Abstract
Heart failure is the final common pathway in many forms of heart disease, and is associated with excessive morbidity and mortality. Pathophysiologic alterations in the interaction between the heart and the autonomic nervous system in advanced heart failure have been noted for decades. Over the last decade, great advances have been made in the medical and surgical treatment of heart failure – and some of these modalities target the neuro-cardiac axis. Despite these advances, many patients progress to end-stage heart failure and death. Recently, device-based therapy targeting the neuro-cardiac axis with various forms of neuromodulatory stimuli has been shown to improve heart function in experimental heart failure models. These include spinal cord stimulation, vagal nerve stimulation, and baroreflex modulation. Human trials are now underway to evaluate the safety and efficacy of these device-based neuromodulatory modalities in the heart failure population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) remains an increasingly demanding clinical problem in the United States and elsewhere. Nearly 6 million people in the United States have heart failure, and over 650,000 people are diagnosed with it each year. One-fifth of patients with HF will die within 1 year of diagnosis, and HF was a contributing cause of over 275,000 deaths in 2006. Heart failure care cost the United States an estimated $39.2 billion in 2010 [1]. The statistics cited above refer to all forms of HF, but systolic HF is responsible for the majority of morbidity and mortality, and will be the primary HF type discussed in this review.
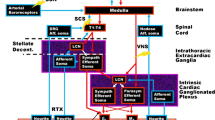
The autonomic nervous system (ANS) regulates cardiac activity via a complex and dynamic interplay and cross-talk between the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS), with endocrine, paracrine, and, immunologic influences.
It has long been recognized that ANS dysfunction occurs in HF [2]. The initial response in early HF is compensatory but later becomes deleterious and part of the disease process itself, characterized by SNS hyperactivity, shown by increased plasma norepinephrine (NE) levels and excessive NE spillover from sympathetic nerve fibers [3]. Despite this, paradoxically decreased responsiveness of the myocardium to adrenergic stimuli exists, with decreased sympathetic neuronal density and neuronal function, as well as decreased myocardial NE concentrations [4]. Decreased responsiveness to beta1 adrenergic receptor (AR) stimulation within the myocardium in HF occurs [4]. Eventually, pathophysiologic cellular processes, such as abnormal calcium handling and apoptosis, result [5–7]. SNS hyperactivity seems to be linked to abnormal cardiac reflexes, in that the inhibitory arterial baroreceptor reflex is suppressed and the excitatory sympathetic afferent and arterial chemoreceptor reflexes are enhanced [8]. Excessive SNS activity is associated with increased propensity for sudden cardiac arrest and ventricular arrhythmias [9, 10], recurrent admission for decompensated HF [1], and mortality [1].
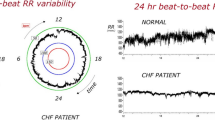
Less is known about the role of the PSNS in the pathophysiology of HF, though our knowledge base is increasing. Decreased parasympathetic tone in patients with HF [11] results in increased heart rates and decreased heart rate variability, both of which are correlated with increased mortality [12]. These changes may occur early and provide a potential diagnostic and therapeutic avenue [13]. Changes in parasympathetic signaling are manifested as changes in parasympathetic ganglionic signaling, decreased postganglionic muscarinic receptor density and function, and decreased acetylcholinesterase activity (role in neurotransmitter inactivation). In addition, increased vagal afferent activation has been noted in HF, which has been shown to pathophysiologically alter cardiac cytokine and neurohumoral activity [14, 15].
Current Clinical Therapy Targeting the ANS in HF
Beta Adrenergic Agents
Therapies for advanced HF directly and indirectly target the ANS and can improve outcomes and reduce morbidity and mortality [16–20]. The importance of blockade with beta1 AR selective agents bisoprolol, metoprolol, and carvedilol, even in the face of target receptor downregulation at the level of the myocardium, is well known to improve outcomes [16–20]. These beneficial effects can reflect inhibition of catecholamine-induced cardiotoxicity and apoptosis, restoration of normal cardiac reflex control mechanisms, and slowing of the heart rate.
