Abstract
Vitamin D is a secosteroid hormone that resembles other nuclear steroid hormones such as thyroid, gluco-, and mineralocorticoids, as well as gonadal effector systems. Primarily understood as a master regulator of bone and calcium/phosphate physiology, it is now increasingly recognized as orchestrating numerous aspects of cell growth and differentiation in many tissues, including those of innate and acquired immunity. This review addresses recently discovered aspects that highlight vitamin D´s potential for immune intervention and how the vitamin D pathway is utilized for anti-infective and antineoplastic immunity. This provides the rationale for novel therapeutic strategies in the context both of prevention and of therapy of immune dysregulation in type 1 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes is an immune-mediated disease where environmental factors and genetic susceptibility interact in the pathophysiology of selective ß-cell destruction. This is similar to other autoimmune diseases that share with type 1 diabetes some of the predisposing gene loci, as well as vitamin D deficiency [1•]. Since genetic susceptibility cannot be altered and is difficult to circumvent, environmental determinants have been the focus for possible preventive action, particularly those with immune effects such as vitamin D (reviewed in [2]).
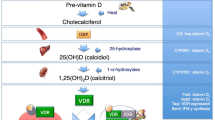
Vitamin D is derived from dietary sources or is synthesized in the skin as the precursor 7-dehydrocholesterol, which then requires conversion to active metabolites. After being converted in the liver to 25-hydroxyvitamin D3 (25D), the major circulating form of vitamin D, it is further activated to the most active metabolite 1,25-dihydroxyvitamin D3 (1,25D) in the kidney or in other cells, such as macrophages and monocytes. The half-life of 1,25D is 4–6 h; that of 25D, however, is as long as 2–3 weeks. Vitamin D circulates as 25D, largely bound (99 %) either to the vitamin D binding protein (DBP; also known as GC protein, which amounts to 80 %–90 % of circulating vitamin D) or to albumin (10 %–20 %). It is then taken up by cells to be activated by 1α-hydroxylation and subsequent binding to the nuclear vitamin D receptor (VDR), which, in turn, activates vitamin D response elements within target genes, whose transcription is then regulated. These genomic effects have long been considered to be the only mode of vitamin D action; it is now apparent that some cells can be targeted by vitamin D, using a membrane effector route prior to intracellular signaling, mediating, for example, photoprotection of the skin [3] or calcium increase in spermatozoa [4]. Whether this nongenomic action is also used in macrophages and T-lymphocytes and, if so, how is unclear at present.
Epidemiologic Evidence Suggesting an Association Between Vitamin D Deficiency and Type 1 Diabetes
Vitamin D deficiency, defined by a serum level of <20 ng/ml (50 nmol/l), is a growing health issue worldwide, with more than 50 % of the population at risk [5]. This has several clinical implications not only for bone health, but also for infections and malignant and immune-mediated disorders [6]. Recent recommendations address adequate vitamin dosing to combat its deficiency [7, 8] and distinguish the vitamin D requirements for bone health and protection from falls (targeting vitamin D levels at 12–16 ng/ml) from the potential benefit of higher doses for other outcomes, which are still subject to ongoing studies.
Large cohort studies also confirmed a significant association of vitamin D deficiency with type 1 diabetes, showing 81.6 % of patients in the U.K. to be deficient in summer and 96.1 % in winter [1•]. Whether this vitamin D deficiency is a primary defect or secondary after chronic disease is uncertain, but a recent large cohort study from the U.S. military on soldiers showed a 3.5 times higher risk to develop type 1 diabetes if 25D levels were <17 ng/ml prior to diagnosis, as compared with >28 ng/ml (Doerr Gorham E, Garland CF, Burgi A, et al., unpublished data). Earlier studies on smaller cohorts had also reported a high prevalence of vitamin D deficiency in newly diagnosed children or adolescents with type 1 diabetes in Italy [9] and across a wider spectrum of ages at manifestation in Sweden [10]. Similar observations have been reported for multiple sclerosis, where lower 25D levels are found before manifestation [11].
