Abstract
Polyunsaturated fatty acids are of particular interest in the nutritional therapy for diabetes, given their potential role in several pathophysiological processes related to cardiovascular disease. Both omega-3 and omega-6 fatty acids are beneficial for improving lipid profiles in healthy individuals and among type 2 diabetic patients: Supplementation with omega-3 fatty acids lowers triglycerides and VLDL-cholesterol. However, they might also increase LDL-cholesterol. Omega-3 fatty acids are, from the latest evidence, not related to mortality and cardiovascular disease. Similarly, glucose control and hypertension, as well as risk of microvascular complications, seem unaffected by omega-3 supplementation. Most studies involved mainly patients with type 2 diabetes, and future research needs to focus on the type 1 diabetic patient. Also, the role of omega-6 fatty acids remains largely unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of type 2 diabetes (T2DM) is increasing worldwide [1]. Diabetes increases the risk of cardiovascular morbidity and mortality [2] through various abnormalities in glucose, lipid, and lipoprotein metabolism, increased platelet aggregation, endothelial dysfunction, and increased risk of cardiac arrhythmia. Diet can play a role in the prevention of T2DM and is also a central component of therapy [3]. Polyunsaturated fatty acids (PUFAs) are of particular interest, given their potential role in several pathophysiological processes related to cardiovascular disease (CVD). Omega-3 fatty acids (FAs) have received substantial attention, initiated by the first studies done by Bang and Dyerberg in Greenland. The two Danish researchers observed that the Inuit diet was high in omega-3 FAs caused by a high intake of traditional marine food [4]. The Inuit suffered mostly from infectious diseases, and not from cardiovascular or metabolic diseases, before the nutrition transition changed the disease pattern among these populations [5]. This led to numerous studies among both healthy individuals and patient groups such as diabetic patients with related cardiovascular risk factors, CVD, mortality, and other endpoints. For patients with CVD, the American Heart Association recommends 1 g of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) daily, preferably by consumption of oily fish [6]. CVD patients are recommended to consume at least 5 %–10 % energy from omega-6 FAs.
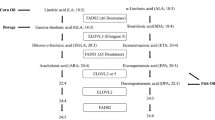
Several omega-3 and omega-6 FAs can be elongated or desaturated endogenously (Fig. 1). In humans, EPA and DHA are synthesized from alfa-linoleic acid (ALA) or are absorbed from the diet. Similarly, linoleic acid as the major dietary omega-6 FA is a source for endogenous formation of longer-chain and/or more unsaturated n-6 FAs. Our aim is to review the evidence for the role of omega-3 FAs and omega-6 FAs in relation to mortality and cardiovascular and microvascular morbidity, as well as intermediate risk factors, among patients with diabetes.
Shows the metabolic process pathway of omega-3 and omega-6 fatty acids. 18:2 n-6 = linoleic acid; 18:3 n-6 = γ-linolenic acid; 20:3 n-6 = dihomo-ɤ-linolenic acid; 20:4 n-6 = arachidonic acid; 22:4 n-6 = adrenic acid; 24:4 n-6 = tetracosatetraenoic acid; 24:5 n-6 = tetracosapentaenoic acid (all-cis-6,9,12,15,18-tetracosapentaenoic acid); 22:5 n-6 = docosapentaenoic acid; 18:3 n-3 = α-linolenic acid; 18:4 n-3 = stearidonic acid; 20:4 n-3 = eicosatetraenoic acid; 20:5 n-3 = eicosapentaenoic acid; 22:5 n-3 = docosapentaenoic acid; 22:6 n-3 = docosahexanoic acid; 24:5 n-3 = tetracosapentaenoic acid (all-cis-9,12,15,18,21-tetracosapentaenoic acid); 24:6 n-3 = tetracosahexaenoic acid
Method
A literature search was conducted in the PubMed database using the key words “diabetes” in combination with the various endpoints within mortality, CVD, microvascular complication, and intermediate endpoint. This was combined with a third search term, which included “fish oil,” “PUFA,” “fatty acids,” “omega-3 fatty acid”/“n-3 fatty acids,” and “omega-6/n-6 fatty acids.” After extraction of literature from databases, we went through the reference lists of reviews to further identify relevant studies. Our aim was to limit the search from references published between 2000 and September 2012; however, papers published before the year 2000 were also included if they provided relevant information. Studies investigating the association between omega-3 FA and omega-6 FA intake were included if they were prospective studies being either observational (cohort studies) or trials, as well as systematic reviews and meta-analyses of such studies. Only studies among type 1 and/or type 2 diabetes patients were included. All randomized controlled trials and meta-analyses included in this review are shown in Table 1. The main outcomes and risk factors are listed in Table 2 with a summary of evidence and main association.
