Abstract
It is now increasingly being appreciated that a substantial proportion of subjects with prediabetes may exhibit peripheral neuropathy and/or neuropathic pain. The reverse is also true, inasmuch as examining patients with idiopathic peripheral neuropathy will frequently reveal prediabetes. In the general population, the prevalence of neuropathy in prediabetes is intermediate between overt diabetes and subjects with normoglycemia. This prediabetic neuropathy is, generally, milder in comparison to diabetic neuropathy and mainly affects small fibers mediating sensory function. Hyperglycemia, microangiopathy, dyslipidemia and the metabolic syndrome have been implicated as pathogenic mechanisms. In practice, therapy of prediabetic neuropathy should be addressed towards normoglycemia and correction of cardiovascular risk factors. However, additional work is needed to establish the long-term results of this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: The Scope of the Problem
Traditionally, neuropathy has been considered a microvascular complication of diabetes developing after long-standing hyperglycemia [1–3]. This complication may lead to debilitating pain, dysautonomia and reduced quality of life [1, 2, 4–6], while it also represents a cardinal factor in the pathogenesis of diabetic foot ulcers [7, 8] and is associated with increased mortality [9, 10]. More recently, however, it became apparent that neuropathy may develop during the earlier stages of glucose dysmetabolism as well [11, 12]. As will be discussed in detail later, this new knowledge was derived from evidence of neuropathy among prediabetic subjects in the general population [13•, 14•, 15, 16], but also from the realization that prediabetes was demonstrable in a substantial proportion of patients referred to specialized clinics for the evaluation of idiopathic peripheral neuropathy [17–23].
In this context, it is important to define prediabetes. This term refers to impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) [24]. The 2003 American Diabetes Association (ADA) Expert Committee report reduced the lower fasting plasma glucose (FPG) cut point to define IFG from 110 mg/dl (6.1 mmol/l) to 100 mg/dl (5.6 mmol/l), in part to ensure that prevalence of IFG was similar to that of IGT [25]. IFG has been defined as fasting plasma glucose 100–125 mg/dl (5.6-6.9 mmol/l) and IGT as glucose concentration 140–199 mg/dl (7.8-11.0 mmol/l) 2 hours following 75 g glucose administration during the standardized oral glucose tolerance test (OGTT) [24, 25]. However, the World Health Organization (WHO) and many other diabetes organizations did not adopt this change in the definition of IFG [26]. Studies of prediabetic neuropathy have focused on IGT rather than IFG, and the OGTT has been proposed as a necessary screening test of glucose metabolism in the differential diagnosis of alleged idiopathic neuropathy [12, 13•, 21, 23]. More simply, subjects may be classified to those with normal glucose tolerance, those with prediabetes (IFG and/or IGT) and those with diabetes [11, 12, 18, 27]. IFG and IGT should not be viewed as clinical entities in their own right but rather as risk factors for diabetes as well as cardiovascular disease [25, 26].
Prediabetic Neuropathy: Clinical Manifestations
In general, prediabetic neuropathy is less severe than genuine diabetic neuropathy. Indeed, Sumner et al. [20] have shown that the former is characterized by a slightly less pronounced impairment in amplitudes (p = 0.056) and conduction velocities (p = 0.03) of the sural nerve. Similarly, in the Singleton et al. [22] study, peroneal nerve amplitude was significantly (p < 0.05) more likely to exhibit reduced values in diabetes than prediabetes (58 % vs. 21 %, respectively). Moreover, prior or currently active denervation in calf muscles was significantly (p < 0.05) more likely in the former than the latter [22]. The relatively more moderate nature of prediabetic neuropathy is also reflected in the significantly (p = 0.01) less severe reduction of intra-epidermal nerve fiber density (IENFD) on skin biopsy [20].
Another feature of prediabetic neuropathy is that it predominantly affects sensory modalities, while motor modalities are relatively spared [22, 28]. Nerve conduction studies (NCS) have provided evidence that sensory nerve attributes are impaired, with little or no involvement of motor nerves [22, 28]. Furthermore, patients exhibit sensory symptoms, notably increased vibration perception threshold and reduced temperature perception, rather than motor symptoms [28].
