activation is an important pathway in modulating the enterohepatic circulation and de novo synthesis of bile acids (from cholesterol precursors) and governs the composition of the bile acid pool [1, 27]. Moreover, bile acid activation of FXR is involved in lipid and glucose metabolism [1].
The TGR5 Receptor
The transmembrane G protein-coupled receptor TGR5 is widely expressed in the gastrointestinal tract and associated glands, including human gallbladder epithelium and cholangiocytes [30], several cell types in the liver [31, 32], and the colon and ileum [33•, 34]. Furthermore, TGR5 is expressed in brown adipose tissue [5] and in the central nervous system [35, 36] of mice, and human skeletal muscle [5]. Interestingly, it was recently shown that TGR5 is expressed in mouse and human beta cells, and that activation of TGR5 increases insulin secretion [37]. The most potent natural TGR5 agonist is the secondary bile acid lithocholic acid [2, 34], whereas the more abundant and hydrophilic bile acids deoxycholic acid, chenodeoxycholic acid and cholic acid, are less potent TGR5 activators [2]. Activation of TGR5 results in cyclic adenosine monophosphate (cAMP) synthesis, activation of protein kinase A, and subsequent down-stream effects [2]. TGR5 activation is involved in incretin hormone secretion [3, 4] and possibly energy metabolism [5].
The Glucose-Lowering Mechanisms of Bile Acid Sequestrants
Despite recent years’ efforts, the glucose-lowering mechanisms of the BASs are incompletely understood. Data from in vitro and animal and human studies have suggested several plausible mechanisms behind the blood glucose-lowering effect of BASs. The 3 predominant hypotheses are that (1) BASs alter the bile acid pool composition, (2) BASs improve hepatic glucose metabolism, and (3) BASs increase release of incretin hormones (Fig. 1).
Bile Acid Pool Composition
The fact that individual bile acids activate FXR [24] and TGR5 [34] with different potency and the observation that BASs alter the bile acid pool composition [38], has led to the hypothesis that the latter might explain the improved glycemic control associated with these drugs. Importantly, peripheral venous concentrations of the individual bile acids are well correlated with those in portal blood [39].
Brufau et al showed that colesevelam treatment (3.75 g daily for 8 weeks) increased bile acid synthesis stabilizing the bile acid pool size, and was associated with decreased FGF-19 levels and a more hydrophilic bile acid pool composition. Colesevelam treatment reduced HbA1c by 0.7 % in patients with type 2 diabetes, but the reduction was not correlated to the change in the bile acid pool composition [40], suggesting that the improvement in glucose control is not directly mediated by serum bile acids. Similarly, colestimide treatment (4 g daily) was associated with a more hydrophilic bile acid pool, and again, this change was not correlated to the reductions in HbA1c in patients with type 2 diabetes [18]. Finally, changes in bile acid pool composition following colesevelam treatment (3.75 g for 8 weeks) did not affect resting energy expenditure in patients with type 2 diabetes and matched healthy control subjects [41]. Taken together, these findings do not support alterations in bile acid pool composition as an important pathway in the glucose-lowering mode of action of BASs.
Hepatic Glucose Metabolism
Several studies have tested the hypothesis that BASs improve glucose control by improving hepatic glucose metabolism. Diabetic db/db mice treated with a diet containing 2 % colesevelam for 2 weeks showed increased glucose clearance rate whereas endogenous glucose production was unaffected [42], and in diet-induced obesity mice, a high-fat diet admixed 2 % colesevelam suppressed glycogenolysis [33•]. Furthermore, in hyperlipidemic transgenic mice, a high-fat diet containing 1.5 % colestimide, decreased blood glucose and insulin levels and hyperinsulinemic-euglycemic clamp experiments showed improved insulin sensitivity [43]. Thus, based on these animal studies it appears that BASs indeed do improve hepatic glucose metabolism. However, the majority of clinical studies have not been able to confirm these findings.