Alpha Adrenergic Agents
Limited studies have examined the role of alpha adrenergic agents in HF patients. In a VA Cooperative Study, the alpha1 AR antagonist prazosin was found to be associated with worse outcomes compared with combination isosorbide and hydralazine [21]. A doxazosin arm was discontinued early in another major trial after it was found to be associated with increased incidence of heart failure [22]. These studies do not support a role for peripherally-acting alpha AR antagonists in HF therapy. Interestingly, carvedilol has both beta1 and alpha1 AR effects, and is thought by some HF experts to have a more pronounced beneficial effect in HF patients. More study is needed to better understand whether combined alpha/beta AR antagonism is of benefit in HF treatment.
Central Sympathetic Agents
The centrally-acting alpha-2 agonist, clonidine, has been shown to improve heart failure symptoms in limited patients [23], perhaps by direct inhibition of sympathetic excitation and reduction in SNS tone/hyperactivity. In canines with HF, intrathecal clonidine infusion reduced ischemic ventricular arrhythmias during coronary artery occlusion [24]. The alpha1 AR agonist moxonidine has been shown to decrease plasma NE levels in HF, but was associated with increased mortality in HF patients, presumably via loss of beta stimulation support of cardiac output [25]. Spinal epidural analgesia has been reported to suppress VT refractory to conventional therapy [26].
Surgical Therapies
Surgical sympathectomy, resecting the lower half of the stellate ganglion and first 2–4 thoracic ganglia, has been employed for almost 45 years [27] to treat ventricular tachycardia refractory to conventional therapy, such as catecholaminergic ventricular tachycardia [28], and in long QT patients using simple thoracoscopic surgery [29].
Parasympathetic Targets
Few trials have used parasympathomimetic agents to restore parasympathetic tone, largely due to the paucity of effective and tolerable agents. In 1 study, oral pyridostigmine therapy reduced ventricular ectopic activity and improved heart rate variability [30]. Pharmacologic therapy targeting the renin-angiotensin system indirectly improves in parasympathetic measures in HF patients [31].
Preclinical Studies Supporting a Potential Role for Neuromodulation Therapy in HF
Therapeutic neuromodulation with device-based therapies has been in clinical use since the 1980s. Targeted autonomic neuromodulation with either spinal cord stimulation or vagal nerve stimulation has been employed routinely in patients with chronic pain or epilepsy and depression, respectively, and more recently in HF.
Spinal Cord Stimulation
Spinal cord stimulation (SCS) has been used clinically to treat chronic pain and peripheral vascular disease, and refractory angina in Europe, using a spinal epidural stimulating lead connected to an implanted pulse generator. Animal studies showed in anesthetized canines that cardiac ischemia caused intracardiac nerve firing suppressed by SCS at spinal segment T1- with no change in other cardiac parameters [32]. In a rabbit model, preemptive SCS at spinal segment T1 (delivered before and during coronary artery occlusion) reduced infarct size, an effect blocked by treatment with alpha or beta AR blockers, leading the authors to conclude that this cardioprotective effect was mediated by adrenergic neurons [33].
Zipes and coworkers reported that epidural spinal stimulation at T1 significantly increased sinus cycle length and the AH interval, effects eliminated by vagal transection but not by ansae subclaviae transection. The authors concluded that SCS enhanced parasympathetic activity via a vagus-dependent mechanism [34•]. In a canine post-infarction heart failure model, acute SCS at T1 during coronary artery balloon occlusion suppressed ventricular arrhythmias [35••]. In these experiments, SCS also significantly decreased sinus rate, increased the PR interval, and reduced systolic blood pressure [35••]. Similar effects of SCS on ischemic VT were seen in porcine HF studies [36].