Mortality
A recent meta-analysis of 97 prospective studies with 820,900 individuals estimated that a 50-year-old person with diabetes and with no history of vascular diseases, on average, would die 6 years before a healthy counterpart. Furthermore, the analysis found that fasting glucose above 100 mg per deciliter was associated with a higher risk of death [2].
The Nurses´ Health Study, a prospective cohort among U.S. female nurses, examined the association between fish intake and omega-3 FA and the risk of coronary heart disease (CHD) and total mortality among women with diagnosed type 2 diabetes. For the analysis (based on data from 1980 to 1996) 5,103 women (1,694 prevalent cases and 3,409 incident cases) were included. Higher consumption of omega-3 FA was associated with a lower total mortality (quintile with highest consumption [average of 0.25 g/d] compared with lowest: RR = .63 [95 % CI 0.45; 0.88], p for trend = .02) [7]. As is the case for observational studies in general, residual confounding cannot be excluded as an alternative explanation for the observed associations.
The GISSI Heart Failure study (Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca) (GISSI-HF study), a randomized double-blind, placebo-controlled trial, investigated whether omega-3 FA could improve morbidity and total mortality in patients with symptomatic heart failure (HF) from any cause [8]. A daily dose of 1 g of omega-3 FA (850–882 mg EPA and DHA as ethyl esters in an average ratio of 1:1.2) was administered to participants. In a subgroup analysis restricted to diabetes patients (average of 3.9 years follow-up), omega-3 FAs were effective in reducing all-cause mortality, as compared with placebo (placebo was not defined) (hazard ratio [HR]: 0.89 [95%CI: 0.80–0.99]) [8]. The study had composite endpoints (all-cause mortality and hospitalization) and included patients with type 1 and type 2 diabetes with preexisting HF, which might make generalization difficult. In contrast, in a subgroup of 1,014 patients with diabetes and myocardial infarction (MI) from the OMEGA trial, 40 months of supplementation with either EPA-DHA, or EPA-DHA plus ALA (223 mg EPA, 149 mg DHA, and/or 1.9 g ALA) did not reduce all-cause mortality (EPA-DHA HR: 0.80 [95 % CI 0.47–1.34], p = .39; EPA-DHA plus ALA HR: 0.78 [95 % CI 0.46–1.33], p = .37) [9•].
In the ORIGIN study, participants supplemented with 1 g/d omega-3 FA (465 mg EPA and 375 mg DHA) were compared with those given olive oil (placebo). The ORIGIN follow-up was, on average, 6.2 years. In accordance with the OMEGA trial, the ORIGIN study showed no significant effect of omega-3 FA supplementation on the risk of death from any cause (HR: 0.98 [95 % CI; 0.89; 10.7], p = .63). In addition, risk of death from cardiovascular causes, from arrhythmia, from MI, or from stroke was unaffected [10•]. Thus, the three clinical trials provide conflicting evidence as to whether patients with T2DM may benefit from supplementation with long-chain omega-3 FAs in terms of reduced total or cause-specific mortality. However, it is important to note that the GISSI-HF study, as well as the OMEGA trial, had a shorter follow-up. The participants in all three studies were at high risk of CVD; the OMEGA and GISSI-HF studies included patients with prior event of MI. The endpoint considered in GISSI-HF was a composite endpoint combining total mortality with hospitalization. Thus, despite the variation in the duration of supplementation, the content of EPA+DHA, and the number of participants, the data from the ORIGIN and OMEGA trials strongly suggest that there are no benefits of omega-3 FA <1 g/d on mortality. Nevertheless, as can be seen in the GISSI-HF study, it cannot be ruled out that a larger dose of omega-3 FA could influence mortality among high-risk patients with preexisting HF. We were not able to identify studies that evaluated the relation of omega-3 and -6 PUFA and mortality among patients with type 1 diabetes.