Henry et al studied patients with type 2 diabetes, randomized to colesevelam (3.75 g daily) or placebo for 12 weeks. Treatment with colesevelam reduced HbA1c and FPG but treatment differences did not reach statistical significance. Colesevelam had no significant effects on basal endogenous glucose production, insulin sensitivity or on maximal steady-state glucose disposal rate during hyperinsulinemic-euglycemic clamp, and oral glucose tolerance test (OGTT) [44]. In line with this, Schwartz et al measured peripheral insulin sensitivity by the insulin clamp method, and found no difference between colesevelam (3.75 g daily) and placebo in patients with type 2 diabetes. The Matsuda Index increased significantly in the colesevelam group, suggesting improved whole-body insulin sensitivity. The postprandial glucose area under curve (AUC) decreased with colesevelam vs placebo, whereas the AUC for insulin did not change. Moreover, single dose colesevelam had no acute effect on postprandial glucose levels at baseline [45]. In an elegant study using a labeled triple-tracer mixed meal test, Smushkin et al randomized patients with type 2 diabetes to 12 weeks of colesevelam (3.75 g daily) or placebo. Colesevelam did not affect endogenous glucose production or glucose disappearance rate [46•]. This was confirmed by Beysen et al who randomized patients with type 2 diabetes to colesevelam (3.75 g daily for 12 weeks) or placebo. Colesevelam did not affect gluconeogenesis, fasting endogenous glucose production, or glycogenolysis [47]. Thus, in clinical settings it does not appear that BASs improve hepatic glucose metabolism in patients with type 2 diabetes.
Incretin Hormone Secretion
Animal and human studies have shown that administration of bile acids in the colon and rectum, areas rich in L cells, leads to glucagon-like peptide 1 (GLP-1) secretion [33•, 48]. Perhaps counterintuitive, it has been speculated that BASs may also increase GLP-1 secretion. Indeed, after an OGTT, colesevelam (2 %) increased portal GLP-1 levels and protected diet-induced obese rats against hyperglycemia and hyperinsulinemia, compared with rats treated with high-energy diet alone or in combination with an apical sodium-dependent bile acid transporter (ASBT) inhibitor [49]. The same authors reported that treatment of Zucker diabetic fatty (ZDF) rats with a combination of 2 % colesevelam and an inhibitor of dipeptidyl peptidase inhibitor 4 (DPP-4), the ubiquitous enzyme that degrades circulating GLP-1, reduced glucose AUC and increased GLP-1 AUC more than colesevelam alone. This indicates that inhibition of DPP-4 extends the half-life of colesevelam-induced GLP-1 [50]. In another study of ZDF rats, cholestyramine treatment (1.5 % or 4.5 %) dose-dependently decreased serum glucose and HbA1c, and significantly increased postprandial levels of GLP-1, peptide YY, and insulin. Quantitative gene expression analysis indicated that cholestyramine treatment decreased FXR activity in the liver and the intestine. Adding an FXR agonist to the cholestyramine treatment did not reduce the glucose-lowering effect of cholestyramine, suggesting that the FXR-SHP pathway is not required for the glycemic effects of cholestyramine [51].
In a study of TGR5 knockout mice and TGR5 overexpressing mice, 2 % colestimide added to the diet enhanced postprandial GLP-1 secretion and improved glycemic control in TGR5 overexpressing mice, whereas the response was blunted in TGR5 knockout mice. It should be noted, however, that the mice were treated with a DPP-4 inhibitor 60 minutes prior to the mixed meal. The increased GLP-1 secretion was associated with an increased TGR5-dependent transcription of proglucagon mRNA (the precursor of GLP-1) [52•]. This points to TGR5 activation as an essential mediator of BAS-induced GLP-1 secretion. Interestingly, both TGR5 knockout and TGR5 overexpressing mice treated with colestimide lost a significant amount of body weight and almost tripled the weight of their fecal output, without any apparent difference between the 2 phenotypes. Furthermore, treatment with colestimide enriched the feces with phospholipids, glycerol derivatives, and nonesterified fatty acids [52•]. This indicates a TGR5-independent mechanism of the weight loss, potentially by supply of fatty acid receptor agonists (see other mechanisms) to the colon, but it may perhaps also be explained by a degree of malabsorption. Finally, the study showed that Chinese hamster ovarian cells transfected with the TGR5 receptor was robustly activated by colon content from mice treated with colestimide, but only moderately activated by colon content from untreated mice. Furthermore, colon explants from TGR5 overexpressing mice showed GLP-1 secretion when exposed to colon content from colestimide-treated wild-type mice [52•]. These data strongly suggest that TGR5 activation in the colon is a key mediator of GLP-1 secretion during BAS treatment, and that BASs increase the supply of TGR5 agonists