In terms of nerve fiber type, it is small nerve fibers that are predominantly and during the earliest stages affected [20, 22, 28]. This predilection is largely attributable to the absence of myelin sheath in small fibers, rendering them more immediately vulnerable than large fibers to direct osmotic injury via glucose toxicity [29]. There is evidence that patients with diabetes exhibit more severe impairment of large fibers (vibration and light touch perception), whereas those with prediabetes mainly present impairment of small fibers (hot/cold and pain perception) [20, 22, 30•]. The preferential involvement of small fibers has now been confirmed by skin biopsy [20, 31]. As part of this small fiber injury, when there are symptoms, pain is the most frequent and worst complaint [14, 15, 28, 32].
Diagnosis of Prediabetic Neuropathy
Diagnosis of prediabetic neuropathy rests on careful clinical examination, as, indeed, is the case for diabetic neuropathy [1, 30•, 33]. According to the Toronto Diabetic Neuropathy Expert Group [33], clinical examination should encompass several modalities, including peripheral sensation (tuning fork, temperature, light touch, pain perception), tendon reflexes, and evaluation of patient symptoms (numbness, causalgia, lancinating or dull pain). The clinician should be particularly careful not to miss signs of early small fiber dysfunction, namely loss of pain and/or temperature perception [30•, 33].
The diagnosis may be confirmed by NCS [18, 20, 22, 33] or skin biopsy [28, 33, 35•]. Given that NCS may be normal in approximately one out of 10 patients with impaired small fiber function [18], skin biopsy quantifying intra-epidermal nerve fiber density (IENFD) and length (IENFL) is the most sensitive modality, although it is, naturally, too demanding to be used outside specialized research centers [34, 35•]. Other confirmatory diagnostic tests have also been used. These include the quantitative sudomotor axon reflex test (QSART) [36], which assesses secretory sweat function, and corneal confocal microscopy (CCM) [37], which enables in vivo examination of corneal nerve fibers. Especially CCM has shown promising accuracy in prediabetic neuropathy [37], but, for the time being, further experience with both tests is warranted. Finally, Neuropad®, a simple adhesive indicator test measuring skin humidity as a surrogate marker of sweat secretion [38], has shown moderately high sensitivity but low specificity as a screening tool of prediabetic neuropathy [27].
Diagnosis of cardiac autonomic neuropathy (CAN) should rely on cardiovascular autonomic tests (Ewing´s battery), heart rate variability (HRV) and QT interval corrected for heart rate (QTc), as standardized [39]. Other forms of autonomic neuropathy should be investigated following the recent guidelines for diabetes [40].
Prediabetic Neuropathy: Pathogenic Mechanisms
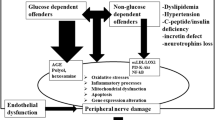
The pathogenic mechanisms of prediabetic neuropathy are multiple, not completely understood and mutually interacting. Chronic hyperglycemia, dyslipidemia, microangiopathy, and factors of the metabolic syndrome have been implicated [12, 17, 29].
-
1)
Hyperglycemia: It appears to be the major etiological factor, increasing oxidative stress, and activating protein kinase C and the polyol pathway, both of which exert direct neurotoxic effects [12, 29, 41]. Neurotoxicity is enhanced by the chronic accumulation of advanced glycation end products as a result of hyperglycemia [12, 29, 41]. The validity of hyperglycemia as a causal factor in prediabetic neuropathy should not be questioned. Certainly, in the initial phases of glucose dysmetabolism, hyperglycemia is mainly encountered postprandially, but this glucose excursion occurs repeatedly. In the experimental setting, acute short-term hyperglycemia has now been documented to harbor a variety of adverse actions, notably apoptosis of dorsal root ganglion neurons and Schwann cells [42], mitochondrial injury in nerve fibers and early neuronal death [43, 44]. In humans, acute, even though transient, hyperglycemia has been shown to produce reactive oxygen species, leading to endothelial dysfunction, impaired neuronal metabolism and function, along with direct DNA injury in nerve fibers [45].
