Abstract
Hyperglycemia is important in the development of microvascular and macrovascular complications from type 2 diabetes. Although there are many oral therapies available to help ameliorate hyperglycemia, it has been found that the competitive inhibition of the sodium-D-glucose cotransporter-2 (SGLT2) in the kidney may be a promising alternative treatment reducing hyperglycemia and potentially helping to lower the risk of diabetes complications. This article reviews clinical trials that have revealed favorable responses to many glycemic and metabolic parameters with SGLT2 inhibition, both as monotherapy and as an adjunct to insulin and oral antidiabetic agents. Additional studies are necessary to further determine the efficacy and long-term safety of SGLT2 inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperglycemia is an important pathogenic component in the development of microvascular and macrovascular complications from type 2 diabetes, including retinopathy, nephropathy, neuropathy, and coronary heart disease. Many treatment modalities have since been developed with the goal of approximating normoglycemia to prevent and diminish these complications. Many therapeutic agents have been developed with various mechanisms of action including the following: enhancing altered insulin secretion and sensitivity, improving glucose disposal, controlling hepatic glucose release, inhibiting intestinal glucose absorption, and restoring glucose-dependent insulin secretion [1]. There are now promising data in a novel alternative route of glucose disposal by inhibiting the sodium-D-glucose cotransporter-2 (SGLT2) in the kidney, resulting in increased glucosuria and the desired effect of reducing circulating levels of glucose in the plasma.
Diabetes and Conventional Therapies for Hyperglycemia
A wide array of therapeutic modalities is available for both types of diabetes with different metabolic targets, aside from insulin replacement, either as monotherapy or combination therapy. Despite the varied mechanisms used to control hyperglycemia and insulin action, it is inevitable that, with the advancing nature of diabetes, there will be a need for multiple medications to control hyperglycemia. We review a novel approach of inhibiting the reabsorption of glucose through renal tubules that may work as an adjunct in managing type 2 diabetes mellitus.
Glucose Homeostasis and the Kidney
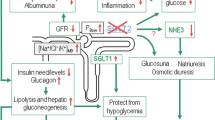
The kidney plays a large role in the metabolic homeostasis of the human body, one of which is glucose regulation. This involves not only glucose uptake and release, but filtration and reabsorption or excretion. It has been suggested that roughly 40% of all gluconeogenesis is of renal origin. In one study, there was a threefold increase of renal glucose production in diabetic patients compared with control subjects [2]. The kidney is also involved in the filtering and reabsorbing/secretion of glucose. More than 99% of filtered plasma glucose is reabsorbed in the kidney by a healthy human [3]. Per day, about 180 g of plasma glucose are filtered by the kidney, which is then physiologically reabsorbed back into the plasma in the proximal tubules [4]. Once the maximum capacity (Tm) has been exceeded (260–350 mg/min/1.73 m2), glucose spills into the urine. The Tm usually corresponds to a plasma glucose of around 200 mg/dL [2]. In diabetes, renal tubular concentrations of glucose are elevated, overwhelming membrane transporters, resulting in glucosuria [5]. In addition, gluconeogenesis in the kidney, which occurs along most of the proximal tubule, uses noncarbohydrate precursors (eg, lactate, glycerol, alanine, and glutamine) as the substrates for de novo glucose synthesis [2]. However, this aspect of physiology is not currently a therapeutic target.
Renal Sodium–Glucose Transport
Glucose is not capable of passively diffusing through cell membranes and depends on transport proteins [6]. Two families of glucose transporters are responsible for this in the kidney: the facilitative glucose transporters (GLUTs) and the active sodium–glucose cotransporters (SGLTs) [3]. The GLUTs are a large family of proteins with at least 13 isoforms, each with different kinetics, substrate specificities, and tissue expression profiles [6], that transport glucose from the epithelial cell to the blood by facilitated diffusion [7]. The two glucose transporters of interest in the renal tubules are the high-affinity GLUT1 in the S3 segment, and the low-affinity GLUT2 in the S1 segment [5, 8].