Cardiovascular Disease
The majority of mortality and morbidity among T2DM patients is caused by CVD. HF is the overall dominating event [11]. The effect of omega-6 FA on CVD risk among patients with diabetes is largely unknown. Among nondiabetic individuals, higher intake of omega-6 FA is related to decreased incidence of CHD according to a recent review [12] that summarized the evidence from both intervention studies (n = 9) and prospective cohort studies (n = 12).
The Nurses’ Health Study found that among women with diabetes, a higher intake of long-chain omega-3 FAs was associated with a lower CHD incidence if adjusted for lifestyle and dietary confounders, although not statistically significant (RR: .69, 95 % [CI: 0.47; 1.03], p for trend: .10 for comparison between the highest quintile [0.25 g/d] and the lowest quintile [average 0.04 g/d] of intake) [7]. However, omega-3 FA supplementation showed no significant effect on the rate of major cardiovascular events in the ORIGIN trial (hazard ratio: 1.01 [95 % CI: 0.93; 1.10], p = .81) [10•]. A stratified analysis in the ORIGIN study on the effect of omega-3 FA supplementation further showed that prior CV event (myocardial infarct, stroke or revascularization procedures (cardiac, carotid, and peripheral) did not change the results regarding new cardiovascular event (prior event, 0.99 [0.86; −1.14]; no prior event, 0.95 [0.77; −1.17], p for interaction .70). In the OMEGA trial, incident CVD was decreased with EPA+DHA supplementation, as compared with ALA supplementation or placebo (HR: 0.65 [95 % CI: 0.43; 0.97], p = .04) [13] in an analysis restricted to diabetes patients. Further analysis showed that the rate of fatal CHD was lower among diabetic patients supplemented with EPA-DHA, as compared with placebo (fatal CHD HR: 0.51 [CI: 0.27–0.97], p = .04) [13].
HF is an inability of the heart to provide sufficient pump action to distribute an adequate blood flow. Most often, HF is caused by MI or cardiac arrest. Risk factors include ischemic heart disease, smoking, obesity, hypertension, and the presence of diabetes. Not many studies have evaluated HF as a main outcome. An inverse association between EPA-DHA intake and HF was observed in a diabetic subgroup analysis in the Rotterdam study. The Rotterdam study investigated HF risk over a follow-up of 11.4 years among 479 diabetes patients free from HF at baseline. The omega-3 FA was calculated from food intake assessed with a food frequency questionnaire. The risk of HF was lower for the group with the highest intake of EPA+DHA (>183 mg/d), as compared with the lowest quartile (average <34 mg EPA+DHA/d), although this association was only borderline significant (RR: 0.58 [95%CI: 0.32; 1.06], p = .08) [14]. In the GISSI-HF study, the risk of HF was similar in the EPA-DHA group, as compared with the placebo group (28 % vs. 28.6 %, p = .147). However, this analysis was not restricted to diabetes patients [8]. The ORIGIN study found no effect on HF incidence with omega-3 FA supplementation (RR: 1.02 [95 % CI: 0.88–1.19]) [10•].
MI is caused by inadequate blood supply and results in muscle damage of the heart. Long clinical trials have shown contradictory results: Patients with diabetes and preexisting MI in the OMEGA trial with EPA, DHA, and ALA supplementation were at lower risk of arrhythmia-related events, as compared with placebo (HR: 0.16 [95 % CI 0.04–0.69], p = .01). The supplementation with all three FAs also reduced the composite endpoint ventricular arrhythmia-related events and fatal MI (HR: 0.28 [95 % CI 0.11–0.71], p = .007), but the lower risk for fatal MI alone observed did not reach statistical significance (HR: 0.53 [95 % CI 0.15–1.81], p = .31) [9•]. Similarly, supplementation with only EPA+DHA appeared also to reduce risk of fatal MI, as compared with placebo (HR: 0.66 [95 % CI: 0.22; 2.07], p = .47).
