Abstract
Prosthesis-patient mismatch (PPM) is present when the effective orifice area of the inserted prosthetic valve is too small in relation to body size. Its main hemodynamic consequence is to generate higher than expected gradients through normally functioning prosthetic valves. The purpose of this review is to present an update on the present state of knowledge with regard to diagnosis, prognosis, and prevention of PPM. PPM is a frequent occurrence (20% to 70% of aortic valve replacements) that has been shown to be associated with worse hemodynamics, less regression of left ventricular hypertrophy, more cardiac events, and lower survival. Moreover, as opposed to most other risk factors, PPM can largely be prevented by using a prospective strategy at the time of operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction: Definition of Valve Prosthesis-patient Mismatch
Valve prosthesis-patient mismatch (PPM) was first described in 1978 by Rahimtoola [1] as follows: “Mismatch can be considered to be present when the effective prosthetic valve area, after insertion into the patient, is less than that of a normal human valve.” However, for all practical purposes, almost all types of valve replacement have an effective valve area that is less than that of a normal human valve and, nowadays, the term PPM is applied to situations in which the effective orifice area (EOA) of the prosthesis is physiologically too small in relation to the patient’s body size thus resulting in abnormally high postoperative gradient [1–5].
Identification of PPM
Consistent with the aforementioned definition, the parameter that has been used to characterize PPM is the indexed EOA (ie, the EOA of the prosthesis divided by the patient’s body surface area [BSA]). It should be emphasized that, for all practical purposes, the indexed EOA as measured in vivo postoperatively is the only parameter that can consistently be correlated with postoperative gradients as well as clinical outcomes [5, 6]. In contrast to EOA, the geometric orifice area (GOA) is a static manufacturing specification based on the ex vivo measurement of the diameter of the prosthesis and the criteria used for its measurement may differ considerably from one prosthesis to the other. Thus, the GOA always overestimates the EOA but in widely varying proportions depending on the type of prosthesis. For instance, it can be observed that, for similar values of indexed GOA, peak and mean gradients are more or less twofold in pericardial valves than in homografts [7]. Likewise, because of different flow conditions, the indexed EOAs calculated based on the manufacturer’s in vitro data are generally too optimistic and also correlate poorly with postoperative hemodynamics [8••]. It thus follows that most studies using GOA or in vitro EOA to identify PPM fail to show any significant relationship between these parameters and adverse clinical outcomes [6, 7]. Unfortunately, some authors still do not make that distinction and indiscriminately regroup all studies using either in vivo EOA, GOA, or in vitro EOA to conclude that the clinical relevance of PPM is still unclear and remains controversial [9]. It is now widely accepted and enshrined in the American Society of Echocardiography/American College of Cardiology/American Heart Association/European Association of Echocardiography/Japanese Society of Echocardiography/Canadian Society of Echocardiography guidelines that the in vivo indexed EOA is the only valid parameter to identify PPM and predict postoperative gradients and/or adverse clinical outcomes [10••].
Aortic PPM
An indexed EOA ≤0.85 cm2/m2 is now widely accepted as the threshold for PPM in the aortic position [2, 3, 5, 6, 10••] with values between 0.65 and 0.85 cm2/m2 being classified as moderate PPM and those below 0.65 cm2/m2 as severe PPM. It should be of no surprise that these values are very close to those used in the case of native aortic stenosis. Depending on studies, the reported prevalence of moderate PPM varies between 20% and 70% whereas that of severe PPM is between 2% and 11%. As for native aortic stenosis, the impact of PPM on clinical outcomes increases with severity and the categorization between moderate and severe PPM is thus essential when studying these phenomena. It should also be noted that the prevalence of severe PPM has had a tendency to decrease substantially over the past decade due to: 1) increased recognition and awareness that, notwithstanding associated conditions, severe PPM is invariably associated with adverse outcomes and that it should thus be avoided as much as possible; 2) more widespread implementation of preventive strategies designed to avoid PPM; and 3) improved design and hemodynamic performance of newer-generation prostheses.