Areas of high incidence rates both of type 1 diabetes and of multiple sclerosis lie in geographical regions of low UV exposure correlating with less cutaneous vitamin D synthesis [12]. Furthermore, high-dose vitamin D supplementation in early childhood was associated with lower incidence rates of type 1 diabetes in Scandinavian countries (reviewed in [13]). Latitudinal gradients in both the northern and southern hemispheres show a higher risk for T-helper 1 mediated disorders such as type 1 diabetes, rheumatoid arthritis, or multiple sclerosis, and UV irradiation may down-regulate cellular immune responses—particularly, T-helper 1 [14]. A recent survey from Australia shows that the age and sex standardized prevalence rates of type 1 diabetes correlate positively with the latitude, increasing 2.97-fold over the north–south gradient, and is inversely related to the regional average solar-noon UV radiation [12]. In Australia, it was also reported that the UV association with type 1 diabetes was influenced by the population density, where rural area better reflected the individual´s UV exposure than did densely populated regions [15]. Similar observations have been reported from Canada [16], where incidence rates are high and even increasing [17]. Since type 1 diabetes' incidence is reported to have increased in many countries worldwide, it can be speculated that the growing problem of vitamin D deficiency may be explained either by reduced dietary intake or by less exposure to UV irradiation. Recent data from the Frankfurt am Main/Hesse region show that declining UV radiation in summer months leads to more pronounced vitamin D troughs in diabetes patients during that period (Langer et al., unpublished data).
The vitamin D status has also been investigated in long-term diabetic complications. A recent survey from Australia involving over 500 pediatric patients with type 1 diabetes showed that there was a significant association of vitamin D deficiency with the risk for diabetic retinopathy; the relative risk conferred was higher than that of diabetes duration or HbA1c levels [18]. Taken together, both vitamin D deficiency and major genes controlling vitamin D status show significant associations with type 1 diabetes and other autoimmune disorders. These observations have supported the concept that vitamin D plays an important immune regulatory role.
Genetic Control of Vitamin D Levels and Link with Autoimmune Disease
Vitamin D status not only is regulated by the environment, but also, to a considerable extent, is inherited (heritability estimate of 28.8 %) [19–21]. Two genome-wide association studies (GWASs) have shown that vitamin D status, as determined by serum levels, is also controlled by gene loci whose proteins are involved in the vitamin D pathway: (1) the gene GC, encoding DBP; (2) CYP2R1, encoding the enzyme catalyzing 25-hydroxylation of the precursor in the liver; (3) CYP24A1, encoding 24-hydroxylase, which deactivates 1,25D to 24,25D3 with less receptor potency; and (4) DHCR7, encoding 7-dehydrocholesterol reductase, an enzyme involved in the early steps of cholesterol synthesis that is independent of the cytochrome p450 reductase. Taken together, variation in these genes accounts for 1 %–4 % of vitamin D level variability [22•, 23]. It may also explain why some individuals remain vitamin D deficient despite supplementation, whereas others have a sufficient concentration without any intake.
Interestingly, three out of these four genes (GC, CYP2R1, and DHCR7) show significant associations with type 1 diabetes both in family and in case/control studies from the U.K. [1•]. A fourth gene, CYP27B1, encoding 1-α-hydroxylase activating the final step to the 1,25D3, which has already been identified as conferring susceptibility to type 1 diabetes [24] and Addison´s disease [25], is also implicated in type 1 diabetes. In addition, it has been shown that CYP27B1 is associated with other autoimmune disorders, such as multiple sclerosis [26, 27].
It is unclear how genetic variation in this steroidogenic enzyme can lead to functional differences in different tissues. 1-α-hydroxylase controls tissue-specific activation of vitamin D and, therefore, the abundance of local receptor binding, whereby the physiological processing of the enzyme in the kidney and macrophages underlies a complex orchestration involving splice variants of the CYP27B1 gene derived from intronic sequences. These are capable of affecting protein synthesis. Transfection experiments with renal tubular as well as monocytic cells, using splice variants, suggest that such nonclassical regulation is recruited as a complementary route to fine-tune tissue-specific vitamin D action [28]. Such alternative routes of vitamin D activation serve also as potential therapeutic targets.