to the colon. Most of these findings were confirmed by Potthoff et al who studied mice with diet-induced obesity and found that 2 % colesevelam admixed a high-fat diet, suppressed glycogenolysis, increased GLP-1 secretion, and improved glycemic control. TGR5 knockout mice treated with colesevelam did not increase GLP-1 secretion and only showed a trend toward reduction in plasma glucose, furthermore, plasma insulin was robustly reduced [33•]. Thus, it appears that the TGR5/GLP-1 pathway is responsible for the reduction of hepatic glycogenolysis and partially responsible for the improved hyperglycemia, and may also explain the reduction of hyperinsulinemia, given that TGR5 is expressed in beta cells [37]. Using the GLP-1 receptor antagonist exendin9-39, it was shown that GLP-1 only explains part of the glucose-lowering effect of colesevelam, as exendin9-39 did not completely abrogate the reduction in plasma glucose and the reduction of plasma insulin following colesevelam treatment [33•]. Interestingly, the authors established the concept that bile acids bound to colesevelam are able to activate TGR5 and elicit downstream effects ie, cAMP production and GLP-1 release. Furthermore, the study found the highest levels of TGR5 mRNA in the distal colon and showed that rectal administration of colesevelam preloaded with bile acid taurocholic acid increased portal GLP-1. Finally, it was shown that FXR knockout mice exhibit the same reduced plasma insulin, as observed after colesevelam treatment [33•].
Taken together, data from these animal studies strongly suggest that the glucose-lowering effect of BASs is mediated through deactivation of FXR and activation of the TGR5/GLP-1 pathway. This is in agreement with the findings of Suzuki et al who introduced the concept that BASs increase circulating levels of GLP-1 in humans. In a 1-week, 1-arm study, treatment with colestimide (3.0 g daily) increased 2 hours postprandial GLP-1 levels significantly, whereas there was no difference during fasting or 1-hour postprandially in patients with type 2 diabetes [53]. In line with this, Beysen et al reported that colesevelam (3.75 g daily for 12 weeks) improved glycemic control and increased fasting GLP-1, and postprandial concentrations of total GLP-1 and glucose dependent insulinotropic peptide (GIP), while concentrations of insulin and glucagon were unchanged, compared with placebo [47]. However, it seems that the difference in postprandial GLP-1 and GIP levels were partly due to lower baseline levels in the group treated with colesevelam vs placebo, and other clinical studies have failed to show an effect of BASs on incretin hormone secretion.
In a 12-week study, in adult patients with type 1 diabetes, colesevelam treatment resulted in a significant reduction in HbA1c at week 4. However, this effect was not sustained throughout the study. There was a significant increase in median plasma GLP-1 concentrations during the first 2 hours of the baseline meal test after a single dose of colesevelam vs placebo, but no difference at 12 weeks [54]. In the study by Smushkin et al colesevelam treatment decreased HbA1c, fasting, and postprandial plasma glucose concentrations without decreasing insulin concentrations, which could suggest an incretin-mediated mode of action, however, postprandial GLP-1 concentrations were not altered by colesevelam [46•]. Finally, a recent 8-week study of subjects with impaired fasting glucose, showed that colesevelam (3.75 g daily) improved FPG and HbA1c vs placebo, whereas fasting insulin did not change [55•]. Puzzling, colesevelam did not affect glucose tolerance after an IV glucose tolerance test, whereas incremental AUC (iAUC) for both glucose and insulin decreased with colesevelam following a mixed meal tolerance test, leaving the insulin-to-glucose ratio unchanged. Interestingly, cholecystokinin (CCK) iAUC increased significantly, while glucagon, GIP, and GLP-1 iAUC did not change [55•].
Thus, it is less clear whether BASs increase GLP-1 secretion in humans. The apparent difference between animal and clinical studies may be explained be several factors: One animal study used a DPP-4 inhibitor [50] and another study sampled portal blood [49]. Furthermore, the 2 % (and 4.5 % in 1 study) dosing of BASs in the animal studies may exceed the dose used in the clinical trials; 1 study reported that 2 % colestimide resulted in a daily dose of 0.1 g colestimide, in diet-induced obesity mice with a body weight between 20–30 g [56], which is likely to overestimate the role of GLP-1 in the glucose-lowering mechanism of BASs. It should be noted, however, that GLP-1, after secretion from the L cell, may elicit local effects in the intestine, by stimulation of local afferent sensory nerve fibers, which via the nucleus of the solitary tract and the hypothalamus, could signal to the pancreas [57]. Hence, the effect of BASs may be GLP-1 mediated without increasing peripheral concentrations of circulating GLP-1.