Mitral PPM
Because of the lower pressure regimen and similarly to native mitral valve stenosis, the threshold values for mitral PPM are higher than for aortic PPM. Thus, mitral PPM is considered moderate when the indexed EOA is ≤1.2 to 1.3 cm2/m2 and severe when it is ≤0.9 to 1.0 cm2/m2 [3, 4, 10••, 11]. Recent studies report that the incidence of mitral PPM is much higher than previously believed: 30% to 70% and 5% to 10% for moderate and severe PPM, respectively [11, 12••, 13–15]. Awareness with regard to mitral PPM is more recent and with applicable strategies being more limited, it is too early to determine to what extent it can be prevented.
Clinical Impact of Aortic PPM
There is now a strong body of evidence showing that aortic PPM is an important risk factor with regard to various clinical outcomes including improvement in functional class, regression of left ventricular (LV) hypertrophy, and both early and late survival.
LV Hypertrophy and Function
Whereas some authors have found that the persistence of PPM results in lesser regression of LV hypertrophy (LVH), others have reported that patients with PPM and/or small prostheses could exhibit significant reductions in LV mass and, on this basis, have concluded that PPM was not an important issue. In this context, the following important pathophysiologic concepts should be remembered [16, 17]. 1) Even in the presence of PPM, surgery normally results in improved hemodynamics, the extent of which can be quite important. 2) A more optimal result can be expected if PPM is completely avoided. 3) In analyzing the results of aortic valve replacement (AVR), it is important to remember that the relationship between gradients and the indexed EOA is curvilinear and that the implications for a given patient will be directly related to his original and final positions on the indexed EOA-gradient curve. Moreover, it is becoming increasingly evident that many patients with aortic stenosis have decreased systemic arterial compliance and concomitant hypertension, resulting in an increased LV hemodynamic load that is only partially relieved by the operation [18]. Thus, these patients often have more severe concentric LVH that only partially regresses after operation. Also, for similar hemodynamic load, patients with hypertension and/or metabolic syndrome have recently been shown to have more interstitial myocardial fibrosis and a relatively greater increase in LV mass [19•]. In this context, Weidemann et al. [20•] reported that myocardial fibrosis is frequent in patients undergoing AVR (22 of 58 patients in their series) and that, in contrast to myocardial cell hypertrophy, it is not reversible for up to 9 months after operation; these patients also have more extensive and irreversible myocardial damage as evidenced by decreases in stroke volume [21], increases in B-type natriuretic peptide levels [22], and selective decreases in LV longitudinal shortening [23, 24] that do not regress after operation. Thus, it is becoming increasingly evident that the regression of LVH after AVR is related to many factors beyond PPM.
LV Function
As for LVH, recent studies also show that in patients with severe aortic stenosis and depressed LV systolic function, postoperative improvements in LV ejection fraction and patient functional capacity are largely dependent on the extent of valve EOA improvement achieved by the operation [25, 26]. Thus, the residual LV afterload imposed by PPM negatively impacts on recovery of LV function in these high-risk patients. Noticeably, a recent multicenter study revealed that transcatheter aortic valve implantation was associated with better and faster postoperative improvement in LV ejection fraction compared with surgical AVR [26] and that this beneficial effect can be attributed in large part to the superior valve hemodynamics and lower incidence of PPM achieved by transcatheter valve implantation [26, 27••, 28••].
Coronary Flow Reserve
In the same context, Rajappan et al. [29] demonstrated that the severity of impairment of coronary flow reserve (CFR) measured by positron emission tomography was related to the severity of valve stenosis (valve EOA, gradient, LV systolic pressure) rather than to LV mass and that changes in CFR after AVR were not directly related to regression of LV mass but were rather dependent on the magnitude of the change in valve EOA achieved with AVR [30]. Accordingly, Garcia et al. [31] have reported that when the aortic valve indexed EOA is larger than 0.8 to 0.9 cm2/m2, there is no significant impact on CFR which decreases sharply when the indexed EOA is lower than this threshold and becomes almost completely exhausted when the indexed EOA is below 0.5 cm2/m2.