Because the above studies examined the cardiac effects of acute SCS in normal, ischemic, or failing animals, we examined the action of chronic SCS in a post-infarction heart failure canine model [37••]. All canines underwent foam embolization of the LAD followed by ventricular high rate pacing for 3 weeks to induce HF. Animals were then randomized to the following treatment groups: Control (no SCS), chronic SCS at spinal segment T4 (delivered at 90 % motor threshold, 50 Hz, 0.2 ms pulse duration, for 2 hrs TID), standard medical therapy (carvedilol + ramipril), or SCS + medical therapy. Animals were followed for 5–10 weeks during the treatment period. The SCS, medical therapy, and SCS + medical therapy groups all demonstrated significant decreases in spontaneous and ischemic ventricular arrhythmias, compared with the control group, with the SCS group showing the greatest decrease in VTs [37••]. It seems likely that this effect was mediated by a treatment-mediated reversal of excessive SCS activation during HF. Interestingly, the SCS and SCS + medical therapy groups also demonstrated significant improvement in clinical HF parameters (resting heart rate, systolic blood pressure, oxygen saturation) compared with the medical therapy and control group [37••]. This clinical improvement was associated with recovery of left ventricular ejection fraction (Fig 1). Further, this left ventricular remodeling was associated with marked and significant reversal of LV dilatation [37••]. The pronounced nature of this beneficial therapeutic effect with both solo SCS therapy and when SCS was combined with standard medical therapy, and the increased degree of this beneficial clinical response when compared with the group with standard medical therapy alone, suggests that SCS must be acting in a distinct manner, and not by just providing additive effects on the same molecular targets as standard HF medical therapy. Put another way, SCS must be altering the pathophysiology of HF in a unique manner to cause these therapeutic responses. This is supported by the additional study finding that both SCS-treated groups also demonstrated decreases in serum NE and B-type natriuretic peptide (BNP) levels after completion of the treatment interval, whereas the standard medical therapy group showed no significant changes in serum NE levels compared with untreated HF animals (Fig 2). We concluded that SCS therapy could improve LV contractile function and suppress VTs, likely by normalization of autonomic tone [37••]. An additional, limited analysis of this dataset has revealed that ambulatory heart rate variability is decreased after HF induction and is restored to normal patterns after completion of 5 weeks of SCS therapy -- further supporting the contention that autonomic tone recovers with SCS therapy (personal communication - Dr. John Lopshire).
Spinal cord stimulation improves LV function in experimental heart failure. BSLN indicates baseline; after HF, after HF induction; 2 wk, after 2 weeks of neuromodulation stage; 5 wk, after 5 weeks of neuromodulation stage; and 10 wk, after 10 weeks (completion) of neuromodulation stage.*P < 0.05 vs group baseline; †P < 0.05 vs group after HF induction; ‡P < 0.05 vs control group at same time point. (With permission from: Lopshire JC, Zhou X, Dusa C, et al.: Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–94) [64]
Spinal cord stimulation improves neurohumoral function in experimental heart failure. BNP indicates B-type natriuretic peptide; BSLN, baseline; After HF, after HF induction; 2 wk, after 2 weeks of neuromodulation stage; 5 wk, after 5 weeks of neuromodulation stage; and 10 weeks, after 10 weeks (completion) of neuromodulation stage. *P < 0.05 vs group baseline; †P < 0.05 vs group after HF induction; ‡P < 0.05 vs control group at same time point. (With permission from: Lopshire JC, Zhou X, Dusa C, et al.: Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;28;120:286-94 [64]
In a similar canine model, we examined whether the VT- suppressing and LV remodeling effects of SCS were dependent on the spinal site and intensity of spinal stimulation. After myocardial infarction and HF induction with high rate pacing, canines received SCS at 90 % motor threshold at spinal segments T1, T4, or T8. Two additional groups received SCS at 60 % and 30 % motor threshold at spinal segment T4 (the other stimulation parameters of stimulation frequency, pulse width, and delivery intervals were unchanged from the prior study). Significant effects on VT suppression and LV ejection fraction were noted with 90 % stimulation at T1 or T4 and 60 % stimulation at T4, with the most profound effects on both parameters noted in the 90 % T4 group. The other groups showed no change in these parameters relative to control (untreated) animals [38].
Beneficial therapeutic effects of chronic SCS have been obtained in HF models in other species. In pigs with ischemic HF, SCS treatment improved LV function (both LV ejection fraction and dp/dt) and regional myocardial strain as assessed by echocardiography. SCS also decreased myocardial oxygen consumption with no change in serum NE levels [39]. Taken together, the above studies indicate the SCS can ameliorate excessive SNS effects and improve HF parameters in animal models. These studies provide compelling support for human trials of SCS in the HF population, which are now underway and will be discussed later in this paper.