-
2)
Dyslipidemia: An adverse lipid profile aggravates the untoward effect of hyperglycemia [12, 17, 46]. After 12 weeks of high-fat diet, rats exhibited significantly (p < 0.05) reduced sciatic nerve conduction velocity, and significantly (p < 0.05) reduced IENFD, along with significant (p < 0.05) sensory deficit, as manifested by increased latency to hind paw withdrawal after painful stimuli [46]. At the same time, they presented significantly (p < 0.05) elevated serum glucose and oxidized low-density lipoprotein (LDL) particles. In vitro, the latter induced direct toxicity in dorsal root ganglion neurons through binding to their lectin-like oxidized LDL receptors, thereby generating oxidative stress, NAD(P)H oxidase activation and neuronal death [46]. LDL-induced oxidative stress was additive to that induced by hyperglycemia [46].
-
3)
Microvascular dysfunction: Both hyperglycemia and dyslipidemia lead to microvascular dysfunction [45]. In subjects with prediabetes, laser Doppler imaging has revealed significantly (p < 0.001) reduced endothelium-dependent vasodilatation in response to iontophoresis of acetylcholine [47] and significantly (p = 0.0001) diminished axonal reflex flare after local skin heating [48]. Impressively, there is evidence that endoneurial microvascular abnormalities may herald the onset of prediabetes [49]. Studying sural nerve biopsies, Thrainsdottir et al. [49] have shown that low total capillary luminal area was associated with progression form normoglycemia to IGT, while increased total capillary membrane area was associated with the development of neuropathy. Their observations support the notion that early development of microangiopathy, largely attributable to glucose-induced oxidative stress [45], may underlie prediabetic neuropathy.
-
4)
Metabolic syndrome: Parameters of the metabolic syndrome have been implicated in the pathogenesis, as well. These mostly include obesity [13•, 14•, 15, 50] and dyslipidemia [51], but also the diagnosis of metabolic syndrome per se [51, 52]. In the KORA surveys [13•, 14•, 15], there was an association between neuropathy and obesity, expressed as waist circumference [13•], body weight [14•] and abdominal obesity [15], in the general population. Herman et al. [50] have also demonstrated impaired pain perception and diminished reflex vasodilatation in subjects with morbid obesity. Arguably, obesity is accountable for elevation of serum tumor necrosis factor alpha and lipids. The former is known to promote oxidative stress and endothelial perturbation [45]. The latter may not only lead to a vicious circle of further hyperglycemia by promotion of hepatic gluconeogenesis, but they probably also exert direct neurotoxicity, as already discussed [46, 51, 53]. Interestingly, there is evidence that patients with idiopathic neuropathy may have significantly more prominent features of the metabolic syndrome other than hyperglycemia (mostly adverse lipid profile) than those without [51], underlining the relationship of prediabetic neuropathy with the metabolic syndrome.
Prediabetic Peripheral Neuropathy
Having clarified the concept of prediabetic neuropathy along with its clinical manifestations and diagnosis, we will now look again at the evidence for its existence. For this purpose, studies will be divided into clinic-based and population-based works.
Clinic-Based Studies.
The first evidence for the association between neuropathy and adverse glucose metabolism came from studies examining neuropathic patients [18–23], in which neurologists investigated whether neuropathy of unknown cause could be attributed to prediabetes in some patients [18–23]. Neuropathy was categorized as peripheral without further specification, sensory (chronic neuropathy manifesting mainly with sensory loss and no motor involvement), painful or axonal (chronic idiopathic axonal neuropathy [CIAP], in which electrophysiology demonstrated mainly axonal loss, without demyelination) [32, 54, 55]. Most studies have found that frequency of prediabetes is increased in neuropathic patients (25-62 %), but one study reported negative results [56].
Among patients with idiopathic neuropathy, Novella et al. [18] found that more than a quarter of patients (13/48, 27 %) had evidence of IGT. IGT was more frequent in comparison to literature-based controls (15.3 % frequency among non-Hispanic whites in NHANES 3) [57]. Similarly, Sumner et al. [20] reported that 26/73 neuropathic patients (35.6 %) had IGT, again higher than historical controls [57]. In another centre, 24.6 % frequency (15/61 patients) of IGT has been found in patients evaluated for idiopathic neuropathy [19].
In a survey of idiopathic painful neuropathy, 36/107 patients (34 %) had IGT [19], higher than matched NHANES 3 subjects [57]. In a second report by the same group, 35 % of patients with painful neuropathy had IGT, after excluding those with frank diabetes [22]. Moreover, IGT showed a significant association with presence of neuropathic symptoms among patients with neuropathy of unknown cause [18].