Early Mortality
The impact of PPM is more important on early rather than late mortality given that the left ventricle is more vulnerable during the early postoperative period than later on. In this regard, there is general agreement that early mortality is significantly increased in patients with PPM particularly if severe or associated with LV dysfunction [6, 32–34]. Thus, in our original series of 1,265 consecutive patients undergoing AVR [32], overall early mortality was 4.6% whereas the risk ratio of moderate PPM compared to no PPM was 2.1 (95% CI, 1.2–3.7) and that of severe PPM was 11.4 (95% CI, 4.4–29.5). Moreover, the adverse impact of PPM was much more evident in patients having impaired LV function (ejection fraction ≤40%). Thus, mortality was 5% in patients with moderate PPM and preserved LV function compared to 16% in patients with moderate PPM and depressed LV function and a prohibitively high 67% in patients with the combination of severe PPM and poor LV function. This strong interaction between PPM and depressed LV function has subsequently been confirmed not only in relation to early mortality but also with regard to the occurrence of heart failure as well as late survival [34–36]. In light of these results, avoidance of potential PPM should become a particularly mandatory consideration in patients with LV dysfunction because these high-risk patients have a decreased ventricular reserve and are thus more vulnerable to the different degrees of PPM particularly in the perioperative period.
Late Mortality
The controversy with regard to this variable continues. Thus, several recent studies confirm that PPM is independently associated with reduced late survival [33–38••], whereas others report the opposite [39–41]. In this context, it is becoming increasingly clear that the analysis of patients’ characteristics is of paramount importance when interpreting such data and in this sense the findings of a recent study from our laboratory [38••] may provide some insight into the discrepancies observed in previous studies. Thus, our results with regard to late mortality in a series of 2,576 patients having survived AVR showed that moderate PPM was detrimental only in patients with pre-existing LV dysfunction but not in those with preserved LV function, whereas severe PPM increased mortality only in patients less than 70 years old and/or with a BMI less than 30 kg/m2 and/or an LV ejection fraction less than 50% but not in patients without these characteristics. Other studies have also reported similar findings with regard to age and LV function [35, 36, 42]. In contrast, studies reporting negative results often do not consider LV function [39] and/or include a large proportion of elderly patients and/or have a high prevalence of obesity in their patients with PPM [41]. The lack of significant impact of PPM on survival in the obese population is most likely related to the fact that the utilization of the BSA for the normalization of EOA may not be appropriate in this population and future studies will be necessary to determine if this purpose would not be better served by the utilization of height or lean body mass.
Exercise Capacity and Quality of Life
Besides the extension of longevity of life, the improvement in the quality of life is an essential and important objective of AVR. Several studies have shown that PPM is associated with reduced functional capacity and quality of life. In a study in which maximum exercise testing was systematically performed at 6 months post AVR in a consecutive series of 312 patients, Bleiziffer et al. [43] found that PPM was a powerful independent predictor of reduced exercise capacity. In the elderly population, several studies have also reported that although moderate PPM does not necessarily alter late survival, it does impair quality of life.
Miscellaneous Outcomes
Other negative outcomes previously reported in association with aortic PPM are a higher occurrence of late congestive heart failure [35] as well as abnormalities of the von Willebrand factor and associated bleeding complications [44]. More recently, Mannacio et al. [45] showed that exercise-induced arrhythmias are more frequent in patients with PPM, whereas Unger et al. [46] observed that there is a tendency for these patients to have more important residual mitral regurgitation after operation. Finally, Flameng et al. [47] showed that PPM is an important risk factor for early (2–3 years after AVR) stenosis-type structural valve deterioration of bioprostheses.
