Abstract
Purpose of Review
This review aims to provide a comprehensive assessment of mitral valve disease, both mitral stenosis and mitral regurgitation, starting with an overview of the valve anatomy.
Recent Findings
The advent of three-dimensional imaging has allowed a better representation of the valve anatomy. Rheumatic disease is still the number one cause of mitral stenosis worldwide and percutaneous balloon mitral valvuloplasty remains the therapy of choice when indicated and in anatomically eligible patients. Mitral regurgitation (MR) is classified as primary (i.e., lesion in the mitral apparatus) or secondary (caused by left ventricular geometrical alterations). While surgery, preferably repair, is still the recommended therapy for severe primary MR, percutaneous approaches to repair and/or replace the mitral valve are being extensively investigated.
Summary
Mitral valve disease is common. A careful understanding of mitral valve anatomy and the disease processes that affect the valve are crucial for providing optimal patient care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Anatomy
The mitral valve (MV) apparatus is a complex structure [1,2,3,4,5] consisting of an annulus, two leaflets, three types of chordae tendineae, and two dominant papillary muscles (Fig. 1).
-
1.
The MV annulus: is in continuity with the aortic valve through the fibrous aortic-mitral curtain. It is a complex, saddle-shaped, nonplanar geometric structure with a greater intercommisural than medio-lateral diameter.
-
2.
The leaflets: the anterior leaflet is long and narrow while the posterior leaflet is shorter and wider. The leaflets meet at two commissures: the anterolateral and posteromedial commissures. For descriptive purposes, each leaflet (A for anterior and P for posterior) is divided into three scallops, 1 to 3, from lateral to medial.
-
3.
The chordae tendineae: are fibrous structures that attach the MV leaflets to the papillary muscles. There are three types of chordae based on their insertion level: the primary chordae insert on the free margin of the leaflets, the secondary chordae insert on the rugged surface of the leaflets, and the tertiary chordae insert only on the basal part of the posterior leaflet.
-
4.
The papillary muscles: there are two dominant papillary muscles, one anterolateral and the other posteromedial. The posteromedial papillary muscle has a single blood supply usually from the posterior descending artery, while the anterolateral papillary muscle is usually supplied by both the left anterior descending artery and left circumflex artery.
Mitral valve anatomy. The mitral valve apparatus is shown here with the annulus, leaflets, chordae tendineae, and papillary muscles. Each leaflet anterior (A) and posterior (P) is divided into three scallops (1 for lateral, 2 for middle and 3 for medial). (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2017. All Rights Reserved)
Pathology at any level of the MV apparatus (one and/or multiple levels) can lead to MV dysfunction. This dysfunction is mainly of two types, which may coexist: MV stenosis and MV regurgitation.
Mitral Valve Stenosis
Etiology
Rheumatic heart disease (RHD) is the most common cause of mitral valve stenosis (MS) worldwide. It is more common and progresses more rapidly in developing countries. It is thought to be related to an exaggerated immune response initiated by cross-reactivity between a streptococcal antigen and the valve tissue [6]. Patients with recurrent bouts of acute rheumatic fever resulting in carditis are particularly prone to develop RHD. Although any cardiac valve may be involved, the mitral valve is almost always affected. Characteristic rheumatic changes include:
-
Commissural fusion
-
“Fish-mouth” appearance of the MV orifice
-
Leaflet thickening, especially at the free edges
-
Shortening and fusion of the chordae
The latter two changes are responsible for the characteristic hockey-stick appearance of the leaflet, particularly the anterior, on echocardiography.
Other etiologies of MS include:
-
Mitral annular calcification (MAC): while MAC is commonly seen in the elderly and patients with advanced renal disease, it rarely causes significant MS [7].
-
Radiation valvulitis: which typically manifests 10 to 20 years after mediastinal radiation therapy [8].
-
Congenital causes: very rare, such as cor triatriatum, parachute mitral valve, double-orifice mitral valve, or supravalvular mitral ring [9].
-
Systemic inflammatory disorders such as lupus erythematous and rheumatoid arthritis may occasionally lead to valvulitis and resulting MS.
-
Obstructing lesions such as a large atrial myxoma or infected vegetation which may cause functional MS.