The frequency of prediabetes has also been examined in patients with idiopathic sensory neuropathy. Using OGTT, Smith et al. [21] have observed 47 % frequency of prediabetes (45 % IGT, 2 % IFG) among patients investigated for the origin of this condition. Finally, in patients with CIAP, the frequency of prediabetes has been found exceedingly high (62 %) [23], much above that reported for the general population [57].
By contrast, Hughes et al. [56] compared 50 consecutive CIAP patients with 50 control subjects from the same region and found a 28.6 % (14/49) frequency of pre-diabetes among patients vs. 12.2 % (6/49) among controls. Frequency of prediabetes was 45.5 % (10/22) in the subgroup of patients with pain vs. 14.8 % (4/27) in those without pain. After adjustment for age and sex, these differences did not reach the level of statistical significance [56].
Based on the above, it appears that prediabetes is frequently encountered among patients with peripheral neuropathy. Nonetheless, this relationship needs to be viewed with caution. Indeed, most researchers have either reported high frequency of prediabetes without comparison or relied on the comparison with literature-based controls [57]. These controls, however, came from population studies that had been conducted some years before and included subjects with extremely wide age ranges (20–74 years) [57]. Hence, the comparison with such controls is not entirely legitimate [58]. Importantly, Hughes et al. [56] included his own matched control group, failing to confirm the relationship of neuropathy with prediabetes. Still, this work may be criticized for its very small number of patients, especially in the subgroup analysis of painful vs. painless subjects [56]. The limited patient series applies to all studies including patients with neuropathy, highlighting the need for a larger enquiry. Naturally, an inherent limitation of such clinic-based studies is the inclusion of selected patients referred to specialized centers.
Population-Based Studies (Table 1).
Vice versa, population-based studies have reported an 11–25 % prevalence of peripheral neuropathy in prediabetes. In a population survey conducted in San Luis Valley, Colorado, between 1984 and 1986 [59], age-adjusted prevalence of neuropathy was 25.8 % in diabetes, 11.2 % in subjects with IGT and 3.9 % in controls [59]. Compared with subjects with normal glucose tolerance, odds ratios (ORs) for neuropathy in those with diabetes and IGT were 10.6 and 3.5, respectively [59].
Ziegler et al. [13•, 14•, 15] have investigated the prevalence of neuropathy and painful neuropathy in subjects with prediabetes as part of the population-based MONICA (Monitorning trends and determinants of cardiovascular disease)/KORA (Cooperative Research in the region of Augsburg) study. In 1997/1998, 195 patients with diabetes and 198 age- and sex-matched controls aged 25–74 years were examined for neuropathy [13•], using a cut point > 2 of the validated Michigan Neuropathy Screening Instrument (MNSI) [60]. An OGTT was performed in controls [13•]. Overall, almost a quarter of subjects with prediabetes (24.3 %) exhibited neuropathy (13 % and 11.3 % frequency in IGT and IFG, as defined by the WHO criteria, respectively) [13•]. This compared with a frequency of 28 % in diabetic subjects and 7.4 % in controls [13•]. In the same sample, prevalence of neuropathic pain was evaluated separately [13•]. Overall, 12.9 % of subjects with prediabetes reported neuropathic pain (8.7 % and 4.2 % frequency in IGT and IFG, respectively), compared with 13.3 % of diabetic subjects and 1.2 % of controls [13•]. In a more selected sample of survivors of acute myocardial infarction aged between 25 and 74 years, 214 subjects with diabetes and 212 age- and sex-matched controls were evaluated for neuropathic pain using the same methodology [15]. It was found that 20.5 % of subjects with prediabetes had neuropathic pain (14.8 % and 5.7 % frequency in IGT and IFG, respectively), compared with 21 % of diabetic subjects and 3.7 % of controls [15].