Vagal Nerve Stimulation
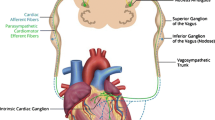
The idea that vagal nerve stimulation (VNS) to restore parasympathetic tone might ameliorate the deleterious effects of excessive SNS hyperactivity in HF was first supported in work by Schwartz and coworkers. In a canine model of post infarction heart failure, VNS decreased the incidence of sudden cardiac arrest [40]. In rat models, other groups demonstrated that right cervical VNS reduced mortality [41] and ventricular arrhythmias [42]. In another canine HF model, VNS was shown to restore autonomic balance, slow systemic inflammatory processes, and heart failure progression [43••]. Finally, a novel VNS system that actively titrated the VNS stimulus output to reduce heart rate to a desired range was employed in canines with HF. This system significantly improved left ventricular ejection fraction and reduced LV volumes, compared with untreated HF animals. Within this study, a separate group received VNS with concurrent beta blocker therapy (metoprolol succinate 100 mg daily) or beta blocker therapy alone. The combination of VNS and beta blocker produced the greatest improvement in LV ejection fraction [44•]. Additionally, VNS dramatically decreased circulating cytokines, myocyte hypertrophy, and restored baroreflex control to normal [44•]. This device is currently being investigated in an open label study of New York Heart Association class II and III patients and will be examined further in a large-scale clinical trial (discussed below).
Baroreflex Stimulation
Animal and human studies have suggested that chronic stimulation of the carotid sinus with baroreflex stimulation devices can lower blood pressure in refractory hypertension [45, 46].
Altered baroreflex control reflects decreased parasympathetic tone seen in HF and restoration of baroreflex sensitivity may improve outcomes in HF. In a canine heart failure model, chronic (3 months) bilateral electrical stimulation of the carotid sinus produced a modest increase in left ventricular function compared with untreated controls. Baroreflex stimulation also promoted reverse remodeling by decreasing LVEDP and reducing fibrosis and cardiomyocyte hypertrophy [47••].
Current Clinical Trials Evaluating Neuromodulatory Therapies for Treatment of HF
Spinal Cord Stimulation
The preclinical studies cited above have provided a strong basis for human trials of SCS in the HF population. The Defeat- HF (Determining the feasibility of spinal cord neuromodulation for the treatment of chronic heart failure) trial is an industry-sponsored, multi-site (Europe and North America), Phase II trial to determine the safety and efficacy of SCS in advanced heart failure patients [48••, 49]. Targeted enrollment will be 70 HF patients with NHYA Class III-IV symptoms, an LVEF ≤35 % and LV end-diastolic diameter between 55–80 mm, no implanted cardiac resynchronization device, and on stable medical HF therapy. All enrolled patients will receive an implanted SCS device with randomization to active treatment with SCS or no therapy groups. The primary endpoint will be change in left ventricular volumes as measured by cardiac echo at 6 and 12 months with secondary outcomes of changes in blood chemistry (pro-BNP) and exercise capacity (peak oxygen uptake). This trial started in 2010, with actively enrolled patients in the United States and Europe, and has a targeted completion date in 2014 [48••].
Smaller trials are also investigating the utility of SCS in HF patients. SCS-Heart [50] is being conducted in Hong Kong and Australia and will enroll 20 NYHA Class III HF patients. A Phase I safety /efficacy trial is also reportedly underway in Texas [51]. Initial results from these trials should be available soon.
Vagal Nerve Stimulation
Preclinical studies demonstrating the potential effectiveness of VNS in experimental heart failure have also spawned human trials. Early human trials demonstrated the potential safety and feasibility of VNS in HF patients [52, 53••]. The CardioFit for the Treatment of Heart Failure safety/efficacy trial utilized a proprietary VNS stimulating device paired with an RV endocardial sensing electrode (CARDIO-FIT; BioControl Medical, Yehud, Israel) to titrate VNS to achieve a pre-set, targeted heart rate reduction. This small Phase 2 trial enrolled 32 HF patients and was recently completed [54, 55••]. This was an open-label study and enrolled HF patients to receive right cervical vagus stimulation. Therapy was well-tolerated with adverse effects of cough, dysphonia, and stimulation-related pain seen early in study but these effects regressed with stimulation titration. There was a net significant improvement in LVEF (from 22 % to 29 %) and reduction in LV systolic volumes at 6 months of therapy. This was maintained at 1 year of treatment [55••]. A controlled, large-scale clinical trial using this device is now underway [56]. The increase of vagal tone in CHF (INOVATE-HF) trial is a randomized, multicenter (US and European sites), open-label Phase III trial. It will enroll 650 patients (NYHA Class III, LVEF ≤40 %, LV end diastolic dimension 50–80 mm) in a 3:2 scheme to active VNS therapy vs standard of care (no implant). The primary efficacy end-point of the study is the composite of all-cause mortality or unplanned heart failure hospitalization. Co-primary safety endpoints will evaluate freedom from procedure-related issues across 90 days and long-term morbidity compared with the control group. Secondary endpoints will include changes in 6-minute walk and left ventricular volumes. This trial is slated for completion in 2017.