Genomic Vitamin D Actions
The vitamin D system is increasingly understood as a highly complex endocrine, paracrine, and autocrine regulator of cell growth, differentiation, and tissue-specific action. Most cells express the VDR, and many also the machinery to activate vitamin D metabolites locally, as, for example, macrophages, dendritic cells, keratinocytes, and proximal tubular cells in the kidney (reviewed in [29]). The vitamin D system has been shown to strongly regulate the immune system at both the innate (macrophages, dendritic cells) and the acquired (T- and B-lymphocytes) levels (reviewed in [30]). These immune effects have been studied in great detail; two studies have been published that address the DNA binding locations of the VDR with and without ligand in human cells. The first study of chromatin immunoprecipitation of lymphoblastoid cell lines followed by massively parallel DNA sequencing (ChIP-seq) revealed that vitamin D is involved in the transcriptional regulation of a large number (n = 229) of genes. In this study, vitamin D bound gene locations were associated with polymorphisms that had been identified in GWASs for autoimmune disorders [31•]. Such regions included IRF8 and PTPN2 and are known to confer susceptibility to autoimmune disorders such as multiple sclerosis, Crohn´s disease, and type 1 diabetes. Whereas the first study used an incubation period of 36 h in lymphoblastoid cells, the second incubated monocytes for only 40 min [32•], identifying 638 1,25D/VDR target genes. Their gene ontology identified pathways in immunity and signaling, pointing to vitamin D´s role in these systems, which are of potential therapeutic interest in cancers and in immune-mediated disorders, including type 1 diabetes. Vitamin D effects are mediated by the VDR, but also by the recruitment of metabolizing enzymes. Many cell types express not only the VDR, but also the enzyme 1-α-hydroxylase, encoded by the CYP27B1 gene, which allows the tissue-specific up-regulation of 1,25D formation. A locally regulated autocrine/paracrine pathway thereby allows fine-tuned adaptation of cell-specific vitamin D actions. One rare, naturally occurring genetic mutation illustrates that loss of vitamin D action may lead to type 1 diabetes: A child with the rare vitamin D-resistant rickets was identified with manifest type 1 diabetes. This child has a mutant VDR with compound heterozygosity (L263R and R391S) causing dissociated responses of the CYP24A1- and rel-B promoters to 1,25D, thus impairing this regulation in all VDR expressing cells [33]. Therefore, the child lacks an important endocrine pathway, affecting not only bone, but many cell systems.
Vitamin D Effects on Innate and Acquired Immunity
Vitamin D deficiency is strongly associated with bacterial infections, the most frequently investigated being the tuberculous and leprous mycobacterial pathologies (reviewed in [34•]). In vitro experiments have shown how antibacterial action can be improved by vitamin D replenishment.
For the innate immune response, vitamin D regulates monocyte responses to bacterial infections through induction of cathelicidin expression, thereby promoting autophagy [35]. Furthermore, the pathogen recognition system of the toll-like receptor 1 and 2 (TLR 1, TLR 2) leads to 1,25 vitamin D production and activation of the intracellular NOD2 (nucleotide-binding oligomerization domain containing 2). Interleukin 1 can be released from monocytes in order to act on adaptive immune cells (T- and B-lymphocytes). Thus, vitamin D opens an antimicrobial pathway not only in tuberculous infections, but also in leprous and, most likely, in other bacterial infections [34•]. Since the VDR and vitamin D enzymes are expressed at several barrier sites of the body (skin, placenta, intestinal mucosa, alveolae), the vitamin D system has been linked to membrane stabilization and is thought to constitute part of the first-line defense against microbial invasion [36].
For the acquired immune response, T cells undergo functional avidity maturation in order to specify their antigen responsiveness. Their signaling pathways use vitamin D receptor expression and vitamin D action in order to increase their sensitivity to antigen stimulation [37•], as has been shown for the key signaling protein phospholipase C gamma1 (PLC-γ1). Induction of the VDR leads to up-regulation of PLC-γ1 in mature T cells, while naive T cells do not even express VDR. Furthermore, vitamin D deficient patients had a lower proliferation index of T cells after stimulation than did T cells from healthy volunteers with normal 25D and 1,25D concentrations, an impairment that could be overcome by in vitro replenishment of 1,25D. This underlines the importance of a replenished vitamin D status for a normalized PLC-γ1 signaling and full activation function of T lymphocytes in humans. VDR signaling was also functional in the alternative p38 signaling pathway, allowing naive T cells to recruit the VDR. This vitamin D action in the early phases of T lymphocyte division after antigen recognition has been interpreted as fine-tuning of an early lag phase to control infection and to reduce the risk of an overreacting immune response.