Clinical Impact of Mitral PPM
For a long time, mitral PPM remained quite unexplored and might have been thought to be a relatively rare phenomenon with minimal impact on postoperative outcomes. However, recent studies demonstrate that this is not the case and that mitral PPM is not uncommon and is independently associated with worse hemodynamic and clinical outcomes following mitral valve replacement (MVR). PPM has been shown to be associated with persisting pulmonary hypertension, increased incidence of congestive heart failure, and reduced survival after MVR [12••, 13, 15]. As for aortic PPM, early and late mortality would seem to be affected mainly by severe PPM but further studies are necessary to determine if, as for aortic prosthesis, moderate mitral valve PPM could not be detrimental in some specific subgroups of patients. For instance, a recent study has suggested a possible interaction between preoperative pulmonary hypertension and either moderate or severe PPM [14].
Prevention of PPM
Aortic Valve Replacement
As opposed to most other risk factors associated with adverse clinical outcomes after AVR, PPM is modifiable and can be largely avoided by using a simple strategy at the time of operation [5, 6]. Our original description of this strategy was as follows:
-
Step 1: Calculate patient’s BSA from patient’s weight and height;
-
Step 2: Multiply BSA by 0.85 cm2/m2, the result being the minimal EOA that the prosthesis to be implanted should have to avoid PPM. For instance, if patient’s BSA is 1.80 m2, then 1.80 × 0.85 = 1.53 cm2 = minimal EOA to completely avoid PPM;
-
Step 3: Chose the prosthesis in light of the result obtained in step 2 and the reference values for the different types and sizes of prosthesis (Table 1). Thus, if the surgeon had intended to implant a Carpentier-Edwards Perimount prosthesis in the example chosen, the minimum size to obtain an EOA greater than 1.53 would have been a size 23 or greater. Fortunately, most prosthesis manufacturers have now made this exercise easier by providing charts that give the projected indexed EOAs for the different levels of patient’s BSA and prosthesis sizes. With regard to reference values for EOA and indexed EOA, there are three important caveats worth reiterating: 1) The values should be derived from in vivo rather than the in vitro data supplied by the manufacturers because the latter are usually too optimistic, particularly in the case of stentless valves. Moreover, the in vivo reference EOAs should be derived from reliable sources (ie, from studies including a sufficient number of patients in each model/size subcategory and using adequate Doppler-echocardiographic methods for EOA measurement [8••]). 2) Reference values derived from geometric measurements (eg, internal diameters or geometric areas) are inadequate because they bear no relation to postoperative hemodynamics and particularly if different types of valves are being compared (see aforementioned considerations). 3) When using these charts (or Tables 1 and 2), it is important to remember that there are often important discrepancies between the sizers supplied by the manufacturers for the different types of prostheses and that for a given patient annulus size, the labeled size that fits may vary from one type of prosthesis to the other.
Table 1 Normal reference values of effective orifice areas for the aortic valve prostheses Table 2 Normal reference values of effective orifice areas for the mitral valve prostheses
The validity and feasibility of this strategy is now widely accepted and we believe that, given its simplicity and rapidity, this exercise should be performed in every patient undergoing AVR. Depending on the result, if moderate PPM is anticipated in a patient with certain characteristics (eg, depressed LV function and/or severe LVH, young age [<70 years old], athletic lifestyle, and an elderly patient seeking enhanced quality of life) or a severe PPM in any given patient, the following strategies can be considered: 1) the implantation of a prosthesis with a better hemodynamic performance (eg, a newer generation of stented bioprosthesis or bileaflet mechanical valve implanted in a complete supra-annular position or a stentless bioprosthesis); or 2) the performance of an aortic root enlargement, to accommodate a larger size of the same type of prosthesis.
Unfortunately, some recent papers have challenged the use of this approach based on the false premise that the first-line strategy, if not the only one, for avoiding PPM would be aortic root enlargement and thus possibly be associated with an increased operative mortality particularly in the elderly. In reality, given the significant improvements in prostheses design, contemporary prevention of PPM can largely be accomplished by the implantation of prosthetic models providing a better hemodynamic performance (Table 1). The study by Bleiziffer et al. [8••] is particularly illustrative in this regard whereby these investigators were able to reduce the incidence of moderate PPM from 44% to 30% and that of severe PPM from 9% to 1% by applying strategy #1 described above. Conversely, there is a variance of opinions with regard to aortic root enlargement for the purpose of avoiding PPM, many groups having reported excellent results using this type of strategy. Furthermore, in patients with a small BSA, the preoperative calculation of the projected indexed EOA at the time of operation can be used to validate the use of a small prosthesis and thus avoid more aggressive procedures such as aortic root enlargement. Whereas some might still advocate systematic avoidance of smaller prostheses, several studies demonstrate that such prostheses may be safe and adequate in patients with smaller BSAs, pending calculation of the projected indexed EOA before operation.