The strengths of the MONICA/KORA surveys lie in the population-based setting, the relatively large number of subjects enrolled, as well as the robust methodology for the diagnosis of neuropathy using the MNSI, which has recently been validated as a diagnostic tool [61]. Essentially, the population-based setting ensures examination of subjects encountered in real-world situations rather than a selected sample from a specialized neuropathy clinic. An additional advantage is the separate evaluation of neuropathy and neuropathic pain. Taken together, the results of the MONICA/KORA study suggest a continuum of neuropathy progression among general population from normal glucose tolerance through prediabetes to manifest diabetes [13•, 14•, 15]. The same holds true for neuropathic pain [13•, 14•, 15]. These observations are in agreement with the Hoorn population study [62]. Prevalence of absent Achilles tendon reflexes was 67.1 % in diabetes, 15.6 % in prediabetes, and 6 % in controls (p < 0.05 for diabetes vs. controls, p < 0.05 for diabetes vs. prediabetes) [62].
By contrast, some studies have not found increased frequency of neuropathy in prediabetes [63–66]. Among Japanese-American (Nisei) men, peripheral neuropathy was diagnosed by nerve conduction study in 46.2 % of diabetic men and in 4.0 % of non-diabetic (normal and IGT) men, without difference between IGT and normal subjects [63]. In Mauritius, there was also no evidence of neuropathy in IGT: mean vibration perception threshold (VPT) was 11.5 V both in subjects with normal glucose tolerance and in those with IGT, whereas it was significantly (p < 0.05) higher in diabetic patients (13 Volts) [64]. However, this study did not aim to examine neuropathy in prediabetes. Further limitations include the very low frequency of neuropathy in the population, as well as the fact that neuropathy was solely diagnosed by VPT, which may not be adequate, at least for small fiber impairment. Not to be ignored, these two works [63, 64] included subjects from very specific populations, which may be different from the usual Caucasian subjects of most studies. Eriksson et al. [65] followed age-matched men with IGT and diabetes mellitus by NCS and sensory function prospectively for 12. After 12–15 years, peripheral nerve function did not differ between IGT and controls [65]. Conversely, diabetic patients exhibited lower conduction velocities than IGT and control subjects, suggesting that diabetes but not IGT is linked with peripheral nerve dysfunction [65]. However, this study addresses the impact of long-term IGT on peripheral nerve function, and not whether neuropathy is frequent in IGT. More recently, in the prospective Olmsted County Impaired Glucose Survey, Dyck et al. [66] studied distal symmetric polyneuropathy and atypical peripheral neuropathy (small fibre sensory or autonomic neuropathies) in subjects with prediabetes (IFG or IGT; n = 174), normal glucose metabolism (n = 150) and new diabetes (n = 218). Numbers (%) with both neuropathy types in subjects with normal glucose metabolism, prediabetes and new diabetes were: 3 (2 %), 3 (1.7 %), and 17 (7.8 %) with neuropathy narrowly defined and 10 (12.7 %), 22 (12.6 %), and 38 (17.4 %) defined broadly [66]. In neither case (narrow or broad) was peripheral neuropathy more frequent in prediabetes than in normal glucose metabolism, suggesting that prediabetes was not associated with increased prevalence of either neuropathy type. However, IFG and IGT were not considered separately, and the same applies to distal symmetric polyneuropathy and atypical neuropathy [66].
Prediabetic Cardiac Autonomic Neuropathy
Population-based studies have provided evidence for prediabetic cardiac autonomic neuropathy. The evidence is, generally, more compelling for IGT than IFG [68–75]. In the former, diminished heart rate variability [64, 70], poor increase of systolic blood pressure during handgrip [16] and decreased parasympathetic activity [72] have now been documented. Some reduction in heart rate variability may also be diagnosed in the latter, but findings have not yet been conclusive [69, 71, 72]. Interestingly, obesity and metabolic syndrome have also been linked with reduced heart rate variability [73, 74], independently of hyperglycemia.
Such changes in cardiovascular reflexes are mostly asymptomatic, and prediabetic cardiac autonomic neuropathy may easily be overlooked. Nonetheless, it is known that autonomic neuropathy is associated with increased mortality in both diabetes [9, 79] and in the general population [80]. Accordingly, the clinician should be familiar with the likelihood of prediabetic cardiac autonomic neuropathy and take care not to miss this diagnosis.