How in vitro treatment of human T cells effectively triggers VDR signaling was explained by Baeke et al. [38], showing that 24-hyroxylase induction leads to an inhibition of cytokines IFN‐γ and IL-10, as well as modulation of chemokine receptors CCR10 and CLA, in T lymphocytes. Observations using in vivo treated NOD mice could be confirmed in the human setting, illustrating that vitamin D treatment is capable of altering the immune phenotype of the effector cell compartment in type 1 diabetes. Furthermore, in mouse T lymphocytes, 1,25D may down-regulate IL-17A by direct and indirect mechanisms, the latter also involving an up-regulation of FOXP3, the transcription factor for T regulatory cells [39].
Mechanisms of Action: How Does Vitamin D Exert its Antiproliferative and Immunomodulatory Effects?
Recent studies by Thomas Lisse and Martin Hewison have found a novel mechanism that integrates both cell proliferation and immunomodulation: Vitamin D plays a role in mTOR signaling, a central pathway integrating many pivotal cellular functions [40•]. It could be shown that 1,25D can also regulate the master regulator of cell function—in particular, the mammalian/mechanistic target of rapamycin (mTOR) signaling pathway by stimulation of DNA-damage-inducible transcript 4 (DDIT4). DDIT4 is regulated in development and by DNA damage response 1 (REDD1) and can potently suppress mTOR activity. The mTOR signaling path itself has complex intracellular effector pathways, by which the mTOR serine/threonine kinase mediates autophagy (inhibition) and cell growth (stimulation) through complex 1 and 2 (mTORC1/2). An up-regulation of DDIT4 by 1,25D may result in reduced cell proliferation and growth [41]. mTOR signaling integrates a large variety of signals, such as nutrients, growth factors, energy consumption and expense, cellular stress, and the immune response, and since studies from several species, including mammals, have suggested a role in longevity, it is provoking to speculate that 1,25D's ability to upregulate DDIT4 and suppress mTOR could provide the rationale for an antiaging application. Due to this potency, vitamin D has a pivotal role in cellular responses and can be used for new immune intervention strategies.
A possible mechanism for vitamin D action is through noncoding microRNAs. They were detected in 1993 and are derived from intronic and exonic gene regions. They are known to be involved in the regulation of several cellular processes, including immunity. miRNA-326 is expressed more strongly in type 1 diabetes patients that were GAD-Ab positive, in comparison with Ab-negative patients [42]. This miRNA had been found earlier to be involved in T-helper 17 activity in multiple sclerosis [43]. Interestingly, one of the potential targets of miRNA-326 is the VDR; however, that assumption needs experimental proof as yet. A possible anti-inflammatory mode of action is also conceivable. Several intervention studies using vitamin D have targeted immune markers. A recent double-blind randomized trial in 75 patients with coronary heart disease failed to show an improvement in endothelial function but demonstrated a significant reduction in C-reactive protein levels at a dose equivalent of 1,800 U/d cholecalciferol [44].
Effects of Vitamin D Supplementation: Prevention in the Animal Model and Effects on Adaptive Immunity in Humans
The potential of vitamin D to prevent type 1 diabetes in the NOD mouse model was first demonstrated by Chantal Mathieu, showing a fourfold reduction of diabetes manifestation after preventive treatment with 1,25 D [45•]. This concept has now been used also in other animal models of autoimmunity, such as induced arthritis or experimental autoimmune encephalomyelitis [46–49], where the protective effect of vitamin D treatment was explained by its effects on T helper 17 lymphocytes and their cytokines [38, 39].