Pathophysiology and Consequences
A normal MV area is 4 to 6 cm2. With progressive MS, and when the area becomes less than 2 cm2, a diastolic pressure gradient develops between the left atrium (LA) and left ventricle (LV) [10]. This leads to increased LA pressures (A) and decreased forward flow (B). Tachycardia is particularly detrimental in MS since it shortens the diastolic filling time, leading to an increase in the the transmitral gradient.
-
(a)
Consequences of increased LA pressures:
-
LA enlargement with increased risk of atrial arrhythmias, particularly atrial fibrillation, and systemic thromboembolism.
-
Increased pulmonary pressures with resultant pulmonary edema and pulmonary hypertension. The latter may lead to right ventricular failure and subsequent tricuspid regurgitation.
-
-
(b)
Consequences of decreased forward flow:
-
Low cardiac output: due to poor LV filling.
-
Quantification of Mitral Stenosis
Echocardiography is the modality of choice. Quantitative assessment of MS should be performed according to the ASE/EAE joint guidelines [11] and is focused on the assessment of three main parameters: (1) mean diastolic transmitral pressure gradient, (2) MV area, and (3) secondary changes including left atrial and right-sided chamber sizes and right-sided pressures.
-
1.
Mean diastolic pressure gradient: This is typically assessed by continuous-wave Doppler through the MV from an apical four-chamber view. By tracing the Doppler-derived diastolic transmitral flow and using the simplified Bernoulli equation to convert velocities into pressures, the mean MV gradient is obtained. Typically, a mean gradient >10 mmHg is consistent with severe MS, 5–10 mmHg with moderate MS, and <5 mmHg with mild MS. It is important to note that the transmitral gradient is strongly influenced by heart rate and forward flow. This is why it has not been included in the criteria for assessment of MS severity in the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease and in the 2017 AHA/ACC Guideline update [12••, 13••].
-
2.
MV area: MV area can be measured directly by either two-dimensional (2D) or preferably three-dimensional (3D) planimetry [14] with the measurements performed in mid-diastole at the leaflet tips. MV area can also be estimated by indirect methods including pressure half time (PHT), deceleration time (DT), continuity equation, and proximal isovelocity surface area (PISA). Although generally not indicated for most patients, mitral valve gradient may also be measured invasively in the cardiac catheterization laboratory and the mitral valve area calculated via the Gorlin equation [15]
-
(a)
Pressure half time method: The PHT is the time required for the maximal early diastolic mitral gradient to reach half of its value. It is quite short in patients without significant stenosis due to rapid equilibration of LA and LV pressures during diastolic filling. In contrast, in patients with severe stenosis, the pressure gradient declines slowly resulting in a prolonged PHT. MV area is inversely related to pressure half and can be estimated by the following empirical equation
$$ \mathrm{MV}\ \mathrm{area}\ \left({\mathrm{cm}}^2\right)=220/\mathrm{PHT}\ \left(\mathrm{ms}\right) $$ -
(b)
Deceleration time method: The DT is the time required for the maximal early diastolic mitral gradient to reach zero. Similarly to PHT, MV area is inversely related to DT and may be estimated according to the following formula
$$ \mathrm{MV}\ \mathrm{area}\ \left({\mathrm{cm}}^2\right)=759/\mathrm{DT}\ \left(\mathrm{ms}\right) $$ -
(c)
Continuity equation method: This method assumes that the stroke volume (SV) through the mitral valve is equal to that through the LV outflow tract in the absence of significant mitral or aortic regurgitation. Stroke volume is calculated as the product of the orifice area by the velocity-time integral (VTI) as obtained by pulsed-wave Doppler. The equation to calculate MV area becomes the following:
$$ \mathrm{MV}\ \mathrm{area}=\frac{\mathrm{LVOT}\ \mathrm{area}\times \mathrm{LVOT}\ \mathrm{VTI}}{\mathrm{MV}\ \mathrm{VTI}} $$The left ventricular outflow tract (LVOT) area is calculated by measuring the systolic LVOT diameter in the parasternal long axis, and assumes a circular shape of the LVOT.