The ORIGIN study found no effect of long-chain omega-3 FA supplementation on risk of fatal and nonfatal MI [10•] (adjusted HR: 1.09 [95 % CI 0.93; 1.27], p = .28).There are several differences between the OMEGA trial and the ORIGIN trial [9•, 10•]: Patients in the ORIGIN trial had impaired glucose tolerance, increased fasting glucose, or diabetes, with a considerable proportion without a prior CVD event, whereas the subgroup analysis in the OMEGA study involved patients with diagnosed diabetes with prior MI. Hence, there is a large difference not only in size of the study population, but also with regard to the level of impaired glucose metabolism and CVD risk between the studies. Furthermore, both studies differ to some extent in the duration (ORIGIN 6.2 years, OMEGA 3.9 years) and in terms of the intervention dose (ORIGIN: 1 g/d and OMEGA: 372 mg/d EPA+DHA). Taken together, the data provide evidence that omega-3 FAs do not protect patients with diabetes or prediabetes against fatal MI in general; however, in high-risk diabetes patients with a history of MI, omega-3 FAs might have beneficial effects.
A stroke is a loss of or a disturbance in blood supply to the brain, causing decreased or ended brain function, and can cause permanent neurological damage, complications, and death. High blood pressure is the most important lifestyle-related risk factor. The only trial on omega-3 FA supplementation among T2DM patients, the ORIGIN trial, showed no significant difference in fatal and nonfatal stoke incidence by supplementation with 1 g/day of omega-3 FA, as compared with placebo (olive oil) (adjusted HR: 0.92 [0.79; 1.08], p = .32) [10•]. Results from the GISSI-HF trial based on analyses among patients with and without diabetes also support the conclusion that omega-3 FAs do not prevent stroke (adjusted HR: 1.16 [95 % CI: 0.89; 1.51], p = .271) [8].
Microvascular Complications
Diabetic Neuropathy
Neuropathy is a common complication of diabetes and increases the risk for other complications, including amputations and food ulcers. Poorer glycemic control, height, and age are factors assumed to increase the risk of neuropathy [15, 16]. Neuropathy is characterized by loss of sensory nerve function, although all nerves, including autonomic nerves, may be damaged [17]. Loss of pain sensation and loss of normal control of skin circulation (required for healing) lead to skin damage and ulceration [17]. Nerve function, conduction velocity and action potential amplitudes, sensory functions, muscle strength, and reflexes were parameters assessed after administration of gamma-linolenic acid (GLA), an omega-6 FA, in two randomized placebo-controlled trials among diabetic patients [18, 19]. In the study by Jamal et al., 22 patients with either T1D or T2DM diabetes received a daily dose of 360 mg GLA (evening primrose oil) or placebo (indistinguishable) capsules for 6 months, and improvements in all the parameters listed above were observed in the GLA group [18]. The study by Keen et al. included 111 patients from seven study centers [19]. The dose of GLA was 480 mg for 12 months, and the patients were evaluated at baseline and at 3, 6, and 12 months. In accordance with Jamal et al., Keen et al. also observed favorable changes in response to GLA, as compared with a placebo group (liquid paraffin). We were not able to identify studies on omega-3 FA and diabetic neuropathy.
Diabetic Nephropathy
According to the American Diabetes Association, diabetes is the most common single cause of end-stage renal disease in Europe and the U.S. Both patients with T2DM and patients with T1D are at risk of nephropathy [20]. In addition, the manifestation of nephropathy is a marker of greatly increased cardiovascular morbidity and mortality for patients with T1DM or T2DM. Risk factors for nephropathy include elevated levels of plasma total cholesterol, high blood pressure, hyperglycemia, high BMI, low levels of HDL-cholesterol, high levels of LDL-cholesterol, smoking, a low socioeconomic status, and male sex [21]. Diabetic nephropathy refers to any deleterious effect on kidney structure and/or function caused by T2DM [22]. Furthermore, nephropathy is defined as a progressive decline in glomerular filtration rate (GFR) in the context of long-standing diabetes, usually accompanied by nephrotic-range proteinuria and other end-organ complications, such as retinopathy [23]. Later, the progressive renal function declines, characterized by decreased GFR, and may result in clinical renal insufficiency and end-stage renal disease. In this way, microalbuminuria (urinary albumin excretion ≥30 mg/24 h–299 mg/24 h) is a symptom of progression toward renal disease.