Another multisite (European only), randomized, double-blind, Phase II trial, neural cardiac therapy for heart failure study (NECTAR-HF), will evaluate direct right vagus stimulation in HF patients (NYHA Class III, LVEF ≤35 %, LV end diastolic dimension ≥55 mm) [57]. Targeted enrollment is 250 patients, with all enrolled patients implanted with VNS system and randomized to active therapy vs no therapy (control). The control group will crossover into active therapy after 6 months. The primary outcome measures will include left ventricular end-systolic dimension (LVESD) at 6 months and all-cause mortality at 18 months. Secondary outcomes will include other echocardiographic measures of LV remodeling, functional capacity and change in NYHA class, and quality of life assessment. It is slated for completion in 2015.
Baroreflex Stimulation
Baroreflex stimulation improved heart failure in a canine experimental model [47••]. Initial human trials in HF patients are now underway [58–61]. Interestingly, early trials have targeted HF patients with preserved ejection fraction. The CVRx Rheos Diastolic Heart Failure Trial is a prospective, randomized, double blind trial that followed 60 subjects (symptomatic HF with elevated BNP, LVEF ≥40 %) at 5 centers in Europe [58]. All subjects received bilateral carotid sinus barostimulator lead implants and were randomized to active therapy vs no therapy (control). The control subjects underwent crossover to active therapy at 6 months. All subjects were followed up to 1 year post-implant. Primary outcomes were safety endpoints and changes in LV mass index. This study was targeted for completion in 2011, but results have not been published to date. The HOPE4HF Trial [61, 62] is a multisite US trial that recruited 540 HF patients (symptomatic HF with elevated BNP, LVEF ≥40 %). Patients will be randomized to active therapy (implanted system with bilateral baroreflex stimulation) or no therapy (standard of care with no implanted system). The primary outcomes will include safety measures, cardiovascular death, or heart failure event with secondary efficacy outcomes. It is closed to enrollment and slated for completion in 2014. Finally, a study of baroreflex stimulation in systolic HF patients is planned to begin enrollment soon [63]. Figure 3 provides a schematic view of the 3 modalities described above -- as they are employed in the active clinical studies.
Schematic demonstrating the location and stimulation sites for each device-based neuromodulation modality. (A) SCS generator implant in abdomen or paraspinous region with stimulation lead (black line) placed in dorsal epidural space at thoracic level 4. (B) Vagal nerve stimulator placed in right subpectoral region with standard transvenous pacing/sensing lead placed in RV and vagal nerve stimulating lead (dotted white lines) tunneled to cervical vagus region. (C) Baroreflex stimulation generator placed in right subpectoral region with bilateral stimulation leads tunneled to the carotid baroreceptor region
Conclusions
Heart failure remains a prevalent and difficult-to-treat condition, with high levels of morbidity and mortality. Despite great advances in pharmacologic and device-based treatment of HF, new lines of therapy are sorely needed. It is clear from the preclinical and clinical studies highlighted above that chronic neuromodulation with device therapies such as SCS, VNS, and baroreflex activation are emerging as potential novel avenues for the treatment of heart failure and other cardiovascular conditions. The growing number of active HF trials involving these modalities is testament to the potential of these therapies to emerge as new treatments for patients with heart failure.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics-2010 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2010;121:e1–170.
Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–23.
Pepper GS, Lee RW. Sympathetic activation in heart failure and its treatment with beta-blockade. Arch Intern Med. 1999;159:225–34.
Regitz V, Leuchs B, Bossaller C, et al. Myocardial catecholamine concentrations in dilated cardiomyopathy and heart failure of different origins. Eur Heart J. 1991;12(Suppl):S171–4.
Piacentino III V, Weber CR, Chen X, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–8.
Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–41.
Communal C, Singh K, Pimentel DR, et al. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–34.
Watson AM, Hood SG, May CN. Mechanisms of sympathetic activation in heart failure. Clin Exp Pharmacol Physiol. 2006;33:1269–74.
Boogers MJ, Borleffs CJ, Henneman MM, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769–77.
Fishman GI, Chugh SS, DiMarco J, et al. Sudden cardiac death prediction and prevention: report from a national heart, lung, and blood institute and heart rhythm society workshop. Circulation. 2010;122:2335–48.