Transport of glucose, as well as osmolytes, vitamins, amino acids, and some ions, through the apical membrane of intestinal and kidney epithelial cells depends on the presence of the GLUTs and secondary active sodium–glucose symporters: SGLT1 and SGLT2. SGLT belongs to the large family of sodium–glucose cotransporter SLC5, and these membrane proteins are located in brush borders of the renal tubules and intestinal epithelium. They concentrate glucose inside the cells, using the energy provided by the cotransport of sodium ions down their electrochemical gradient [3]. Two SGLT isoforms have identified functions in the human kidney that mediate renal tubular glucose resorption in humans: SGLT1 and SGLT2 [9].
SGLT1 and SGLT2 are distinct in substrate affinity characteristics. Despite a 59% homology in their amino acid sequence, there are functional differences between them [9]. SGLT1 is expressed primarily in the small intestine, as well as the more distal S3 segment of the proximal tubule of the kidney and cardiomyocytes [10, 11], where it transports glucose and galactose. SGLT1 is a high-affinity/low-capacity transporter, which works along with the high-affinity GLUT1, and accounts for a small fraction of renal glucose reabsorption, roughly 10% [8, 10].
SGLT2, however, is expressed almost exclusively in the kidney, specifically the apical domains of epithelial cells in the S1 and S2 segments of the proximal renal tubule [7, 9], with trivial intestinal expression [12]. Around 90% of filtered glucose is absorbed by the low-affinity/high-capacity SGLT2 in the early proximal tubule, with the help of the low-affinity GLUT2 [8, 10]. Plasma glucose concentration is an important modulator of expression and activity of the SGLT2 [13].
Diabetic animal models were found to be associated with an increase in SGLT activity and SGLT2 mRNA expression by approximately 50% [13, 14], and SGLT2 and GLUT2 mRNA were found to be increased in human renal proximal tubular cells isolated from the urine of diabetic patients compared with those of healthy individuals [8]. Although this is abnormality is unlikely to be a primary cause of hyperglycemia in diabetes, it may represent an adaptation to excess glucose in the filtered urine. Thus, the renal threshold may be increased and patients with hyperglycemia may not have the classical symptom of polyuria until hyperglycemia is at a much higher level than would be expected from normal physiology. An attempt to correct this process could lead to the development of selective SGLT2 inhibitors. Such compounds may be a novel and beneficial approach to treating diabetes.
The importance of SGLT2 became recognized through the syndrome of familial renal glucosuria (FRG) [15], considered to be a benign disorder [16]. Although the molecular basis of inherited FRG varies between families that are affected, the outcome is a defect of the SGLT2 gene, resulting in glucosuria. The vast majority of affected individuals are asymptomatic, but there is a rare propensity to develop hypoglycemia and hypovolemia [17]. This condition is a potential demonstration for the safety of SGLT2 inhibitors, as these individuals maintain normal renal function, almost never develop hypoglycemia, and have no electrolyte imbalance [18]. Thus, by mimicking the syndrome of renal glucosuria, SGLT2 inhibitors have a clinical phenotype that has been well recognized as being safe. Of course, any drug treatment may also have unanticipated additional side effects.
Development of SGLT Inhibitors for Clinical Use
Phlorizin and T-1095
The first and classic inhibitor of SGLT is phlorizin. It is a naturally occurring O-glucoside, first isolated from the bark of the apple tree in 1835. In 1886, the observation was made that ingestion of more than 1 g of phlorizin produced glucosuria. It consists of a glucose moiety with two aromatic rings joined by an alkyl spacer [19, 20]. In the intestine, β-glucosidase cleaves the O-linkage of phlorizin, which leads to the formation of the active substrate phloretin [20–22]. Phloretin acts as a potent nonselective competitive inhibitor of SGLT1 and SGLT2, resulting in glucosuria along with malabsorption of glucose and galactose, leading to diarrhea [20]. In euglycemic–hyperinsulinemic clamp studies, phlorizin-treated diabetic rats, with normalized fasting glucose concentrations, showed normalization of insulin sensitivity. When the phlorizin was discontinued in this group of diabetic rats, their plasma glucose response during the meal tolerance test was nearly identical to the control diabetic rat group, in that there was a re-emergence of insulin resistance [23]. It can be speculated that these results may be secondary to reversal of glucose toxicity.