Whether omega-3 FAs could prevent increased albuminuria is still debated [24]. A meta-analysis evaluated the effect of omega-3 FA supplementation on proteinuria and GFR [25]. In all trials, long-chain omega-3 FAs were supplemented to patients with progressive kidney disease with a single causative disorder (IgA nephropathy, diabetes, or lupus nephritis). Time periods differed from 6 weeks to 12 months, and the number of participants varied from 18 to 45. The pooled effect size among diabetes patients (Cohen´s d and 95 % CI) was calculated for differences in proteinuria or GFR between the omega-3 FA supplementation and control groups. Proteinuria was nonsignificantly reduced (Cohn´s d: −0.21 [95%CI: −0.46; 0.04], p = .10), and the GFR (based on three studies) was similar between groups (Cohn´s d: −0.02 [95%CI: −0.42; 0.38]). It should be noted that the assessment of proteinuria and GFR differed between the studies, which might complicate the pooling of study results. Still, the available evidence from these studies suggests that omega-3 FA supplementation has no effect on renal function in diabetes patients with existing kidney disease. Whether omega-3 FAs prevent the development of nephropathy among diabetes patients with normal kidney function has not been investigated so far.
Intermediate Risk Factors
Blood Glucose Control
Whether omega-3 FAs affect blood glucose control among diabetes patients has been investigated in several randomized trials. A meta-analysis of studies published up to 2008 showed no effect of omega-3 supplementation on plasma glucose or HbA1c [26•]. Similarly, a recent double-blinded placebo (olive oil) controlled trial with 12-weeks duration among 97 T2DM patients without prior CVD found no significant effect of supplementation with 4 g fish oil/d on fasting plasma glucose [27].
Blood Lipids
Dyslipidemia is one of the strongest risk factors for the development of cardiovascular disease among patients with type 2 diabetes. Whether omega-6 FAs are related to dyslipidemia among patients with diabetes has not been investigated in detail. However, trials among healthy individuals strongly support the conclusion that replacing saturated fat with PUFA (largely omega-6 FA) lowers total cholesterol and LDL-cholesterol and leads to a reduction of the total cholesterol:HDL-cholesterol ratio [12].
The effects of omega-3 FAs on blood lipids among diabetes patients have been evaluated in a number of randomized trials, summarized recently in a meta-analysis (studies published until 2008) [26•]. The average dose of omega-3 FA was 2.4 g/d, and average duration 24 weeks. As compared with placebo, omega-3 supplementation resulted in a significant decrease in TG (−0.17 mmol/L [95 % CI: −0.23;−0.11], p < .00001), a nonsignificant decrease in VLDL-cholesterol (supplementation vs. controls: 0.02 mmol [95 % CI −0.06; 0.02], p = .43), and a significant increase in LDL-cholesterol (supplementation vs. control: 0.08 mmol/L [95 % CI 0.02; 0.13], p = .006).
A few trials have been published after the meta-analysis by Hartwig et al. Wong et al. showed, in a study with 97 T2DM patients without prior CVD, decreased TG after 12-weeks supplementation with omega-3 FA (4 g/d), as compared with olive oil (4 g/d) (treatment effect −0.47 mmol/L [95 % CI −0.71;0.23], p < .001) but no significant effects on LDL-cholesterol or total cholesterol [27]. Long-term supplementation with omega-3 FA reduced TG (difference between intervention and controls: 0.16 mmol/L, p < .001) but had no effects on LDL-cholesterol or total cholesterol in the ORIGIN trial [10•]. Thus, while the TG-lowering effect of omega-3 FA appears to be convincing, it cannot be ruled out that higher doses lead to undesired increases of LDL-cholesterol among patients with diabetes.
Regarding HDL-cholesterol, the results are more consistent and show, overall, no effect of omega-3 supplementation [10•, 26•].