Other Potential Mechanisms
Glucose Absorption
It has been hypothesized that BASs decrease glucose absorption [58]. However, 2 clinical studies using stable isotopes, did not find differences in glucose appearance after treatment with colesevelam in patients with type 2 diabetes [44, 47] suggesting that BASs do not affect glucose absorption. In another clinical study, using triple-tracer mixed meal test, the rate of meal appearance was reported to be significantly decreased by colesevelam in patients with type 2 diabetes, and the authors suggested that colesevelam may increase splanchnic utilization of meal-derived glucose and/or decrease gastric emptying [46•].
Gastric Emptying
Gastric emptying was examined in a clinical 2-week study, showing that colesevelam treatment (3.75 g daily (tablets)) reduced solid food gastric emptying slightly in patients with diarrhea-predominant irritable bowel syndrome [59]. Another study reported that daily cholestyramine (4 g and 12 g (powder), respectively) slowed gastric emptying significantly for liquid meals and reduced appetite in healthy subjects in a dose-dependent manner [60]. The differences between the 2 studies may arise from the different administration-form, and further studies are needed to draw conclusions about the role of gastric emptying in the treatment with BASs.
Energy Expenditure
It has previously been shown that bile acids promote energy expenditure in mice, primarily in brown adipose tissue, by activation of TGR5 [5]. Although this finding was not reproduced by other animal studies [61, 62], it has been hypothesized that BASs may affect energy expenditure. Watanabe et al administered a high-fat diet supplemented with colestimide (2 %) or cholic acid to a mice model of diet-induced obesity. These interventions improved glucose tolerance and decreased triglyceride levels. Furthermore, both colestimide and cholic acid curbed weight gain without increasing fecal lipid excretion or food intake, and indirect calorimetry showed increased energy expenditure accompanied by increased activity in brown adipose tissue [56]. In a similar study, hyperlipidemic transgenic mice, fed a high-fat diet with 1.5 % colestimide for 8 weeks, showed decreased energy expenditure along with decreased body weight, visceral and subcutaneous fat, total cholesterol, and triglycerides levels, while food intake was increased. Furthermore, the treatment was associated with increased fecal excretion of lipids [43]. The significance of these contradictive findings in relation to human physiology is not clear. First of all, both studies found decreased triglyceride levels (a finding also described in db/db mice treated with colesevelam [42]), which is the opposite effect of what consistently has been reported in human trials. Second, the studies reported a weight-lowering effect of BASs, whereas a weight neutral effect has been associated with BAS treatment in human trials [15-17]. In an 8-week clinical study addressing the impact of BAS treatment on energy expenditure, patients with type 2 diabetes and healthy control subjects treated with colesevelam (3.75 g daily) showed no difference in resting energy expenditure measured by indirect calorimetry [41]. Thus, it does not appear that BAS treatment have a major impact on human energy expenditure.
CCK
CCK has been suggested as a potential mediator of the glucose-lowering effect of colesevelam [55•], and studies have shown that cholestyramine increases CCK concentrations [63, 64], while intraluminal bile acids may inhibit CCK release [64]. However, in 1 study examining the effect of colesevelam, the increase in CCK was not correlated with concurrent reductions in glucose, insulin, C-peptide, or glucagon [55•], and in another study, the CKK levels gradually returned to baseline levels during 4-week treatment with cholestyramine [63], questioning CCK as the mediator of the glucose-lowering effect of BASs. Kogire et al examined isolated perfused pancreata from rats, treated with cholestyramine or subcutaneous CCK for 2 weeks, and found that both interventions seemed to exert stimulatory effects on beta cell function, while pancreatic insulin content was not affected, indicating that BASs may increase beta cell glucose sensitivity through a CKK-mediated mechanism [65].