-
(d)
Proximal isovelocity surface area (PISA) method: this method is also based on the continuity equation. As the blood approaches an orifice, it accelerates leading to the formation of a series of hemispheric isovelocity surfaces with smaller areas and higher velocities as the flow approaches the orifice. According to the continuity equation, the amount of flow at the level of any of the hemispheres should equal the flow across the stenotic mitral valve [16]. In addition, since the orifice in case of MS is funnel shaped and not circular, an angle correction should be introduced and the PISA method formula becomes as follows:
$$ \mathrm{MV}\;\mathrm{area}=2\times \pi \times {r}^2\times {V}_{\mathrm{alias}}/{V}_{\max}\times \varphi /180 $$Where r = PISA radius, V alias = aliasing velocity, V max = maximal transmitral velocity, and ϕ = angle between the two mitral leaflets in diastole
According to the 2014 valvular heart disease guidelines and 2017 guideline update [12••, 13••], MS is considered very severe if MVA ≤1 cm2, severe if it is ≤1.5 cm2.
-
(a)
-
3.
Secondary changes from chronic MS: These changes should be carefully assessed by echocardiography and include LA enlargement, pulmonary hypertension, right heart dilation, and functional tricuspid regurgitation.
Role of Stress Testing
Stress testing, most commonly in the form of exercise echocardiography, is particularly helpful in cases when there is discrepancy between the reported symptoms and the severity of MS. For instance, in those patients reportedly asymptomatic with severe MS, exercise testing can help confirm if the patient is able to achieve an adequate workload without the development of symptoms. Similarly, in patients with moderate MS and severe symptoms, stress testing can unmask hemodynamically significant MS during exercise. Typically, in addition to recording symptoms, MV gradients and estimated right-sided pressures should be measured during the stress test and these results included in the decision-making regarding intervention.
Management
-
1)
Medical therapy: includes diuretics and rate controlling agents, mainly beta-blockers, for symptomatic relief. Anticoagulation with warfarin is recommended in cases of atrial fibrillation. Novel oral anticoagulants should not be used in cases of MS.
-
2)
Intervention: indications for intervention include:
-
Symptomatic patients with severe MS (Class I recommendation).
-
Asymptomatic patients with very severe MS (Class IIA recommendation).
-
Asymptomatic patients with severe MS who have new onset of AF (Class IIB recommendation).
-
Symptomatic patients with moderate MS if there is evidence of hemodynamically significant MS during exercise (Class IIB recommendation).
-
Preoperatively, in asymptomatic severe MS undergoing elective moderate- or high-risk non-cardiac surgery.
-
Preconception, in symptomatic women with moderate or severe MS (mitral valve area ≤1.5 cm2). The preconception management of asymptomatic women with moderate to severe MS is more controversial.
-
Percutaneous mitral balloon valvuloplasty (PMBV) is the procedure of choice in eligible patients with surgery reserved for those who cannot undergo valvuloplasty. Eligibility for PMBV is based on the Wilkins score [17] which includes four parameters (each being scored from 0 to 4): leaflet thickening, mobility, and calcification and degree of chordal/subvalvular thickening. Good candidates are those with a score of 8 or less. In cases where surgery is indicated, surgical options include commissurotomy (closed or open), mitral valve replacement, and, rarely, mitral valve repair if there is significant mitral regurgitation.
Mitral Valve Regurgitation
Etiology
The causes of mitral valve regurgitation (MR) can be broadly classified as primary or secondary without being mutually exclusive.
-
1.
Primary causes: refer to abnormalities at any level of the mitral valve apparatus (leaflets, chordae tendineae, papillary muscles, annulus)
-
(a)
MV prolapse: It is the most common cause of MR requiring surgery in developed countries [18]. Pathologically, two distinct degenerative changes have been described with significant overlap between the two entities: Barlow disease, which results from myxomatous degeneration characterized by abnormal accumulation of mucopolysaccharides and fibroelastic deficiency, which results from abnormalities of connective tissue structure that lead to loss of mechanical integrity [19]. On echocardiography, MV prolapse is defined as an abnormal leaflet systolic displacement of ≥2 mm above the mitral annulus plane in a long-axis view.
-
(b)
Other etiologies that can lead to primary MR include the following: infective endocarditis, mitral annular calcification (MAC), rheumatic heart disease, connective tissue disorders, congenital malformations (a cleft for example) and certain drugs (such as anorectics).