However, phlorizin and its analogues could not be developed for clinical use because of concomitant side effects caused by its nonselective inhibition of SGLTs in other tissues [16], which ultimately led to the development of selective SGLT2 inhibitors. Several SGLT2 inhibitors, most of which are similar molecules to phlorizin, but more selective for the renal transporter, are in early clinical development (Table 1; [24, 25]) and have been reported to be safe and well tolerated in general. Only some human data are available.
Sergliflozin
Sergliflozin is an O-glucoside SGLT2 inhibitor and, thus, must be administered as a prodrug to avoid intestinal deactivation by glucosidase [21, 22]. Sergliflozin has greater than 90-fold specificity for SGLT2 over SGLT1 [2]. It has been shown to improve postprandial glucoses in diabetic rats independent of insulin secretion. There was no evidence of inappropriate insulin secretion; therefore, hypoglycemia was not encountered [22, 26]. The degree of antihyperglycemic effects of sergliflozin correlated with the severity of the diabetic condition [26]. In one protocol, a single dose given to Zucker diabetic rats showed upregulation of several genes involving glucose and fatty acid metabolism in the liver, kidney, muscle, and adipose tissue [27]. Sergliflozin’s development was halted during phase 2 trials, and its indication was changed to obesity [2, 28].
Dapagliflozin
Dapagliflozin, developed by Bristol-Myers Squibb/AstraZeneca, is a C-aryl glucoside that is resistant to intestinal degradation and can be administered orally without the need of a prodrug. Dapagliflozin has a 1200-fold selectivity for SGLT2 over SGLT1 compared with phlorizin. After a single dose of dapagliflozin to streptozotocin-induced diabetic rats, there was an approximate 55% reduction in glucose compared with control in a 5-hour interval [21].
Remogliflozin
Remogliflozin, a benzylpyrazole glucoside, is a potent and selective SGLT2 inhibitor. Chronic treatment with remogliflozin in db/db mice exhibited an antidiabetic profile including reduced fasting plasma glucose and hemoglobin A1c (HbA1c). This study also confirmed that placebo-treated diabetic animals lost weight in correspondence to reduced plasma insulin. The remogliflozin-treated group had preservation of insulin levels, improving hyperglycemia [29]. A similar observation was reported in db/db mice treated with T-1095 [14]. The acute treatment of animals with this compound increased glucosuria; however, with chronic administration, there was an amelioration of glucosuria secondary to improving glycemic control. There was improved hyperglycemia, hypertriglyceridemia, fasting glucoses and insulin levels, homeostasis model assessments/insulin sensitivities, and reduction in epididymal and retroperitoneal fat densities in high-fat diet-fed Goto-Kakizaki rats [29]. Recently, the development of remogliflozin was discontinued in phase 2 trials [30]. If similar weight and adiposity benefits occur in humans, there may be a potential for SGLT2 inhibitors in treating the metabolic syndrome.
ISIS-388626
ISIS-388626, an antisense oligonucleotide, is currently in phase 1 clinical trials. It is yet another novel potent compound that selectively knocks out renal SGLT2 gene expression in vivo without any effect on SGLT1. Several animal studies were completed showing that this antisense oligonucleotide compound reduced renal SGLT2 mRNA expression by up to 80%, compared with control, with a once-weekly injection. Improvements in glucose tolerance, HbA1c, and insulin sensitivity were observed. There were findings that showed a delay in the progression of ocular cataract formation and renal glomerular and pancreatic islet cell deterioration. Upon cessation of ISIS-388626, glucose-lowering effects were sustained for more than 4 weeks. If such therapy were to be possible in humans, one might speculate that once-weekly (or less frequent) administration could be achieved in the outpatient setting. There was no hypoglycemia (in euglycemic or hyperglycemic animal models), hepatotoxicity, or renal functional or histologic changes [31, 32]. It has been suggested that the data presented are consistent with expectations based on human subjects who have mutations in the SGLT2 gene, have increased urine glucose levels, and are otherwise asymptomatic.