Treatment of Prediabetic Neuropathy
Treatment of prediabetic neuropathy should address the following issues:
-
1)
Correction of hyperglycemia: The Impaired Glucose Tolerance Neuropathy Study [28, 76] examined the effect of normoglycemia and weight loss on the natural course of neuropathy in IGT. At 12 months, significant weight reduction, significant diminution of LDL cholesterol and increased weekly exercise were accomplished [28]. Glucose at 2 hours post OGTT had also been significantly reduced at 24 months [28]. These changes were accompanied by the following improvements: a significant (p < 0.05) increase in proximal IENFD; an insignificant increase in distal IENFD; a significant (p < 0.05) increase in peroneal motor conduction velocity; an insignificant reduction in pain intensity; a significant (p < 0.05) increase in foot sweat secretion [28]. Nonetheless, at 36 months only significant lipid improvements were sustainable, and the benefit previously seen in small nerve fibers had reversed [76]. Hence, no permanent effect of normoglycemia could be documented.
Similarly, the China Da Qing Diabetes Prevention Outcome Study [77•] employed a 6-year lifestyle intervention program to reduce microvascular complications in adults with IGT followed for up to 20 years. At the end of the study, no difference was seen in neuropathy between the intervention and control arms (8.6 % vs. 9.1 %, p = 0.89) [77•]. Again, this work questioned the long-term efficacy of glucose reduction, but its limitations must not be overlooked. These include: use of the monofilament to diagnose neuropathy, which cannot pick up early neuropathic changes; low frequency of neuropathy, limiting the statistical power of comparisons; absence of data on neuropathy at study entry; potential selection bias, inasmuch as only survivors were finally assessed [77•].
-
2)
Correction of hyperlipidemia: In the study by Hughes et al. [54], elevated serum triglycerides, rather than hyperglycemia, were strongly associated with chronic idiopathic axonal neuropathy, highlighting the importance of serum lipids.
-
3)
Correction of other cardiovascular risk factors: Antihypertensive treatment, weight loss and smoking cessation might ameliorate prediabetic neuropathy, as already reported in diabetic microvascular disease [78]. This now remains to be explored in prediabetic neuropathy.
Conclusions and Clinical Implications
Several lines of evidence indicate that prediabetic neuropathy exists [11, 12, 13•, 14•, 15–23]. It is more closely associated with IGT rather than with IFG [12, 13•, 14•, 15, 68–75]. In general, it is less severe than genuine diabetic neuropathy and affects sensory modalities, mostly those mediated by small fibers including pain [22, 24, 28, 29, 30•, 31, 32]. This has important clinical implications, and the diagnosis will not be missed on careful physical examination [30, 32]. Prediabetic neuropathy is largely attributable to hyperglycemia, microangiopathy, dyslipidemia and the metabolic syndrome [12, 29, 41, 45, 46, 51, 52, 56]. Treatment rests primarily on establishment of normoglycemia, but this appears to offer only short-term improvement [28, 76, 77•]. The therapeutic contribution of hypolipidemic and antihypertensive treatment weight loss, and smoking cessation needs to be further defined [56, 78].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–62.
Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–97.
Fioretto P, Dodson PM, Ziegler D, Rosenson RS. Residual microvascular risk in diabetes: unmet needs and future directions. Nat Rev Endocrinol. 2010;6:19–25.
Ziegler D. Current concepts in the management of diabetic polyneuropathy. Curr Diabetes Rev. 2011;7:208–20.
Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care. 2009;32 Suppl 2:S414–9.
Várkonyi T, Kempler P. Diabetic neuropathy: new strategies for treatment. Diabetes Obes Metab. 2008;10:99–108.
Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24 Suppl 1:S3–6.
Papanas N, Maltezos E. The diabetic foot: established and emerging treatments. Acta Clin Belg. 2007;62:230–8.
Vinik AI, Maser RE, Ziegler D. Neuropathy: the crystal ball for cardiovascular disease? Diabetes Care. 2010;33:1688–90.
Hsu WC, Chiu SY, Yen AM, Chen LS, Fann CY, Liao CS, Chen HH. Somatic neuropathy is an independent predictor of all- and diabetes-related mortality in type 2 diabetic patients: a population-based 5-year follow-up study (KCIS No. 29). Eur J Neurol 2012; Jan 31. [Epub ahead of print].
Dyck PJ, Dyck PJ, Klein CJ, Weigand SD. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve. 2007;36:536–41.
Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7:682–90.
• Ziegler D, Rathmann W, Dickhaus T, et al. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–9. Evidence of peripheral neuropathy in subjects with prediabetes from the general population.