Fatty Acid Receptors
By binding bile acids in the intestine, BASs disrupt micelle formation and shift the absorption of free fatty acids (FFAs) to more distal, and L cell rich, parts of the intestine [52•]. This would allow FFAs to activate fatty acid receptors, such as GPR40, on the L cells in the more distal gut with subsequent release of GLP-1 [66]. This theory was originally proposed by Hofmann [67] and has gained support from others [50], and could also help explain why the animal studies using high fat diets, seem to report a more pronounced effects of BASs treatment. Currently, however, no studies have to our knowledge tested the hypothesis in detail.
Summary and Conclusions
Type 2 diabetes is frequently associated with overweight and dyslipidemia and carries long-term risk of micro- and macrovascular disease [68]. HbA1c is established as an independent predictor of cardiovascular disease [69-71], and individualized glycemic control is key in reducing morbidity and mortality [72]. Treatment of hyperlipidemia with lipid-lowering drugs reduce cardiovascular mortality in type 2 diabetes [68]. Therefore, given the dual glucose and LDL cholesterol-lowering action of BASs, these drugs may be beneficial in the treatment of patients with type 2 diabetes. In line with this notion, cholestyramine has proven to reduce cardiovascular disease (CVD) in patients with hypercholesterolemia [13]. A randomized clinical trial evaluating the effect on CVD in patients with type 2 diabetes is, however, needed to establish the efficacy in this population.
Based on human clinical trials alone, it is difficult to pinpoint the exact mechanism(s) by which BASs promote their glucose-lowering effect, whereas animal studies indicate that at least part of the glucose-lowering effect of BASs is mediated through TGR5 activation on the L cell and subsequent GLP-1 secretion. This mode of action is supported by the fact that colesevelam improve oral glucose tolerance, but not IV glucose tolerance. As mentioned, GLP-1 may stimulate local afferent sensory nerve fibers in the intestine, and via the nucleus of the solitary tract and the hypothalamus, signal to the pancreas [57]. Thus, the glucose-lowering effect of BASs could be GLP-1 mediated, without affecting plasma GLP-1 concentrations in the peripherally circulation. Finally, the hypothesis that BASs may, indirectly, activate fatty acid receptors in the distal part of the intestine, and promote GLP-1 secretion, seems plausible, but studies are needed to address this question.
In conclusion, it appears that the dual-action of BASs is mediated through both FXR deactivation and TGR5 activation. However, the exact mechanisms underlying this interesting pharmacologic concept remain to be fully elucidated, and despite recent years, growing knowledge about the effect of BASs, further studies are warranted to unravel the full potential of this drug class.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi:10.1152/physrev.00010.2008.
Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi:10.1074/jbc.M209706200.
Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–90. doi:10.1016/j.bbrc.2005.01.139.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi:10.1016/j.cmet.2009.08.001.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi:10.1038/nature04330.
Out C, Groen AK, Brufau G. Bile acid sequestrants: more than simple resins. Curr Opin Lipidol. 2012;23:43–55. doi:10.1097/MOL.0b013e32834f0ef3.
Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–37. doi:10.1194/jlr.R024794.
Rosenbaum DP, Petersen JS, Ducharme S, et al. Absorption, distribution and excretion of GT31-104, a novel bile acid sequestrant, in rats and dogs after acute and subchronic administration. J Pharm Sci. 1997;86:591–5. doi:10.1021/js9603820.
Heller DP, Burke SK, Davidson DM, Donovan JM. Absorption of colesevelam hydrochloride in healthy volunteers. Ann Pharmacother. 2002;36:398–403.
Davidson MH, Dillon MA, Gordon B, et al. Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159:1893–900.
Lyons D, Webster J, Fowler G, Petrie JC. Colestipol at varying dosage intervals in the treatment of moderate hypercholesterolaemia. Br J Clin Pharmacol. 1994;37:59–62.
Zieve FJ, Kalin MF, Schwartz SL, et al. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29:74–83. doi:10.1016/j.clinthera.2007.01.003.
The Lipid Research Clinics Coronary Primary Prevention Trial results. II The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–74.
Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in noninsulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Ann Intern Med. 1994;121:416–22.
Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168:1975–83. doi:10.1001/archinte.168.18.1975.
Fonseca VA, Rosenstock J, Wang AC, et al. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–84. doi:10.2337/dc08-0283.
Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–40. doi:10.1001/archinte.168.14.1531.
Neda T, Inukai K, Kurihara S, et al. Hypoglycemic effects of colestimide on type 2 diabetic patients with obesity. Endocr J. 2012;59:239–46.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–36.