-
(a)
-
2.
Secondary causes are those caused by left ventricular geometrical alterations that impede proper function of the MV apparatus. Various mechanisms have been implicated, including adverse LV remodeling and dyssynchrony, mitral annular dilatation, and impaired LV contractility with loss of the forces necessary for MV closure during systole, and tethering of mitral valve leaflets due to changes in geometry of MV and its papillary muscles. Secondary causes can be further categorized into ischemic MR and non-ischemic MR.
-
(a)
Ischemic MR: where LV dysfunction is secondary to coronary artery disease. It portends a poor prognosis in terms of survival and risk for developing heart failure.
-
(b)
Non-ischemic MR: the form of MR found in all types of non-ischemic cardiomyopathy, including dilated cardiomyopathy, restrictive cardiomyopathy, and hypertrophic cardiomyopathy. Non-ischemic MR can also be secondary to atrial fibrillation (with resultant annular dilation) and right ventricular pacing (with resultant dyssynchrony).
-
(a)
Pathophysiology and Consequences
Carpentier has developed a functional classification of mitral regurgitation based on leaflet motion that aids in surgical planning (Fig. 2) [20]:
-
Type I: normal leaflet motion (annular dilatation, leaflet perforation)
-
Type II: excess leaflet motion (prolapse, flail, papillary muscle rupture)
-
Type IIIa: restricted leaflet motion during systole and diastole (thickening, retraction) such as rheumatic disease
-
Type IIIb: restricted leaflet motion during systole only (tethering) in ischemic and functional disease
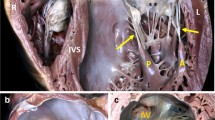
Carpentier functional classification of mitral regurgitation. Type I: normal leaflet motion—an example of mitral annular dilatation with normal leaflets is shown; Type II: excess leaflet motion—note the ruptured chordae and the posterior leaflet flail; Type III a: Restricted leaflet motion during systole and diastole—the leaflets are shown thickened and retracted; Type III b: restricted leaflet motion during systole only—there is tethering of the posterior leaflet secondary to myocardial infarct (note the gray thinned myocardium). (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2017. All Rights Reserved)
Mitral regurgitation causes left atrial and left ventricular volume overload, the impact of which is being determined by the time course of the development of the regurgitation (acute vs. chronic) and the magnitude of the regurgitant volume
-
(a)
Acute MR: When the MR is acute, there is a sudden marked increase in preload and since the ventricle did not have time to accommodate for the increased volume, there is a significant increase in LV filling pressures which are transmitted to the pulmonary circulation leading to pulmonary edema. Also, forward output significantly declines, since now, much of the flow is directed to the left atrium. This may culminate in cardiogenic shock. It may occur with infective endocarditis, chordal rupture, or post-myocardial infarction papillary muscle rupture.
-
(b)
Chronic MR: The natural history of chronic MR may be divided in three stages: an early compensated stage during which most patients are asymptomatic, a transitional stage where there is progressive and adverse LV remodeling, and a decompensated stage marked by the development of symptoms. This progression may be insidious. It is therefore extremely important to identify early deleterious LV changes so that intervention is recommended prior to the development of irreversible LV damage. Chronic MR also leads to LA enlargement which forms the nidus for the development of atrial arrhythmias.
Quantification of Mitral Regurgitation
Echocardiography is also the modality of choice for MR evaluation. Multiple parameters are available for quantification and these should be integrated in a comprehensive assessment, according to the ASE guidelines [21]. Sole reliance on Color Doppler is discouraged. These parameters can be grouped into those based on color Doppler, pulsed-waved (PW) Doppler, continuous-wave (CW) Doppler, PISA, volumetric methods, and other supportive echo findings.
-
1.
Color Doppler: Assessment by color Doppler includes the jet area, with its ratio to the LA area, and the vena contracta. A jet occupying >40% of the LA area and a vena contracta >7 mm are the cutoffs adopted for severe MR. It is important to note that numerous technical, hemodynamic, and anatomic factors affect the jet area [22]; therefore, determining severity solely on this measure is discouraged.
-
2.