Recently, the same group showed that a vitamin D analogue (TX527) in combination therapy of an NOD islet transplantation model prevents recurrent insulitis by co-immunosuppression with low doses of anti-CD3 and cyclosporin A [50]. Another vitamin D analog, calcipotriol, was used in mice with a topical application and led to an expansion of antigen-specific T regulatory cells [51].
Few studies have investigated the adaptive-immunological sequelae of vitamin D treatment in humans. An Austrian study on 46 healthy volunteers found a significant increase in percentage of T regulatory lymphocytes following supplementation with 140 000 U twice 12 and 8 weeks previously, although the number of T regulatory lymphocytes and the vitamin D levels did not correlate [52]. This increase of T regulatory cells may lead to an improved suppression of local T effector lymphocytes at the pancreatic ß-cell and the draining lymph node. The kinetics of T regulatory lymphocytes in this study showed a decline 4 weeks after the second dose of vitamin D; it is, however, unclear whether a specific dose is critical or whether tachyphylaxis occurred after two shots of a high dose. It is not understood whether the difference may be due to variation in conversion rates, since the 1,25D levels had not been measured. Regulatory T cells mostly originate in the thymus, and this profile is primed through epigenetic effects in utero; thus, the vitamin D status of the mother during pregnancy may be decisive for the offspring´s immunity. This could be demonstrated for invariant natural killer T-lymphocytes (iNKT) in mice that were depleted of vitamin D during development in utero, and this reduction of iNKT cells could not be corrected later in life with vitamin D interventions [53•]. iNKT cells are crucial for a healthy immune system, and this vitamin D requirement early in thymic development highlights the epigenetic effect of vitamin D that needs to be viewed separately from the direct action. Nevertheless, studies of iNKT cells in humans have not been carried out, but low maternal vitamin D levels during pregnancy increased the child´s risk of developing type 1 diabetes [54], and, reversely, vitamin D intake of mothers throughout pregnancy reduced the rate of islet autoantibodies in offspring by half (OR 0.49), as reported from the DAISY study (Diabetes Autoimmunity Study in the Young) [55]. The same study, however, found that neither vitamin D intake nor vitamin D levels in childhood affected the risk of developing type 1 diabetes or its antibodies [56]. Moreover, a pilot trial using 1,25D in patients recently diagnosed with type 1 diabetes found no improvement of residual insulin secretion [57].
Open Questions
Vitamin D and the Free Hormone Hypothesis
Vitamin D is bound to DBP and, to a lesser extent, to albumin, so that only around 1 % is freely available. Fluctuations in the concentration of unbound vitamin D have been reported during periods of acute illness, trauma, or surgery. The unbound vitamin D cannot be measured by routine assays; the biochemical screening of total 25D, DBP, and albumin has to be performed and an algorithm applied to give the free 25D levels [58•]. This analysis can be used to analyze monocyte responses to both 25D and 1,25D that depend on the free vitamin D concentration. It is unclear, however, whether the free hormone concentrations lead to different biological actions apart from gross changes in protein balance (such as protein loss or hypoalbuminemia in hepatic or renal disorders).
Vitamin D and Pregnancy
The pathophysiology leading to type 1 diabetes may start already in utero, thereby marking the mother´s pregnancy a critical period [59]. During pregnancy, many mothers suffer from vitamin D deficiency, as well as hypocalcemia; however, their optimal treatment is subject to debate. Vitamin D has been found to be decreased in severe early onset preeclampsia and smallness for gestational age after controlling for hypertension pregestational diabetes, lupus, kidney disease, and smoking, in comparison with normal delivery and fetal growth [60]. An ongoing trial is investigating whether supplementing with 2,000 or 4,000 U/d cholecalciferol can improve pregnancy outcome both for the mother and for the newborn. These investigators have set a threshold of 25D serum levels at 32 ng/ml, which differentiates the outcome parameters and is just below the level of 40 ng/ml, at which the concomitant increase of 1,25D starts to level off [61].
Can Vitamin D Treatment Cause Harm? A Place for Individualized Medicine?