Braunlin W, Zhorov E, Smisek D, et al. In vitro comparison of bile acid binding to colesevelam HCl and other bile acid sequestrants. Polym Prepr. 2000;41:708–9.
Steinmetz KL. Colesevelam hydrochloride. Am J Health Syst Pharm. 2002;59:932–9.
Di Angelantonio E, Sarwar N, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi:10.1001/jama.2009.1619.
Donovan JM, Stypinski D, Stiles MR, et al. Drug interactions with colesevelam hydrochloride, a novel, potent lipid-lowering agent. Cardiovasc Drugs Ther. 2000;14:681–90.
Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5.
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8.
Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–53.
Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–71. doi:10.1152/physrev.00027.2002.
Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi:10.1016/j.cmet.2005.09.001.
Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26.
Keitel V, Ullmer C, Häussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785–9. doi:10.1515/BC.2010.077.
Keitel V, Donner M, Winandy S, et al. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi:10.1016/j.bbrc.2008.04.171.
Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi:10.1002/hep.21458.
Potthoff MJ, Potts A, He T, et al. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G371–80. doi:10.1152/ajpgi.00400.2012. This study establishes the concept that bile acids bound to a BAS can activate TGR5.
Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298:714–9.
Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22(814–25):e227–8. doi:10.1111/j.1365-2982.2010.01487.x.
Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–54. doi:10.1053/j.gastro.2012.09.055.
Kumar DP, Rajagopal S, Mahavadi S, et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun. 2012;427:600–5. doi:10.1016/j.bbrc.2012.09.104.
Garbutt JT, Kenney TJ. Effect of cholestyramine on bile acid metabolism in normal man. J Clin Invest. 1972;51:2781–9. doi:10.1172/JCI107100.
Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–31.
Brufau G, Stellaard F, Prado K, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455–64. doi:10.1002/hep.23831.
Brufau G, Bahr MJ, Staels B, et al. Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab. 2010;7:73. doi:10.1186/1743-7075-7-73.
Meissner M, Herrema H, van Dijk TH, et al. Bile acid sequestration reduces plasma glucose levels in db/db mice by increasing its metabolic clearance rate. PLoS ONE. 2011;6:e24564. doi:10.1371/journal.pone.0024564.
Sugimoto-Kawabata K, Shimada H, Sakai K, et al. Colestilan decreases weight gain by enhanced NEFA incorporation in biliary lipids and fecal lipid excretion. J Lipid Res. 2013;54:1255–64. doi:10.1194/jlr.M032839.
Henry RR, Aroda VR, Mudaliar S, et al. Effects of colesevelam on glucose absorption and hepatic/peripheral insulin sensitivity in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:40–6. doi:10.1111/j.1463-1326.2011.01486.x.
Schwartz SL, Lai Y-L, Xu J, et al. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab Syndr Relat Disord. 2010;8:179–88. doi:10.1089/met.2009.0049.
Smushkin G, Sathananthan M, Piccinini F, et al. The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes. 2013;62:1094–101. doi:10.2337/db12-0923. This study suggests that BASs may decrease meal appearance rate.
Beysen C, Murphy EJ, Deines K, et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomized controlled study. Diabetologia. 2012;55:432–42. doi:10.1007/s00125-011-2382-3.
Adrian TE, Gariballa S, Parekh KA, et al. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia. 2012;55:2343–7. doi:10.1007/s00125-012-2593-2.
Shang Q, Saumoy M, Holst JJ, et al. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–24. doi:10.1152/ajpgi.00362.2009.
Shang Q, Liu MK, Saumoy M, et al. The combination of colesevelam with sitagliptin enhances glycemic control in diabetic ZDF rat model. Am J Physiol Gastrointest Liver Physiol. 2012;302:G815–23. doi:10.1152/ajpgi.00295.2011.
Chen L, McNulty J, Anderson D, et al. Cholestyramine reverses hyperglycemia and enhances glucose-stimulated glucagon-like peptide 1 release in Zucker diabetic fatty rats. J Pharmacol Exp Ther. 2010;334:164–70. doi:10.1124/jpet.110.166892.
Harach T, Pols TWH, Nomura M, et al. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep. 2012;2:430. doi:10.1038/srep00430. This study show that BASs promote GLP-1 secretion in a TGR5-dependent manner, and that the colon is the major source of the enhanced GLP-1 secretion.