Clinical Implications of SGLT2 Inhibitor Therapy
Because of the advancing nature of diabetes, it is difficult for patients to remain controlled on unchanging therapeutic management. As new modalities become available, it is important to consider the myriad issues surrounding safety and tolerability.
Energy and Glucose Metabolism
Urinary glucose excretion increased in patients receiving SGLT2 inhibitors compared with placebo [33•, 34]. This has implications not only for blood glucose concentrations but also for overall energy balance. Thus, SGLT2 inhibitors cause a net loss of calories from the body through glucosuria. In early animal models, there was no difference in food intake between controls and chronically treated diabetic animals, with amelioration of body weight gain in addition to improved glycemic control [26, 35]. All doses of dapagliflozin were shown to induce weight loss in the first week of clinical treatment, consistent with steady caloric loss through glucosuria, but possibly also some fluid loss. If glucosuria calorie losses in animal studies are relevant to humans it might be that continued gradual weight loss may decrease adiposity. Additional long-term clinical and body composition studies are planned to further establish diuresis versus adiposity reduction as the cause of weight loss [34]. In preclinical animal studies, there was significant reduction in fasting and postprandial glucoses, insulin concentrations, and HbA1c levels [26, 35–37]. Phase 3 studies showed that in 12 weeks’ time, up to 59% of subjects were able to reach a goal of HbA1c less than 7%, compared with 54% in the metformin group and 32% in the placebo group [34], with starting levels of HbA1c between 7.6% and 8%. Long-term studies are needed to determine the extent of predictable HbA1c reduction. A series of animal studies have shown that treatment with an SGLT inhibitor normalized insulin resistance and prevented the exhaustion of pancreatic β-cells [36]. There was evidence in one animal study of partial restoration of β-cell function in nonobese diabetic rats [38].
Chronic treatment in genetically obese and diabetic mouse models was associated with reduced triglycerides [35]. Treated Goto-Kakizaki rats on a high-fat diet showed improved triglyceride levels [29], and another study showed that fatty amino acid levels also decreased [36]. However, there was no clear fasting lipids effect with the 12-week phase 3 study of dapagliflozin [34].
Dapagliflozin is currently in a phase 3 study. During a short-term 14-day phase 2 study, significant improvements in oral glucose tolerance test excursions were observed from day 2 to 13, compared with placebo. On day 13, fasting glucose levels were significantly reduced in the treated group, with absolute mean reductions of 18.8 to 38.7 mg/dL in a dose-dependent manner, compared with the placebo group that had no significant reductions. There was no apparent effect on serum insulin, fructosamine, or C-peptide levels, or on body weight. Type 2 diabetic patients who had the highest fasting glucose values had a greater response to dapagliflozin [33•].
In a long-term 12-week trial, dapagliflozin (2.5–50 mg) decreased the HbA1c (−0.55 to −0.90), fasting glucose (−16 to −31), and postprandial glucoses. Although fasting glucoses were dose-related and apparent by the first week, even the lowest dose of dapagliflozin produced a near-maximal effect on the postprandial glucose. Total body weight reduction was up to twice (−3.4 vs −1.7 kg) that of the metformin group, and waist circumference reduction was also marked [34], presumably related to glucosuria (32–65 g of creatinine/day)-induced calorie loss.
In a continuation phase 3 study, dapagliflozin was studied in the diabetic cohort that was uncontrolled on insulin therapy in addition to oral antidiabetic medications (thiazolidinediones and sulfonylureas). The insulin doses in this group of patients were reduced in half and, in addition to the oral antidiabetic agents, the treatment arms were placebo, 10 mg, and dapagliflozin, 20 mg. The primary end point of reduced HbA1c was met with 65% of the dapagliflozin group achieving a ≥0.5% decrease, compared with 16% of placebo. Although only the 20-mg dose of dapagliflozin was shown to improve the fasting glucose, the postprandial glucose excursions were dose dependently improved with dapagliflozin. Compared with placebo, another finding was an additional weight loss of about 2.5 kg in the treatment arms. Despite a 50% dose reduction of insulin in addition to an oral antidiabetic agent, the addition of an SGLT2 inhibitor to the regimen of the uncontrolled diabetic patient in this cohort could improve glycemic control and have weight loss, negating the weight gain typically associated with insulin [39••]. The limitations of this study are the small size and the short duration of 12 weeks.