Finally, recent studies have reported that valve hemodynamics are superior with transcatheter aortic valve implantation than with surgical AVR, especially in the subset of patients with small aortic root [26, 27••, 28••]. Thus, transcatheter valve implantation should provide another valuable alternative to avoid PPM in high-risk patients and yet provide a less invasive procedure.
Mitral Valve Replacement
The rationale for the prevention of mitral PPM is the same as for the aortic valve. However, it is a much more demanding challenge given that the techniques allowing the implantation of a larger-size prosthesis are much more complex and as of yet unproven with regard to their risk/benefit ratio [15]. For the time being, the preventive strategy should therefore be focused on the implantation of the prosthesis having the largest EOA for a given size (Table 2). This observation also underlines the need for the development of better performing prostheses and provides further motivation for proceeding to repair rather than replacement whenever possible.
PPM in the Context of the Interpretation of High Postoperative Gradients
PPM is by far the most frequent cause of high gradient after valve replacement, the other potential causes being a central jet artifact in bileaflet prosthesis, intrinsic valve dysfunction, high flow states, or technical errors [10, 48]. Various algorithms aimed at better interpreting these high gradients have previously been proposed by us and others [6, 10••, 48, 49•]. However, their acceptability by clinicians has varied and they have been criticized for being either too complicated for routine clinical use or too simplistic to cover all possibilities.
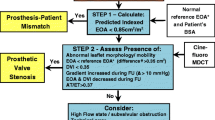
In this paper, we present yet another alternative that is somewhat a compendium of previous algorithms and that can hopefully answer both criticisms (Fig. 1).Thus, after having excluded possible technical errors and given that PPM is by far the most frequent cause of high gradients, the first step would be to simply calculate the projected indexed EOA (ie, EOA/BSA using the normal reference value of EOA (Tables 1 and 2) for the type and size of prosthesis having been implanted). If the result is less than 0.85 cm2/m, one can then surmise that PPM is present and that depending on its degree of severity, it may be partially or totally responsible for the high gradient. In this context, it is important to keep in mind that both phenomenon (ie, PPM and intrinsic dysfunction) may coexist. To fully appreciate the clinical impact of the gradient, it should also be remembered that the net gradient is less in patients with a small aortic diameter (ie, <3.0 cm) due to pressure recovery and it might be useful in such cases to calculate the extent of pressure recovery and the energy loss index [50]. If PPM is not present or not severe enough to totally explain the increased gradient, the next step would be to thoroughly evaluate leaflet morphology and mobility; in the case of mechanical valves, cine-fluoroscopy should mandatorily be performed in addition to echocardiography [10••]. If leaflet mobility is abnormal, dysfunction should be suspected and this can be corroborated if the EOA actually measured on the echo is much lower than the normal reference value for the same type and size of prosthesis (Tables 1 and 2) and/or has decreased progressively during follow-up. If, conversely, there is still uncertainty with regard to possible abnormal leaflet mobility, one can then proceed to calculate the Doppler velocity index (DVI), a readily available dimensionless quantity obtained by dividing maximal Doppler velocity in the LV outflow tract by the maximal velocity through the valve. If DVI is less than 0.35 and the prosthesis is a bileaflet valve with normal leaflet mobility, the high gradient is in all likelihood due to a central jet artifact; however, if the valve is not bileaflet, intrinsic dysfunction should be re-considered as above and the possibility of a technical error should also be envisioned. If the DVI is greater than 0.35, possible etiologies for the high gradient include high flow states, aortic regurgitation, subvalvular obstruction, and again a technical error. It should be noted that technical errors particularly with regard to measurements in the LV outflow tract diameter are frequent and that this possibility should never be neglected. Finally, it should be remembered that in patients with low flow states due to LV dysfunction, transprosthetic gradients may be only slightly or moderately elevated despite the presence of significant PPM or prosthesis dysfunction. Gradients are highly flow-dependent and may thus be “pseudo-normalized” in the presence of low flow states. After adjusting indexed EOA and DVI values, the same rationale as above can be applied to the interpretation of high gradients after MVR [10••, 48].