Blood Pressure
While it is generally believed that PUFAs, including omega-6 FAs, are beneficial in relation to blood pressure [12], trials on omega-3 supplementation largely indicate no effect. Hartweg et al. found, in their meta-analysis of randomized trials, no effect of omega-3 FA supplementation on systolic blood pressure (effect size: −0.78 mm Hg [95 % CI: −2.74; 1.19], p = .44) or diastolic blood pressure (effect size: −0.79 mm Hg [95 % CI: −1.96; 0.37], p = .18) [26•]. Similarly, in the ORIGIN trial [10•], after a median follow-up of 6.2 years, there was no significant difference between the omega-3 intervention group and the control group in either systolic blood pressure (mean change during intervention period: −4.37 vs. −4.51 mm Hg, p = .75) or diastolic blood pressure (−4.93 vs. −4.96 mm Hg, p = .91).
Carotid Intima-Media Thickness
Carotid intima-media thickness (IMT) is a surrogate marker of atherosclerosis and is a strong predictor of future cardiovascular events. It is a measurement of the thickness of tunica intima and tunica media, two layers of the arterial wall. Systolic blood pressure has been shown to be associated with severe carotid intima-media thickening [28]. IMT is also higher in individuals with diabetes, as compared with the healthy controls (0.13 [95 % CI: 0.12–0.14] mm) [29]. Only one trial has investigated omega-3 FA intake among diabetes patients in relation to IMT. Supplementation with 1,800 mg EPA/d significantly decreased average IMT (mean IMT EPA treatment: −0.029 ± −0.112 mm vs. control: 0.016 ± −0.109, p = .029 ) and max IMT (max IMT EPA treatment: −0.084 ± −0.113 mm vs. control: −0.005 ± 0.108 mm, p = .0008) over 2.1 years in this study conducted in Japan [30].
Platelet Function and Inflammation
According to a meta-analysis of randomized trials [26•], several markers related to platelet aggregation are affected by supplementation with long-chain omega-3 FAs. On the basis of two studies with a total of 64 participants with type 2 diabetes, omega-3 FA supplementation (average dose 2.4 g/d) decreases platelet aggregation to ADP (weighted mean difference: −10.30 %,p = .02) and to collagen (−10.55 %, p < .00001). Also, plasma fibrinogen is reduced by omega-3 supplementation (−0.96 μmol/L, p = .02). In the same meta-analysis, no effect on either C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), or interleukin-6 (IL-6) was observed [26•]. In a more recent randomized, double-blind, placebo-controlled trial conducted among 84 subjects with T2DM (diabetes for at least 2 years), supplementation with omega-3 FA (EPA 1,548 mg; DHA 828 mg; other omega-3 FAs 338 mg/d) reduced TNF-α ( p = .002) and IL-2 (p = .0001), as compared with placebo (sunflower oil 2,100 mg/d), during the 8 weeks of intervention. No significant effect on serum-CRP was detected [31].
Conclusions
For many patients with type 2 diabetes, dietary modification is a common component of advice given by health practitioners. The potential benefit of PUFA has been investigated intensively, but almost exclusively in regard to long-chain omega-3 FAs (Table 2). Dyslipidemia, as one of the main CVD risk factors frequently present in diabetes, is a major target for nutrition therapy. Dyslipidemia in diabetes is characterized by low HDL-cholesterol and high triglyceride levels and higher concentrations of atherogenic lipoprotein molecules, the latter frequently not detectable by elevated LDL-cholesterol. It is very evident that omega-3 FAs can decrease triglycerides with virtually no effect on HDL-cholesterol in patients with diabetes. From this perspective, a supplementation with omega-3 FA might have benefits. Still, although evidence for increases in LDL-cholesterol with omega-3 supplementation is contradictive, it cannot be ruled out that such an undesired effect is present particularly at high doses of supplementation. Whether the triglyceride-lowering effect is beneficial among diabetes patients is questionable. While triglycerides are not directly atherogenic, they are associated with atherogenic remnant particles derived from chylomicrons and VLDL. However, supplementation with omega-3 FAs in diabetes patients seems not to affect the relative proportion of small-dense LDL [26•]. Omega-3 FAs appear to have no effect on other major risk factors for CVD, such as blood pressure, blood glucose and HbA1c, or insulin levels. Evidence for carotid IMT, platelet function, and inflammatory markers was insufficient to draw firm conclusions.