Hypoglycemia, Electrolytes, Volume Status, and Blood Pressure
Urinary glucose excretion increased in patients receiving SGLT2 inhibitors compared with placebo [33•, 34]. Preclinical studies showed no evidence of hypoglycemia, even after prolonged fasting [21, 26]. In the phase 3 dapagliflozin study, two patients were reported to have hypoglycemia, both of whom were receiving concomitant metformin [33•]. There was a modest increase in serum magnesium of 0.1 mEq/L and a decrease of serum uric acid by about 1.0 mg/dL. Discontinuation of the medication resulted in normalization [34]. There were no clinically relevant mean changes from baseline of serum sodium, potassium, and calcium. There was a mild increase in serum phosphate, although it was not statistically significant, and small increases in parathyroid hormone. Serum 1,25-dihydroxyvitamin and 25-hydroxyvitamin D levels remained unchanged [34].
Temporary diuretic effects have been observed with no significant changes in urine volume over the long term [22, 26]. Initially, an acute transient increase in urine sodium occurs, which normalizes after 2 weeks [33•]. In the phase 3 study of dapagliflozin, there was a small dose-dependent diuretic effect seen equivalent to 0.3 to 1.5 voids/day, small increases in blood urea nitrogen, and small dose-dependent increases in hematocrit, with no clinical signs of dehydration observed. There was one described event of acute renal failure from dehydration in a patient chronically treated with a diuretic, promptly resolved with oral rehydration [39••]. There was also a small non-dose-dependent decrease in systolic blood pressure from −2.6 to −6.4 mm Hg, consistent with diuretic action [34]. Across multiple dose ranges, there were also no apparent changes in physical examination, vital signs, or electrocardiograms [33•].
Microvascular and Macrovascular Effects
Plasma glucose concentration is an important modulator of the expression and activity of the SGLT2. Enhanced activity of SGLT2 may contribute to the development of tubular and glomerular disease of the kidney [13]. SGLT2 inhibitors possibly may offer renoprotection by increasing extracellular glucose concentrations and blocking glucose fluxes transcellularly, which suppresses intracellular formation of advanced glycation end products, and reduction of protein kinase C activation and oxidative stress [14], although there are no clinical outcome studies yet. This may prevent the progression of diabetic nephropathy, development of albuminuria, and expansion of glomerular mesangial area [14]. There are no data for the role of SGLT2 inhibitors in the macrovascular complications of diabetes.
Genitourinary Infections
Adverse effects reported frequently in phase 2 and 3 trials include constipation, diarrhea, nausea, urinary frequency, and genitourinary infections [33•], involving urinary tract infections (UTIs) and vulvovaginal infections. In phase 2 trials, 5% to 12% of dapagliflozin-treated patients, compared with 6% of placebo and 9% of metformin groups, had UTIs [34]. However, the relative severity of the infections and the need for the antibiotic therapy in these trials is unclear. Two subjects receiving dapagliflozin had vulvovaginal mycotic infections on day 11 of treatment, one of which was also on metformin. More detailed assessments for these infections are planned in longer-term clinical trials that will take place with larger cohorts [33•]. Symptomatic vulvovaginal Candida infections are known to be more prevalent in diabetic patients, and it is well known that these patients are prone to recurrent vaginal candidiasis [33•]. Although diabetic women are more prone to UTI than nondiabetic individuals, a prospective study of around 600 diabetic women showed that glucosuria did not increase the risk for developing asymptomatic bacteriuria or UTI [40]. In additional long-term trials, more careful detailed cataloguing of this adverse event is necessary, as the top risk factors of such infections in all diabetic patients are asymptomatic bacteriuria and recent sexual activity [40], and with SGLT2 inhibitor treatment, an increased and localized glucose concentration in the genitourinary tract may pose as another risk.