Newton GE, Parker AB, Landzberg JS, et al. Muscarinic receptor modulation of basal and beta-adrenergic stimulated function of the failing human left ventricle. J Clin Invest. 1996;98:2756–63.
Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–30.
Kinugawa T, Dibner-Dunlap ME. Altered vagal and sympathetic control of heart rate in left ventricular dysfunction and heart failure. Am J Physiol. 1995;268:R317–23.
Damy T, Ratajczak P, Shah AM, et al. Increased neuronal nitric oxide synthase-derived no production in the failing human heart. Lancet. 2004;363:1365–7.
Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–96.
Dargie HJ, Lechat P. The cardiac insufficiency bisoprolol study (CIBIS-II): a randomized trial. Lancet. 1999;353:9–13.
Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353:2001–7.
Eichorn E, Domanski M, Krause-Steinrauf H, Anderson J, Boardman K, Bristow M. The beta-blocker evaluation of survival trial investigators. A trial of the beta–blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67.
Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–55.
Packer M, Coats AJ, Fowler MB, et al. Effect on carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8.
Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. results of a Veterans Administration cooperative study. N Engl J Med. 1986;314:1547–52.
ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2000;283:1967–75.
Giles TD, Thomas MG, Quiroz A, et al. Acute and short-term effects of clonidine in heart failure. Angiology. 1987;38:537–48.
Issa ZF, Ujhelyi MR, Hildebrand KR, et al. Intrathecal clonidine reduces the incidence of ischemia-provoked ventricular arrhythmias in a canine post infarction heart failure model. Heart Rhythm. 2005;2:1122–7.
Cohn JN, Pfeffer MA, Rouleau J, et al. on behalf of the MOXCON Investigators. Adverse mortality effect of central sympathetic inhibition with sustained–release moxonidine in patients with heart failure (MOXCON). Eur J Heart Fail. 2003;5:659–67.
Mahajan A, Moore J, Cesario DA. Use of thoracic epidural anesthesia for management of electrical storm: a case report. Heart Rhythm. 2005;2:1359–62.
Zipes DP, Festoff B, Schaal SF, et al. Treatment of ventricular arrhythmia by permanent atrial pacemaker and cardiac sympathectomy. Ann Intern Med. 1968;68:591–7.
Wilde AA, Bhuiyan A, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–9.
Collura CA, Johnson JN, Moir C, et al. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–9.
Behling A, Moraes RS, Rohde LE, et al. Cholinergic stimulation with pyridostigmine reduces ventricular arrhythmias and enhances heart rate variability in heart failure. Am Heart J. 2003;146:494–500.
Binkley PF, Haas GS, Starling RC, et al. Sustained augmentation of parasympathetic tone with angiotensin–converting enzyme inhibition in patients with congestive heart failure. J Am Coll Cardiol. 1993;21:655–61.
Foreman R, Linderoth B, Ardelt J, et al. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res. 2000;47:367–75.
Southerland EM, Milhorn DM, Foreman RD, et al. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol. 2007;292:H311–7.
• JE Olgin, Takahashi T, Wilson E, et al. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. J Cardiovasc Electrophysiol. 2002;13:475–81. This work provided evidence of the vagomimetic actions of SCS.
•• Issa ZF, Zhou X, Ujhelyi MR, et al. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a post-infarction heart failure canine model. Circulation. 2005;111:3217–20. This work provided evidence that SCS can reduce ischemic ventricular arrhythmias.
Odenstedt J, Linderoth B, Bergfeldt L, et al. Spinal cord stimulation effects on myocardial ischemia, infarct size, ventricular arrhythmia, and noninvasive electrophysiology in a porcine ischemia-reperfusion model. Heart Rhythm. 2011;8:892–8.
•• Lopshire JC, Zhou X, Dusa C, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–94. This work demonstrated that chronic SCS can improve LV function in heart failure.
Lopshire JC, Zhou X, Mullen T, et al. The site and intensity of spinal cord stimulation determines remodeling response in canine heart failure. Hear Rhythm. 2011;8:S78.
Liu Y, Yue WS, Liao SY, et al. Thoracic spinal cord stimulation improves cardiac contractile function and myocardial oxygen consumption in a porcine model of ischemic heart failure. J Cardiovasc Electrophysiol. 2012;23(5):534-540.