For the major cardiovascular outcomes, the recent evidence indicates that MI might be affected by omega-3 FAs. However, effects could differ between patient groups according to the level of cardiovascular risk. The evidence from prospective studies is heterogeneous in regard to size of study populations, follow-up duration, and dose of supplementation. The most striking difference between the studies, however, is the difference in cardiovascular history. The latter point makes it difficult to clearly conclude benefits of supplementation for diabetic patients in general. Nevertheless, it seems that high-risk patients with former MI could benefit in relation to reinfarction.
Microvascular complications have largely not been studied so far. There is suggestive evidence that omega-3 FAs do not improve kidney function among patients with kidney disease; however, the role of either omega-3 or omega-6 FAs for the prevention of nephropathy in diabetes patients remains unclear. Diabetic neuropathy has been considered only in terms of the benefits of supplementing omega-6 FAs, while we were not able to identify studies on diabetic retinopathy. Nonetheless, given that omega-3 FAs are unlikely to affect blood glucose control and blood pressure, it seems also unlikely that they have a profound effect on risk of microvascular complications.
Furthermore, future supplementation studies, no matter the outcome, should carefully consider appropriate study power, duration of supplementation, and dosage of omega-3 FA or omega-6 FA supplementation. Some studies have had very small study populations, which limits the power of the studies.
The literature is dominated by studies on omega-3 FAs, providing very little evidence for omega-6 FAs. Omega-6 FAs have not been systematically studied among type 2 diabetes patients, except for a few studies evaluating GLA, which is not a typical dietary FA. However, among healthy subjects, omega-6 FAs are beneficial in relation to LDL- and total cholesterol, especially when substituted for carbohydrates or saturated fat. Considering that omega-6 Fas are considered to be proinflammatory, these Fas should be further investigated. Furthermore, research is underrepresented regarding patients with type 1 diabetes. While diabetes patients most likely benefit from substitution of saturated fat by PUFA in their diet in terms of a less atherogenic lipid profile and lower CVD risk, a supplementation with omega-3 FAs might be relevant only for sub/groups of patients with preexisting CVD events.
Abbreviations
- FA:
-
fatty acids
- EPA:
-
eicosapentaenoic acid
- DHA:
-
docosahexaenoic acid
- ALA:
-
α- linoleic acid
- PUFA:
-
polyunsaturated fatty acids
- T1D:
-
type 1 diabetes
- T2DM:
-
type 2 diabetes
- CVD:
-
cardiovascular disease
- MI:
-
Myocardial infarction
- CHD:
-
coronary heart disease
- TIA:
-
transient ischemic attack
- DN:
-
Diabetic nephropathy
- GFR:
-
glomerular filtration rate
- LDL:
-
low density lipoprotein
- VLDL:
-
very low density lipoprotein
- HDL:
-
high density lipoprotein
- IMT:
-
Carotid intima-media thickness
- ESRD:
-
End-stage renal disease
- UPE:
-
urinary protein excretion
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
IDF: http://www.idf.org/diabetesatlas. International Diabetes Federation (2011); 5. Accessed Nov 2012.
Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41.
ADA. http://www.diabetes.org/. American Diabetes Association (2012). Accessed Nov 2012.
Dyerberg J. H.O B. Fatty acid composition of the plasma lipids in Greenland Eskimos. Am J Clin Nutr. 1975;28:958–66.
Young TK, Bjerregaar P, editors. Health transitions in arctic populations. Toronto: Toronto Press; 2008.
AHA. American Heart Association: http://www.heart.org/HEARTORG/General/Frequently-Asked-Questions-About-Fish_UCM_306451_Article.jsp (2012). Accessed Nov 2012.
Hu FB, Cho E, Rexrode KM, et al. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107(14):1852–7.
Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–30.
• Kromhout D, Geleijnse JM, de Goede J, et al. n-3 fatty acids, ventricular arrhythmia-related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes Care. 2011;34(12):2515–20. Contains a sub-group analysis of the two-by-two factorial deigned OMEGA trial including 1014 high-risk diabetic patients with previous MI. The participants were supplemented with 223 mg EPA + 149 mg DHA and/or 1.9 g ALA. The study found a protective effect against ventricular arrhythmia related events and fatal MI.