Suzuki T, Oba K, Igari Y, et al. Colestimide lowers plasma glucose levels and increases plasma glucagon-like PEPTIDE-1 (7-36) levels in patients with type 2 diabetes mellitus complicated by hypercholesterolemia. J Nihon Med Sch. 2007;74:338–43.
Garg SK, Ritchie PJ, Moser EG, et al. Effects of colesevelam on LDL-C, A1c and GLP-1 levels in patients with type 1 diabetes: a pilot randomized double-blind trial. Diabetes Obes Metab. 2011;13:137–43. doi:10.1111/j.1463-1326.2010.01320.x.
Marina AL, Utzschneider KM, Wright LA, et al. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β-cell function. Diabetes Care. 2012;35:1119–25. doi:10.2337/dc11-2050. This study show that colesevelam improves oral but not IV glucose tolerance.
Watanabe M, Morimoto K, Houten SM, et al. Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS ONE. 2012;7:e38286. doi:10.1371/journal.pone.0038286.
Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36) amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–63.
Thomson AB, Keelan M. Feeding rats diets containing cheno- or ursodeoxycholic acid or cholestyramine modifies intestinal uptake of glucose and lipids. Digestion. 1987;38:160–70.
Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–65. doi:10.1016/j.cgh.2009.10.020.
Psichas A, Little T, Lal S, McLaughlin J. Cholestyramine slows gastric emptying of liquids and reduces appetite in healthy subjects. Neurogastroenterol Motil. 2012;24:1095–101. doi:10.1111/j.1365-2982.2012.01988.x.
Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205. doi:10.1677/joe.1.06546.
Vassileva G, Hu W, Hoos L, et al. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J Endocrinol. 2010;205:225–32. doi:10.1677/JOE-10-0009.
Koop I, Fellgiebel A, Koop H, et al. Effect of cholestyramine on plasma cholecystokinin and pancreatic polypeptide levels, and exocrine pancreatic secretion. Eur J Clin Invest. 1988;18:517–23.
Koop I, Dorn S, Koop H, et al. Dissociation of cholecystokinin and pancreaticobiliary response to intraduodenal bile acids and cholestyramine in humans. Dig Dis Sci. 1991;36:1625–32.
Kogire M, Gomez G, Uchida T, et al. Chronic effect of oral cholestyramine, a bile salt sequestrant, and exogenous cholecystokinin on insulin release in rats. Pancreas. 1992;7:15–20.
Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–7. doi:10.2337/db08-0307.
Hofmann AF. Bile acid sequestrants improve glycemic control in type 2 diabetes: a proposed mechanism implicating glucagon-like peptide 1 release. Hepatology. 2011;53:1784. doi:10.1002/hep.24100.
Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi:10.1056/NEJMoa021778.
Gall MA, Borch-Johnsen K, Hougaard P, et al. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44:1303–9.
Agewall S, Wikstrand J, Ljungman S, Fagerberg B. Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Risk Factor Intervention Study Group. Am J Cardiol. 1997;80:164–9.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Ray KK, Seshasai SRK, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomized controlled trials. Lancet. 2009;373:1765–72. doi:10.1016/S0140-6736(09)60697-8.
Compliance with Ethics Guidelines
Conflict of Interest
M. Hansen has received an unrestricted educational stipend from the Novo Nordisk Foundation. D. P. Sonne has received an unrestricted educational stipend from the Novo Nordisk Foundation. F. K. Knop has received research funding from Sanofi-Aventis Deutschland GmbH and lecture fees from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Gilead Sciences, Merck Sharp & Dohme, Novo Nordisk, Ono Pharmaceuticals, Sanofi, and Zealand Pharma. He is part of the Advisory Boards of Eli Lilly Denmark, Bristol-Myers Squibb/AstraZeneca, and Zealand Pharma. He has consulted for AstraZeneca, Gilead Sciences, Novo Nordisk, Ono Pharmaceuticals, and Zealand Pharma. He also has 2 pending patents.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Hansen, M., Sonne, D.P. & Knop, F.K. Bile Acid Sequestrants: Glucose-Lowering Mechanisms and Efficacy in Type 2 Diabetes. Curr Diab Rep 14, 482 (2014). https://doi.org/10.1007/s11892-014-0482-4
Published:
DOI: https://doi.org/10.1007/s11892-014-0482-4