Vanoli E, De Ferrari GM, Stramba-Badiale M, et al. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–81.
Li M, Zheng C, Sato T, Kawada T, et al. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4.
Zheng C, Li M, Inagaki M, Kawada T, et al. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc. 2004;7:7072–5.
•• Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high–rate pacing model. Circulation. 2009;2:692–9. This work demonstrated that VNS can improve LV function.
• Sabbah HN, Ilsar I, Zaretsky A, et al. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 2011;16:171–8. This work utilized a novel VNS system in HF and found improved LV function with therapy.
Filippone JD, Bisognano JD. Baroreflex stimulation in the treatment of hypertension. Curr Opin Nephrol Hypertens. 2007;16:403–8.
Heusser K, Tank J, Engeli S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–26.
•• Sabbah HN, Gupta RC, Imai M, et al. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011;4(1):65–70. This work demonstrated a therapeutic benefit of baroreflex stimulation in experimental heart failure.
•• Determining the feasibility of spinal cord neuromodulation for the treatment of chronic heart failure (defeat-HF). Available at: http://clinicaltrials.gov/ct2/show/NCT01112579. This work is the first large human trial of SCS in heart failure patients.. Accessed 1 Apr 2012.
Cornelussen RN, Splett V, Klepfer RN, et al. Electrical modalities beyond pacing for the treatment of heart failure. Heart Fail Rev. 2011;16:315–25.
Spinal cord stimulation for heart failure (SCS HEART). Available at: http://clinicaltrials.gov/ct2/show/NCT01362725. Accessed 1 Apr 2012.
Neurostimulation of spinal nerves that affect the heart. Available at: http://clinicaltrials.gov/ct2/show/NCT01124136. Accessed 1 Apr 2012.
De Ferrari GM, Ezra OB, Ajmone-Marsan N, et al. Chronic vagal stimulation in patients with heart failure is feasible, safe and appears beneficial. Eur Heart J. 2006;27:330–7.
•• Schwartz PJ, Gaetano M, De Ferrari GM. Vagal stimulation for heart failure: Background and first in–man study. Heart Rhythm. 2009;6(11):S76–81. This study was the first report on the use of vagal stimulation in heart failure patients.
CardioFit™ for the treatment of heart failure. Available at: http://clinicaltrials.gov/ct2/show/NCT00461019. Accessed 1 Apr 2012.
•• De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation; a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32(7):847–55. This paper reported on significant early trials of vagus nerve stimulation in heart failure.
Increase of vagal tone in chf (inovate–hf). Available at: http://clinicaltrials.gov/ct2/show/NCT01303718. Accessed 1 Apr 2012.
Neural cardiac therapy for heart failure study (NECTAR–HF). Available at: http://clinicaltrials.gov/ct2/show/NCT01385176. Accessed 1 Apr 2012.
Rheos® diastolic heart failure trial. Available at: http://clinicaltrials.gov/ct2/show/NCT00718939. Accessed 1 Apr 2012.
Lovett EG, Schafer J, Kaufman CL. Chronic baroreflex activation by the Rheos system: an overview of results from European and North American feasibility studies. Proceedings of the Engineering in Medicine and Biology Society, 2009 Annual International Conference of the IEEE, pp 4626–30.
Baroreflex activation therapy in heart failure. Available at: http://clinicaltrials.gov/ct2/show/NCT01484288. Accessed 1 Apr 2012.
Georgakopoulos D, Little WC, Abraham WT, et al. Chronic baroreflex activation: a potential therapeutic approach to treat heart failure with preserved ejection fraction. J Card Fail. 2011;17:167–78.
Rheos HOPE4HF trial. Available at: http://clinicaltrials.gov/ct2/show/NCT00957073. Accessed 1 Apr 2012.
Barostim neo system in the treatment of heart failure. Available at: http://clinicaltrials.gov/ct2/show/NCT01471860. Accessed 1 Apr 2012.
Lopshire JC, Zhou X, Dusa C, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–94.
Disclosures
Conflicts of interest: D.P. Zipes is the US Principal Investigator and J.C. Lopshire is a site Investigator for DEFEAT-HF, sponsored by Medtronic, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopshire, J.C., Zipes, D.P. Device Therapy to Modulate the Autonomic Nervous System to Treat Heart Failure. Curr Cardiol Rep 14, 593–600 (2012). https://doi.org/10.1007/s11886-012-0292-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-012-0292-8