PW Doppler: Mitral inflow and pulmonary veins PW Doppler can provide supportive elements. In severe MR, peak mitral inflow velocity is typically >120 cm/s. Pulmonary venous flow is normally antegrade during both ventricular systole and diastole with a short retrograde flow during atrial systole. With increasing MR, there is a progressive blunting of the systolic component, with systolic flow reversal in cases of severe MR.
-
3.
CW Doppler: The CW jet profile is a useful adjunct in MR assessment. A very dense jet supports severe MR—the density of the jet being a reflection of the number of red blood cells. A triangular early peaking jet is typically seen in acute MR, due to the rapid equilibration of pressures between the LV and the LA.
-
4.
PISA method: As previously discussed, when blood rushes through an orifice, it forms hemispheres with increasing velocity and decreasing surface area. Based on the continuity equation, the effective regurgitant orifice area (EROA) of MR can be calculated as follow:
$$ \mathrm{EROA}\ \left({\mathrm{cm}}^2\right)=2\times \pi \times {r}^2\times {V}_{\mathrm{alias}}/{V}_{\max } $$where r = PISA radius, V alias = aliasing velocity, and V max = peak velocity through the regurgitant orifice.
Based on the EROA, a regurgitant volume (R Vol) and regurgitant fraction (RF) can be calculated as follows:
$$ \mathrm{R}\ \mathrm{Vol}=\mathrm{EROA}\times \mathrm{VTI} $$where VTI = regurgitant jet velocity-time integral (VTI)
$$ \mathrm{RF}=\left(\mathrm{R}\ \mathrm{Vol}/{\mathrm{SV}}_{\mathrm{MV}}\right)\times 100 $$where SVMV = mitral valve stroke volume = π × MV annulus radius2 (cm2) × MV inflow VTI (cm)
According to the 2014 valvular heart disease guidelines [12••], there was a difference in the cutoffs of severe MR based on whether the MR is primary or secondary. However, this difference in cutoffs has been removed in the 2017 valvular heart disease guidelines update [13••] and the definition of severe secondary MR is now the same as for primary MR: EROA ≥0.4 cm2, regurgitant volume ≥60 mL, or regurgitant fraction ≥50%.
-
5.
Three-dimensional echocardiography (3DE): 3DE allows better representation of the 3D nature of the MR jet and can help in understanding MV pathology. It also allows 3D quantitative assessment of the vena contracta, PISA, and stroke volumes [23], overcoming the limitations of two-dimensional imaging.
Management
-
1.
Primary MR:
-
(a)
Medical therapy: has limited role in acute MR, where surgery is urgently indicated. In chronic MR, there is no drug proven to improve long-term outcomes.
-
(b)
Surgery: If the morphology of the valve is suitable for repair, mitral valve repair is preferred to replacement since its operative risks and outcomes are better [12••]. Features suggestive a high likelihood of successful repair are summarized in Table 1. The indications for surgery along with the strength of the indication, according to the European Society of Cardiology (ESC) [24] and ACC/AHA [12••] guidelines, are summarized in Table 2.
-
(c)
Transcatheter therapies:
-
Transcatheter mitral valve repair: While a number of devices are in clinical development, the only one that has current United States Food and Drug Administration (FDA) approval is the MitraClip system (Abbott Laboratories, IL) which is an edge-to-edge leaflet repair device. It is based on the surgical Alfieri repair, which involves suturing the regurgitant orifice of the mitral valve leaflets together, creating a “double-orifice” mitral valve [25]. This intervention receives a IIB recommendation in the recent AHA/ACC valve guideline update [13••] for patients with NYHA class III-IV symptom in setting of chronic severe MR despite optimal guideline directed medical heart failure therapy with favorable mitral valve anatomy, a reasonable life expectancy, and deemed extreme risk for mitral valve surgery.
-
Transcatheter mitral valve replacement: Similar to mitral valve repair, many technologies are currently under development [26] with none being approved by the FDA so far.
-
-
(a)
-
2.