Conclusions
The kidneys play a central role in the homeostasis of plasma glucose levels. SGLT2 is located in the plasma membrane of proximal tubular cells and mediates the majority of renal glucose reabsorption from the tubular fluid, which normally prevents the loss of glucose into the urine. The pursuit of SGLT2 inhibitor development has been ongoing for more than 20 years with the intention of treating diabetes. The competitive inhibitors of SGLT2 provoke glucosuria, providing a unique mechanism to lower plasma glucose and rid the body of excess calories in a way that is not insulin mediated. Many new compounds are in the late stages of clinical development, which will further clarify the safety and efficacy of SGLT2 inhibitors.
Chronic glucosuria associated with chronic inhibition of renal glucose reabsorption has been shown to have relative safety in those individuals with FRG. Human renal tubular cells isolated from urine demonstrated increased renal glucose transporter expression and activity in type 2 diabetic patients [8]; thus, SGLT2 inhibitors appear to be a logical and unique adjuvant therapy. Because it helps in postprandial glucose excursions, it may be helpful in type 1 diabetic patients as its mechanism is insulin independent [18, 25]. In one small study, there was significant weight loss despite simultaneous insulin therapy [39••].
Long-term studies should aim to determine if there are any contraindications to use of this type of medicine, such as renal insufficiency or in those patients at risk of dehydration. It would also be interesting to see how SGLT2 inhibitors affect adiposity, and if successful whether it is subcutaneous or visceral in nature. Future studies of SGLT2 inhibition might examine whether they have a role in treating prediabetes, polycystic ovarian disease, or the metabolic syndrome. Overall, the urinary elimination of excess glucose is a new and exciting potential target in the effective treatment of diabetes. We await further studies on efficacy and long-term safety.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as follows: • Of importance •• Of major importance
Wagman A, Nuss JM: Current therapies and emerging targets for the treatment of diabetes. Curr Pharm Des 2001, 7:417–450.
Marsenic O: Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 2009, 53:875–883.
Wright EM, Turk E: The sodium/glucose cotransport family SCL5. Pflugers Arch 2004, 447:510–518.
Wright EM: Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol 2001, 280:F10–F18.
Bakris GL, Fonseca V, Sharma K, Wright EM: Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009, 75:1272–1277.
Wood IS, Trayburn P: Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 2003, 89:3–9.
Castaneda F, Burse A, Boland W, Kinne RK: Thioglycosides as inhibitors of hSGLT1 and hSGLT2: potential therapeutic agents for the control of hyperglycemia in diabetes. Int J Med Sci 2007, 4:131–139.
Rahmoune H, Thompson PW, Ward JM, et al.: Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005, 54:3427–3434.
Kanai Y, Lee WS, You G, et al.: The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 1994, 93:397–404.
Hediger MA, Rhoads DB: Molecular physiology of sodium–glucose cotransporters. Physiol Rev 1994, 74:993–1026.
Zhou L, Cryan EV, D’Andrea MR, et al.: Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem 2003, 90:339–346.
Thomson AB, Wild G: Adaptation of intestinal nutrient transport in health and disease. Part I. Dig Dis Sci 1997, 42:453–469.
Freitas HS, Anhe GF, Melo KF, et al.: Na(+)-glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 2008, 149:717–724.
Arakawa K, Ishihara T, Oku A, et al.: Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na(+)-glucose cotransporter inhibitor T-1095. Br J Pharmacol 2001, 132:578–586.
NCBI: OMIM–Online Mendelian Inheritance in Man. Available at http://www.ncbi.nlm.nih.gov/omim. Accessed December 2009.
Wright EM, Hirayama BA, Loo DF: Active sugar transport in health and disease. J Intern Med 2007, 261:32–43.
Santer R, Kinner M, Lassen CL, et al.: Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 2003, 14:2873–2882.
Jabbour SA, Goldstein BJ: Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract 2008, 62:1279–1284.
Idris I, Donnelly R: Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug. Diabetes Obes Metab 2009, 11:79–88.
Ehrenkranz JR, Lewis NG, Kahn CR, Roth J: Phlorizin: a review. Diabetes Metab Res Rev 2005, 21:31–38.