Hypercalcemia has been feared as the classical adverse event in vitamin D supplementation. A recent documentation of a case report and a literature review provided evidence that vitamin D induced hypercalcemia was reported if comorbidity existed, mostly in granulomatous but also in inflammatory and in some neoplastic conditions [62•], which needed to be treated for the underlying cause. This led the authors to conclude that high-dose vitamin D supplementation may unmask these conditions and, therefore, calcium screening is mandatory for vitamin D intervention trials. Vitamin D supplementation with cholecalciferol is considered to be safe if the adequate dose is chosen. Rarely, hypercalcemia may be due either to severe inflammatory disorder or to a rare variant or mutation of the CYP24A1 gene that has been discovered as the cause of idiopathic hypercalcemia in infants [63•], where defective vitamin D inactivation is expected in the order of 1 %. Thus, some hypercalcemias are to be expected from higher dose supplementation regimens once larger groups of patients are enrolled over sufficient time periods. Ongoing pharmacogenetic studies will determine whether patients at risk can be identified by genetic screening.
Vitamin D levels show not only seasonal, but also geographical and ethnical variation, and in addition, the physiological capacity to both synthesize and resorb vitamin D through nutrition differs among individuals. Therefore, it has been argued that doses for supplementation may need to be adjusted for personalized profiles including age, gender, regions, and the underlying disorder, as well as the genetics of vitamin D pathway polymorphisms. Studies addressing the differential impact of such SNPs on the synthesis of vitamin D metabolites are underway in type 1 diabetes, as well as in colorectal cancer patients.
ß-Cell Effects of Vitamin D
Metabolic effects of vitamin D have also been investigated on hepatic and muscular insulin action, as well as on ß-cell secretion. Increasing 25D levels from 10 ng/dl to 30 ng/dl can improve insulin sensitivity by 60 % [64]. In addition, vitamin D facilitates the biosynthetic capacity of ß cells and accelerates the conversion of proinsulin to insulin [65]. Furthermore, earlier experiments in vitamin D deficient animals showed that insulin secretion was impaired and gradually restored after vitamin D supplementation [66, 67]. Mice with a mutant, nonfunctioning VDR have an impaired insulin secretion and glucose tolerance, thus supporting the hypothesis for a role of the VDR in the endocrine function of the ß-cell [68]. Taken together, this line of evidence suggests that both insulin sensitivity and secretion may benefit from vitamin D repletion and, therefore, improve glycemia.
Conclusions
Animal models, human genetic data, and the deficiency of both innate and adaptive immunity in vitamin D depletion, with reversal on repletion, as well as epidemiologic data, provide indirect evidence on how vitamin D is involved in the immune pathophysiology of type 1 diabetes. Strategies for immune modulation utilizing vitamin D may need to be stratified due to the complexity of the hormonal vitamin D system and its individual variability. The different steps for vitamin D´s involvement and its potential use in intervention are depicted in Fig. 1, summarizing the pleiotropic and protective actions of vitamin D for a normal ß-cell. Randomized controlled intervention trials addressing this mode of action are currently underway for several disorders, including type 1 diabetes.
Modes of protection for ß-cells by vitamin D actions. Pancreatic ß-cells are targets of multiple and frequent attacks by environmental adverse events ranging from infections and inflammatory conditions to metabolic stress, with all contributing to their dysfunction, apoptosis, and cell death. At the same time, regeneration of the ß-cells occurs. Vitamin D can attenuate detrimental immune reactions such as antigen and MHC class II expression, and chemotaxis of macrophages; moreover, it can induce regulatory T cells and support the balance between T helper 1 and T helper 2 cells. During thymic maturation, the autoreactive thymocytes undergo apoptosis, a process induced by dendritic cells also primed through vitamin D. Furthermore, ß-cell regeneration is coregulated by vitamin D and impaired in vitamin D deficiency
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Cooper JD, Smyth DJ, Walker NM, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60(5):1624–31. Both vitamin D deficiency and four genetic polymorphisms are significantly associated with type 1 diabetes.
Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16(6):261–6.
Bikle DD. Vitamin D, and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13(1):3–19.
Blomberg JM, Bjerrum PJ, Jessen TE, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26(6):1307–17.
Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–6S.
Holick MF. Vitamin D, deficiency. N Engl J Med. 2007;357(3):266–81.
Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–52.
Pozzilli P, Manfrini S, Crino A, et al. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2005;37(11):680–3.
Littorin B, Blom P, Scholin A, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia. 2006;49(12):2847–52.
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–8.
Staples JA, Ponsonby AL, Lim LL, McMichael AJ. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: latitude, regional ultraviolet radiation, and disease prevalence. Environ Health Perspect. 2003;111(4):518–23.
Hypponen E. Preventing vitamin D deficiency in pregnancy: importance for the mother and child. Ann Nutr Metab. 2011;59(1):28–31.
Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–68.
Elliott JC, Lucas RM, Clements MS, Bambrick HJ. Population density determines the direction of the association between ambient ultraviolet radiation and type 1 diabetes incidence. Pediatr Diabetes. 2010;11(6):394–402.
Sloka S, Grant M, Newhook LA. Time series analysis of ultraviolet B radiation and type 1 diabetes in Newfoundland. Pediatr Diabetes. 2008;9(2):81–6.
Newhook LA, Grant M, Sloka S, et al. Very high and increasing incidence of type 1 diabetes mellitus in Newfoundland and Labrador, Canada. Pediatr Diabetes. 2008;9(3 Pt 2):62–8.
Kaur H, Donaghue KC, Chan AK, et al. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34(6):1400–2.
Shea MK, Benjamin EJ, Dupuis J, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63(4):458–64.
Orton SM, Morris AP, Herrera BM, et al. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr. 2008;88(2):441–7.
Hunter D, de Lange M, Snieder H, et al. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res. 2001;16(2):371–8.
• Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. This first genome-wide association study proves that four genes regulate vitamin D levels.
Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–45.
Bailey R, Cooper JD, Zeitels L, et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007;56(10):2616–21.
Lopez ER, Zwermann O, Segni M, et al. A promoter polymorphism of the CYP27B1 gene is associated with Addison's disease, Hashimoto's thyroiditis, Graves' disease and type 1 diabetes mellitus in Germans. Eur J Endocrinol. 2004;151(2):193–7.
Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–9.
Sundqvist E, Baarnhielm M, Alfredsson L, Hillert J, Olsson T, Kockum I. Confirmation of association between multiple sclerosis and CYP27B1. Eur J Hum Genet. 2010;18(12):1349–52.
Wu S, Ren S, Nguyen L, Adams JS, Hewison M. Splice variants of the CYP27b1 gene and the regulation of 1,25-dihydroxyvitamin D3 production. Endocrinology. 2007;148(7):3410–8.
Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–76.
Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76(3):315–25.
• Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20(10):1352–60. This is the first paper describing genome-wide vitamin D effects om liganded VDR at vitamin D regulated gene loci in lymphoblast cells.
• Heikkinen S, Vaisanen S, Pehkonen P, Seuter S, Benes V, Carlberg C. Nuclear hormone 1alpha,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011;39(21):9181–93. This paper addresses vitamin D effects with an approach similar to that in [7] but treats its monocytic cell lines differently, arriving at some different gene transription profiles. Taken together, these two papers illustrate the pleiotropic actions of vitamin D for the first time on the genomic level.
Nguyen M, d'Alesio A, Pascussi JM, et al. Vitamin D-resistant rickets and type 1 diabetes in a child with compound heterozygous mutations of the vitamin D receptor (L263R and R391S): dissociated responses of the CYP-24 and rel-B promoters to 1,25-dihydroxyvitamin D3. J Bone Miner Res. 2006;21(6):886–94.
• Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7(6):337–45. This review explains the complex regulation of innate immunityby vitamin D.
Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3.
Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2012;523(1):37–47.
• von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11(4):344–9. Vitamin D is required for T lymphocyte signaling, thus fine-tuning the adaptive immunity, avoiding an overreaction.
Baeke F, Korf H, Overbergh L, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121(1–2):221–7.
Joshi S, Pantalena LC, Liu XK, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31(17):3653–69.
• Lisse TS, Hewison M. Vitamin D: a new player in the world of mTOR signaling. Cell Cycle. 2011;10(12):1888–9. A new mechanism for vitamin D effects in the antiproliferative action and on immune cells is reviewed, based on [41].