Secondary MR:
-
(a)
Medical therapy: The underlying LV dysfunction should be managed according to current guidelines (Class I) with consideration for cardiac resynchronization therapy when indicated. Improvement in LV function commonly translates in reduction in the severity of MR
-
(b)
Surgery and transcatheter therapies: There is no evidence that surgery [27•, 28•] or any transcatheter therapy aimed at correcting the MR severity is helpful in patients with secondary MR, mainly because secondary MR is a disease of the ventricle rather than the valve itself. As a result, intervention on the valve is rarely recommended and may be considered in severely symptomatic patients with severe secondary MR despite appropriate medical management (Class IIB recommendation). MV surgery is indicated in patients with ischemic MR undergoing surgical revascularization if the regurgitation is more than moderate (Class IIA recommendation). Recent studies suggest that MV repair and replacement have similar outcomes in this setting with replacement associated with less likelihood of recurrence of MR [29•, 30].
-
(a)
Novel Prognostic Markers in Patients with Asymptomatic Severe MR and Preserved Ejection Fraction
Asymptomatic patients with severe MR and preserved ejection fraction are of particular interest since the timing for surgery in these patients is crucial to prevent adverse LV remodeling. Recent research has identified the following markers as of prognostic significance in these patients:
However, these variables have not yet been incorporated into society guidelines.
Conclusion
The mitral valve apparatus is a complex structure consisting of the annulus, leaflets, chordae, and papillary muscles. Pathology at any level can lead to mitral valve dysfunction. Mitral stenosis and mitral regurgitation can have significant impact in terms of morbidity and mortality. Echocardiography remains the mainstay modality for diagnosis, and a comprehensive assessment integrating multiple parameters is recommended. Careful understanding of these disease processes is crucial in selecting the appropriate management strategy and for careful timing of the intervention. While percutaneous balloon valvuloplasty is the preferred approach in eligible patients with mitral stenosis, surgery remains the cornerstone therapy for mitral regurgitation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Van Mieghem NM, Piazza N, Anderson RH, Tzikas A, Nieman K, De Laat LE, et al. Anatomy of the mitral valvular complex and its implications for transcatheter interventions for mitral regurgitation. J Am Coll Cardiol. 2010;56(8):617–26. doi:10.1016/j.jacc.2010.04.030.
Perloff JK, Roberts WC. The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation. 1972;46(2):227–39.
Anwar AM, Soliman OI, ten Cate FJ, Nemes A, McGhie JS, Krenning BJ, et al. True mitral annulus diameter is underestimated by two-dimensional echocardiography as evidenced by real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Card Imaging. 2007;23(5):541–7. doi:10.1007/s10554-006-9181-9.
Muresian H. The clinical anatomy of the mitral valve. Clin Anat. 2009;22(1):85–98. doi:10.1002/ca.20692.
Ho SY. Anatomy of the mitral valve. Heart. 2002;88(Suppl 4):iv5–10.
Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366(9480):155–68. doi:10.1016/S0140-6736(05)66874-2.
Pressman GS, Agarwal A, Braitman LE, Muddassir SM. Mitral annular calcium causing mitral stenosis. Am J Cardiol. 2010;105(3):389–91. doi:10.1016/j.amjcard.2009.09.042.
Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290(21):2831–7. doi:10.1001/jama.290.21.2831.
Ruckman RN, Van Praagh R. Anatomic types of congenital mitral stenosis: report of 49 autopsy cases with consideration of diagnosis and surgical implications. Am J Cardiol. 1978;42(4):592–601.
Hugenholtz PG, Ryan TJ, Stein SW, Abelmann WH. The spectrum of pure mitral stenosis. Hemodynamic studies in relation to clinical disability. Am J Cardiol. 1962;10:773–84.
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22(1):1–23; quiz 101-2. doi:10.1016/j.echo.2008.11.029.
•• Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63(22):e57–185. doi:10.1016/j.Jacc.2014.02.536. This report constitues the AHA/ACC recommendations for the management of patients with valvular heart disease.
•• Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017, 2017; doi:10.1016/j.Jacc.2017.03.011. This report is the recent (2017) AHA/ACC focused update of the valvular guidelines.
Schlosshan D, Aggarwal G, Mathur G, Allan R, Cranney G. Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. JACC Cardiovasc Imaging. 2011;4(6):580–8. doi:10.1016/j.jcmg.2010.12.009.
Gorlin R, Gorlin SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I. Am Heart J. 1951;41(1):1–29.
Rodriguez L, Thomas JD, Monterroso V, Weyman AE, Harrigan P, Mueller LN, et al. Validation of the proximal flow convergence method. Calculation of orifice area in patients with mitral stenosis. Circulation. 1993;88(3):1157–65.
Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60(4):299–308.
Luxereau P, Dorent R, De Gevigney G, Bruneval P, Chomette G, Delahaye G. Aetiology of surgically treated mitral regurgitation. Eur Heart J. 1991;12(Suppl B):2–4.
Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow's disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg. 2007;19(2):90–6. doi:10.1053/j.semtcvs.2007.04.002.
Carpentier A. Cardiac valve surgery—the “French correction”. J Thorac Cardiovasc Surg. 1983;86(3):323–37.
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi:10.1016/S0894-7317(03)00335-3.
Chen CG, Thomas JD, Anconina J, Harrigan P, Mueller L, Picard MH, et al. Impact of impinging wall jet on color Doppler quantification of mitral regurgitation. Circulation. 1991;84(2):712–20.
Marsan NA, Westenberg JJ, Ypenburg C, Delgado V, van Bommel RJ, Roes SD, et al. Quantification of functional mitral regurgitation by real-time 3D echocardiography: comparison with 3D velocity-encoded cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2(11):1245–52. doi:10.1016/j.jcmg.2009.07.006.
Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33(19):2451–96. doi:10.1093/eurheartj/ehs109.
Maisano F, La Canna G, Colombo A, Alfieri O. The evolution from surgery to percutaneous mitral valve interventions: the role of the edge-to-edge technique. J Am Coll Cardiol. 2011;58(21):2174–82. doi:10.1016/j.jacc.2011.07.046.
De Backer O, Piazza N, Banai S, Lutter G, Maisano F, Herrmann HC, et al. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv. 2014;7(3):400–9. doi:10.1161/Circinterventions.114.001607.
• Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371(23):2178–88. doi:10.1056/NEJMoa1410490. This study, along with the study referenced below, showed that surgical treatment of ischemic mitral regurgitation did not provide a clinically meanigful benefit.
• Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2016;374(20):1932–41. doi:10.1056/NEJMoa1602003. This study reports the 2-year outcomes of the surgical treatment of ischemic mitral regurgitation, similarly showing the lack of benefit.
• Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370(1):23–32. doi:10.1056/NEJMoa1312808. This study shows no significant difference between patients who underwent mitral-valve repair and those who underwent mitral-valve replacement.
Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374(4):344–53. doi:10.1056/NEJMoa1512913.
Alashi A, Mentias A, Patel K, Gillinov AM, Sabik JF, Popovic ZB, et al. Synergistic utility of brain natriuretic peptide and left ventricular global longitudinal strain in asymptomatic patients with significant primary mitral regurgitation and preserved systolic function undergoing mitral valve surgery. Circ Cardiovasc Imaging. 2016;9(7) doi:10.1161/CIRCIMAGING.115.004451.
Mentias A, Naji P, Gillinov AM, Rodriguez LL, Reed G, Mihaljevic T, et al. Strain echocardiography and functional capacity in asymptomatic primary mitral regurgitation with preserved ejection fraction. J Am Coll Cardiol. 2016;68(18):1974–86. doi:10.1016/j.jacc.2016.08.030.
Mentias A, Naji P, Barr T, Gillinov AM, Rodriguez LL, Mihaljevic T, et al. Incremental prognostic utility of LV global longitudinal strain and functional capacity in asymptomatic patients with significant primary mitral regurgitation and preserved left ventricular ejection fraction undergoing rest-stress echocardiography. Circulation. 2015;132
Mentias A, Patel K, Patel H, Gillinov AM, Sabik JF, Mihaljevic T, et al. Effect of pulmonary vascular pressures on long-term outcome in patients with primary mitral regurgitation. J Am Coll Cardiol. 2016;67(25):2952–61. doi:10.1016/j.jacc.2016.03.589.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Serge C. Harb and Brian P. Griffin declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Valvular Heart Disease
Rights and permissions
About this article
Cite this article
Harb, S.C., Griffin, B.P. Mitral Valve Disease: a Comprehensive Review. Curr Cardiol Rep 19, 73 (2017). https://doi.org/10.1007/s11886-017-0883-5
Published:
DOI: https://doi.org/10.1007/s11886-017-0883-5