Meng W, Ellsworth BA, Nirschl AA, et al.: Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 2008, 51:1145–1149.
Katsuno K, Fujimori Y, Takemura Y, et al.: Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther 2007, 320:323–330.
Rossetti L, Smith D, Shulman GI, et al.: Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987, 79:1510–1515.
U.S. National Institutes of Health: Registry of ClinicalTrials.gov. Available at http://www.clinicaltrials.gov. Accessed December 2009.
Kipnes M: Dapagliflozin: an emerging treatment option in type 2 diabetes. Expert Opin Investig Drugs 2009, 18:327–334.
Fujimori Y, Katsuno K, Ojima K, et al.: Sergliflozin etabonate, a selective SGLT2 inhibitor, improves glycemic control in streptozotocin-induced diabetic rats and Zucker fatty rats. Eur J Pharmacol 2009, 609:148–154.
Ghosh S, Shen Y, Harrington WW, et al.: Metabolic responses to a sodium–glucose transporter-2 inhibitor, GW869682, assessed by whole-genome transcript profiling. Diabetologia 2007, 50(Suppl 1):S376.
DiabesityDigest.com, your online resource for obesity and type 2 diabetes. Available at http://www.diabesitydigest.com. Accessed December 2009.
Fujimori Y, Katsuno K, Nakashima I, et al.: Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 2008, 327:268–276.
Kissei Pharmaceutical Co., Ltd. Available at http://www.kissei.co.jp. Accessed November 2009.
Wancewicz EV, Siwkowski A, Meibohm B, et al.: Long-term safety and efficacy of ISIS 388626, an optimized SGLT2 antisense inhibitor, in multiple diabetic and euglycemic species. Presented at the 68th Scientific Sessions of the American Diabetes Association. San Francisco, CA; June 6–10, 2008.
Bhanot S, Murray SF, Booten SL, et al.: ISIS 388626, an SGLT2 Antisense drug, causes robust and sustained glucosuria in multiple species and is safe and well tolerated. Presented at the 69th Scientific Sessions of the American Diabetes Association. New Orleans, LA; June 5–9, 2009.
• Komoroski B, Vachharajani N, Feng Y, et al.: Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009, 85:513–519. This paper describes a phase 3 clinical trial showing favorable results of SGLT2 inhibition on glycemic parameters.
List JF, Woo V, Morales E, et al.: Sodium–glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009, 32:650–657.
Oku A, Ueta K, Arakawa K, et al.: T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 1999, 48:1794–1800.
Tsujihara K, Hongu M, Saito K, et. al.: Na(+)-glucose cotransporter (SGLT) inhibitors as antidiabetic agents. 4. Synthesis and pharmacological properties of 4′-dehydroxyphlorizin derivatives substituted on the B ring. J Med Chem 1999, 42:5311–5324.
Adachi T, Yasuda K, Okamoto Y, et al.: T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats. Metabolism 2000, 49:990–995.
Ueta K, Ishihara T, Matsumoto Y, et al.: Long-term treatment with the Na+-glucose cotransporter inhibitor T-1095 causes sustained improvement in hyperglycemia and prevents diabetic neuropathy in Goto-Kakizaki Rats. Life Sci 2005, 76:2655–2668.
•• Wilding JP, Norwood P, T’Joen C, et al.: A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 2009, 32:1656–1662. This paper describes a phase 3 clinical trial in which SGLT2 inhibitor use with insulin and oral antidiabetic agents showed improvement in glycemic parameters alone with promising data on weight loss.
Geerlings SE, Stolk RP, Camps MJ, et al.: Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care 2000, 23:1737–1741.
Disclosure
Dr. Vivian Fonseca has served as a paid consult to AstraZeneca and Bristol-Myers Squibb in the development of dapagliflozin and is the chair of the principal investigator of a clinical trial involving this drug. No other potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, A.K., Fonseca, V. Turning Glucosuria into a Therapy: Efficacy and Safety with SGLT2 Inhibitors. Curr Diab Rep 10, 101–107 (2010). https://doi.org/10.1007/s11892-010-0095-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-010-0095-5