• ORIGIN JB, Gerstein HC, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–18. A large randomized supplementation trial among 12,536 patients with impaired glucose tolerance and type 2 diabetic patients with a follow-up period of 6.2 years. The participants received 1 g omega-3 FA/d (465 mg EPA and 375 mg DHA) or placebo (olive oil 1 g/d). The study found no significant effect on rates of major vascular events, death from any cause or death from arrhythmia. Only triglycerides were reduced in the omega-3 FA group compared to olive oil, but no other lipids were affected.
AHA. American Heart Association: http://www.heart.org/HEARTORG/Conditions/Diabetes/WhyDiabetesMatters/Cardiovascular-Disease-Diabetes_UCM_313865_Article.jsp. Accessed December 2012.
Czernichow S, Thomas D, Bruckert E. n-6 Fatty acids and cardiovascular health: a review of the evidence for dietary intake recommendations. Br J Nutr. 2010;104(6):788–96.
Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–26.
Dijkstra SC, Brouwer IA, van Rooil FJA, et al. Intake of very long chain n-3 fatty acids from fish and the incidence of heart failure: the Rotterdam Study. Eur J Heart Fail. 2009;11:922–8.
Adler AI, Boyko EJ, Ahroni JH, et al. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20(7):1162–7.
Bruce SG, Young TK. Prevalence and risk factors for neuropathy in a Canadian First Nation community. Diabetes Care. 2008;31(9):1837–41.
Horrobin DF. Essential fatty acids in the management of diabetic neuropathy. In: Huang Y-S, Mills DE, editors. Gamma-Linolenic Acid Metabolism and Its Roles in Nutrition and Medicine. Champaign, Illinois: AOCS Publishing; 1996. doi:10.1201/9781439831939.ch21
Jamal GA, Carmichael H. The effect of gamma-linolenic acid on human diabetic peripheral neuropathy: a double-blind placebo-controlled trial. Diabet Med J Br Diabet Assoc. 1990;7(4):319–23.
Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16(1):8–15.
Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S79–83.
Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158(9):998–1004.
Evans TC, Capell P. Diabetic Nephropathy. Clin Diabetes. 2000;18. http://journal.diabetes.org/clinicaldiabetes/v18n12000/Pg7.htm. Accessed December 2012.
O'Connor AS, Schelling JR. Diabetes and the kidney. Am J Kidney Dis. 2005;46(4):766–73.
Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105(3):428–40.
Miller ER, Juraschek SP, Appel LJ, et al. The effect of n-3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: meta-analysis of clinical trials. Am J Clin Nutr. 2009;89(6):1937–45.
• Hartweg J, Farmer AJ, Holman RR, et al. Potential impact of omega-3 treatment on cardiovascular disease in type 2 diabetes. Curr Opin Lipidol. 2009;20(1):30–8. A meta-analysis on omega-3 FA treatment among T2DM patients on cardiovascular risk factors. The analysis pooled data from 24 trials from 1966–2008 involving 1533 patients receiving 2.4 g omega-3 FA/d for average 24 weeks. The study found that triglycerides, fibrinogen, ADP platelet aggregation and collagen were decreased with omega-3 supplementation whereas LDL-cholesterol was increased.
Wong CY, Yiu KH, Li SW, et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with Type 2 diabetes mellitus. Diabet Med J Br Diabet Assoc. 2010;27(1):54–60.
Rajala U, Paivansalo M, Laakso M, et al. Associations of blood pressure with carotid intima-media thickness in elderly Finns with diabetes mellitus or impaired glucose tolerance. J Hum Hypertens. 2003;17:705–11.
Brohall G, Schmidt C, Behre CJ, et al. Association between impaired glucose tolerance and carotid atherosclerosis: a study in 64-year-old women and a meta-analysis. Nutr Metab Cardiovasc Dis. 2009;19(5):327–33.
Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191:162.
Moghadam M, Saedisomeolia A, Djalali M, et al. Efficacy of omega-3 fatty acid supplementation on serum levels of tumor necrosis factor- alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singap Med J. 2012;53:615–9.
Acknowledgment
The study was supported by the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD eV) and a grant from the German Research Foundation (DFG, SCHU 1516/5-1).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeppesen, C., Schiller, K. & Schulze, M.B. Omega-3 and Omega-6 Fatty Acids and Type 2 Diabetes. Curr Diab Rep 13, 279–288 (2013). https://doi.org/10.1007/s11892-012-0362-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-012-0362-8