Lisse TS, Liu T, Irmler M, et al. Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB J. 2011;25(3):937–47.
Sebastiani G, Grieco FA, Spagnuolo I, Galleri L, Cataldo D, Dotta F. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab Res Rev. 2011;27(8):862–6.
Du C, Liu C, Kang J, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–9.
Witham MD, Dove FJ, Khan F, Lang CC, Belch JJ, Struthers AD. Effects of Vitamin D supplementation on markers of vascular function after myocardial infarction-A randomised controlled trial. Int J Cardiol 2012.
• Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37(6):552–8. This landmark paper demonstrated for the first time the ß-cell protective of vitamin D in the NOD mouse model.
Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85(11):2480–90.
Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5(9):e12925.
Cantorna MT, Hayes CE, Deluca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128(1):68–72.
Cantorna MT, Hayes CE, Deluca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93(15):7861–4.
Baeke F, Van Belle TL, Takiishi T et al. Low doses of anti-CD3, ciclosporin A and the vitamin D analogue, TX527, synergise to delay recurrence of autoimmune diabetes in an islet-transplanted NOD mouse model of diabetes. Diabetologia 2012.
Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin d analog calcipotriol. J Immunol. 2009;182(10):6071–8.
Prietl B, Pilz S, Wolf M, et al. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J. 2010;12(3):136–9.
• Yu S, Cantorna MT. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol. 2011;186(3):1384–90. The vitamin D deficiency and its effects on iNKT cells experienced in utero cannot be overcome by treatment of mice after birth. This illustrates the importance of pregnancy-related imprinting of the vitamin D system.
Sorensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61(1):175–8.
Fronczak CM, Baron AE, Chase HP, et al. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26(12):3237–42.
Simpson M, Brady H, Yin X, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia. 2011;54(11):2779–88.
Bizzarri C, Pitocco D, Napoli N, et al. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33(9):1962–3.
• Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by mathematical modeling. PLoS One. 2012;7(1):e30773. An algorithm is presented for calculating the free, unbound fraction of 25D in serum or plasma.
Burgess MA, Forrest JM. Congenital rubella and diabetes mellitus. Diabetologia. 2009;52(2):369–70.
Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am J Obstet Gynecol. 2011;204(6):556–4.
Hollis BW, Wagner CL. Vitamin D and Pregnancy: Skeletal Effects, Nonskeletal Effects, and Birth Outcomes. Calcif Tissue Int 2012.
• Kallas M, Green F, Hewison M, White C, Kline G. Rare causes of calcitriol-mediated hypercalcemia: a case report and literature review. J Clin Endocrinol Metab. 2010;95(7):3111–7. Hypercalcemia may be a rare adverse event of vitamin D treatment, hereby unmasking an underlying neoplastic or granulomatous condition.
• Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365(5):410–21. Polymorphisms or mutations of the CYP24A1 gene encoding a vitamin D inactivating enzyme may be the cause of idiopathic hypercalcemia, potentially aggravated by vitamin D treatment.
Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5.
Bourlon PM, Faure-Dussert A, Billaudel B. The de novo synthesis of numerous proteins is decreased during vitamin D3 deficiency and is gradually restored by 1, 25-dihydroxyvitamin D3 repletion in the islets of langerhans of rats. J Endocrinol. 1999;162(1):101–9.
Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209(4458):823–5.
Chertow BS, Sivitz WI, Baranetsky NG, Clark SA, Waite A, Deluca HF. Cellular mechanisms of insulin release: the effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology. 1983;113(4):1511–8.
Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17(3):509–11.
Acknowledgment
This work is supported by a grant from the European Community´s Health Seventh Framework Programme (FP7/2009-2014 under grant agreement 241447 with the acronym NAIMIT). We thank Brigitte Held for critical reading of the manuscript.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badenhoop, K., Kahles, H. & Penna-Martinez, M. Vitamin D, Immune Tolerance, and Prevention of Type 1 Diabetes. Curr Diab Rep 12, 635–642 (2012). https://doi.org/10.1007/s11892-012-0322-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-012-0322-3