• Ziegler D, Rathmann W, Dickhaus T, et al. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400. Evidence that neuropathic pain may be encountered in subjects with prediabetes from the general population.
Ziegler D, Rathmann W, Meisinger C, et al. Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain. 2009;13:582–7.
Isak B, Oflazoglu B, Tanridag T, et al. Evaluation of peripheral and autonomic neuropathy among patients with newly diagnosed impaired glucose tolerance. Diabetes Metab Res Rev. 2008;24:563–9.
Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist. 2008;14:23–9.
Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve. 2001;24:1229–31.
Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve. 2001;24:1225–8.
Sumner CJ, Sheth S, Griffin JW, et al. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–11.
Smith AG, Singleton JR. The diagnostic yield of a standardized approach to idiopathic sensory-predominant neuropathy. Arch Intern Med. 2004;164:1021–5.
Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24:1448–53.
Hoffman-Snyder C, Smith BE, Ross MA, et al. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol. 2006;63:1075–9.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29 Suppl 1:S43–8.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35 Suppl 1:S64–71.
Report of a WHO/IDF Consultation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf
Ziegler D, Papanas N, Heier M, et al. Evaluation of the Neuropad sudomotor function test as a screening tool for polyneuropathy in the elderly population with diabetes and prediabetes. The KORA F4 survey. Diabetologia 2011;54:suppl 1:A 177.
Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–9.
Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Invest. 2011;2:18–32.
• Putz Z, Tabák AG, Tóth N, et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care 2009;32:181–3. Study on the reliable diagnosis of prediabetic neuropathy by clinical examination with emphasis on small fiber function.
Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57:1701–4.
Haanpää ML, Backonja MM, Bennett MI, et al. Assessment of neuropathic pain in primary care. Am J Med. 2009;122(10 suppl):S13–21.
Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93.
Hays AP. Utility of skin biopsy to evaluate peripheral neuropathy. Curr Neurol Neurosci Rep. 2010;10:101–7.
• Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–7. Large series providing reference values of intraepidermal nerve fiber density to diagnose neuropathy.
Peltier A, Smith AG, Russell JW, et al. Reliability of quantitative sudomotor axon reflex testing and quantitative sensory testing in neuropathy of impaired glucose regulation. Muscle Nerve. 2009;39:529–35.
Tavakoli M, Marshall A, Pitceathly R, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223:245–50.
Papanas N, Papatheodorou K, Christakidis D, et al. Evaluation of a new indicator test for sudomotor function (Neuropad®) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2005;113:195–8.
Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011 Jun 22; [Epub ahead of print].
Kempler P, Amarenco G, Freeman R, et al. Gastrointestinal autonomic neuropathy, erectile-,bladder- and sudomotor dysfunction in patients with diabetes mellitus: clinical impact, assessment, diagnosis and management. Diabetes Metab Res Rev 2011; Jul 11. [Epub ahead of print].
Dobretsov M, Romanovsky D, Stimers JR. Early diabetic neuropathy: triggers and mechanisms. World J Gastroenterol. 2007;13:175–91.
Russell JW, Sullivan KA, Windebank AJ, et al. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–63.
Edwards JL, Quattrini A, Lentz SI, et al. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;53:160–9.
Vincent AM, Edwards JL, McLean LL, et al. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol. 2010;120:477–89.
Heine RJ, Balkau B, Ceriello A, et al. What does postprandial hyperglycaemia mean? Diabet Med. 2004;21:208–13.
Vincent AM, Hayes JM, McLean LL, et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–85.
Caballero AE, Arora S, Saouaf R, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–62.
Green AQ, Krishnan S, Finucane FM, Rayman G. Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care. 2010;33:174–6.
Thrainsdottir S, Malik RA, Dahlin LB, et al. Endoneurial capillary abnormalities presage deterioration of glucose tolerance and accompany peripheral neuropathy in man. Diabetes. 2003;52:2615–22.
Herman RM, Brower JB, Stoddard DG, et al. Prevalence of somatic small fiber neuropathy in obesity. Int J Obes (Lond). 2007;31:226–35.
Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci. 2008;273:25–8.
Pittenger GL, Mehrabyan A, Simmons K, et al. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord. 2005;3:113–21.
Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257–67.
Rota E, Quadri R, Fanti E, et al. Clinical and electrophysiological correlations in type 2 diabetes mellitus at diagnosis. Diabetes Res Clin Pract. 2007;76:152–4.
Smith AG, Bromberg MB. A rational diagnostic approach to peripheral neuropathy. J Clin Neuromuscul Dis. 2003;4:190–8.
Hughes RA, Umapathi T, Gray IA, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain. 2004;127:1723–30.
Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–24.
Kissel JT. Peripheral neuropathy with impaired glucose tolerance: a sweet smell of success? Arch Neurol. 2006;63:1055–6.
Franklin GM, Kahn LB, Baxter J, et al. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131:633–43.
Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9.
Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108:477–81.
de Neeling JN, Beks PJ, Bertelsmann FW, et al. Peripheral somatic nerve function in relation to glucose tolerance in an elderly Caucasian population: the Hoorn study. Diabet Med. 1996;13:960–6.
Fujimoto WY, Leonetti DL, Kinyoun JL, et al. Prevalence of complications among second-generation Japanese-American men with diabetes, impaired glucose tolerance, or normal glucose tolerance. Diabetes. 1987;36:730–9.
Shaw JE, Hodge AM, de Courten M, et al. Diabetic neuropathy in Mauritius: prevalence and risk factors. Diabetes Res Clin Pract. 1998;42:131–9.
Eriksson KF, Nilsson H, Lindgärde F, et al. Diabetes mellitus but not impaired glucose tolerance is associated with dysfunction in peripheral nerves. Diabet Med. 1994;11:279–85.
Dyck PJ, Clark VM, Overland CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG survey. Diabetes Care. 2012;35:584–91.
Barr EL, Wong TY, Tapp RJ, et al. Is peripheral neuropathy associated with retinopathy and albuminuria in individuals with impaired glucose metabolism? The 1999–2000 AusDiab. Diabetes Care. 2006;29:1114–6.
Grandinetti A, Chow DC, Sletten DM, et al. Impaired glucose tolerance is associated with postganglionic sudomotor impairment. Clin Auton Res. 2007;17:231–3.
Gerritsen J, Dekker JM, TenVoorde BJ, et al. Glucose tolerance and other determinants of cardiovascular autonomic function: the Hoorn Study. Diabetologia. 2000;43:561–70.
Singh JP, Larson MG, O'Donnell CJ, et al. Association of hyperglycemia with reduced heart rate variability: the Framingham Heart Study. Am J Cardiol. 2000;86:309–12.
Schroeder EB, Chambless LE, Liao D, et al. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28:668–74.
Wu JS, Yang YC, Lin TS, et al. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J Clin Endocrinol Metab. 2007;92:3885–9.
Wu JS, Lu FH, Yang YC, et al. Epidemiological evidence of altered cardiac autonomic function in overweight but not underweight subjects. Int J Obes (Lond). 2008;32:788–94.
Stein PK, Barzilay JI, Domitrovich PP, et al. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med. 2007;24:855–63.
Perciaccante A, Fiorentini A, Paris A, et al. Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc Disord. 2006;6:19.
Singleton JR, Bixby B, Feldman EL, et al. Diet and exercise counseling alone does not prevent long term neuropathy progression in IGTN. [Abstract]. Neurology. 2007;68:A410.
• Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–7. Long-term follow-up of subjects with IGT to determine the effect of lifestyle intervention on the development of microvascular complications.
Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93.
Ziegler D, Zentai CP, Perz S, et al. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care. 2008;31:556–61.
Beijers HJ, Ferreira I, Bravenboer B, et al. Microalbuminuria and cardiovascular autonomic dysfunction are independently associated with cardiovascular mortality: evidence for distinct pathways: the Hoorn Study. Diabetes Care. 2009;32:1698–703.
Disclosure
Conflicts of interest: N. Papanas: has board membership with SAB (TrigoCare International, manufacturer of Neuropad); D. Ziegler: has board membership with SAB (TrigoCare International, manufacturer of Neuropad).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papanas, N., Ziegler, D. Prediabetic Neuropathy: Does It Exist?. Curr Diab Rep 12, 376–383 (2012). https://doi.org/10.1007/s11892-012-0278-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-012-0278-3