Conclusions
PPM is a frequent and modifiable risk factor leading to more frequent adverse clinical outcomes in patients undergoing valve replacement. The risk of PPM should be systematically evaluated at the time of operation by calculating the projected indexed EOA of the prosthesis to be implanted and in the case of anticipated PPM, alternative options should be considered in light of patient’s overall clinical condition and risk/benefit ratio. Awareness of the concept of PPM is also essential to correctly interpreting abnormally high gradients that may be recorded after valve replacement.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. 1978;58:20–4.
Dumesnil JG, Honos GN, Lemieux M, Beauchemin J. Validation and applications of indexed aortic prosthetic valve areas calculated by Doppler echocardiography. J Am Coll Cardiol. 1990;16:637–43.
Dumesnil JG, Yoganathan AP. Valve prosthesis hemodynamics and the problem of high transprosthetic pressure gradients. Eur J Cardio-thorac Surg. 1992;6(Suppl I):S34–8.
Dumesnil JG, Honos GN, Lemieux M, Beauchemin J. Validation and applications of mitral prosthetic valvular areas calculated by Doppler echocardiography. Am J Cardiol. 1990;65:1443–8.
Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol. 2000;36:1131–41.
Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart. 2006;92:1022–9.
Koch CG, Khandwala F, Estafanous FG, Loop FD, Blackstone EH. Impact of prosthesis-patient size on functional recovery after aortic valve replacement. Circulation. 2005;111:3221–9.
•• Bleiziffer S, Eichinger WB, Hettich I, Guenzinger R, Ruzicka D, Bauernschmitt R, et al. Prediction of valve prosthesis-patient mismatch prior to aortic valve replacement: which is the best method? Heart 2007;93:615–20. This paper is very important from two standpoints: 1) it shows that the calculation of the projected indexed EOA from in vivo values is by far the best method to predict PPM; 2) it shows that the prevalence of PPM can significantly be reduced by the use of a preventive strategy based on the calculation of this parameter.
Monin JL. Prosthesis-patient mismatch: myth or reality? Heart. 2009;95:948–52.
•• Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975–1014. These are the first comprehensive guidelines with regard to valve prosthesis evaluation by Doppler-echocardiography. Consensus recommendations with regard to assessment and impact of aortic and mitral PPM are presented. This article should be read in priority by all those concerned with this topic.
Li M, Dumesnil JG, Mathieu P, Pibarot P. Impact of valve prosthesis-patient mismatch on pulmonary arterial pressure after mitral valve replacement. J Am Coll Cardiol. 2005;45:1034–40.
•• Magne J, Mathieu P, Dumesnil JG, Tanné D, Dagenais F, Doyle D, et al. Impact of prosthesis-patient mismatch on survival after mitral valve replacement. Circulation 2007;115:1417–25. This is the first evidence of the negative impact of mitral PPM on survival.
Lam BK, Chan V, Hendry P, Ruel M, Masters R, Bédard P, et al. The impact of patient-prosthesis mismatch on late outcomes after mitral valve raplacement. J Thorac Cardiovasc Surg. 2007;133:1464–73.
Jamieson WR, Germann E, Ye J, Chan F, Cheung A, MacNab JS, et al. Effect of prosthesis-patient mismatch on long-term survival with mitral valve replacement: assessment to 15 years. Ann Thorac Surg. 2009;87:1135–41.
Aziz A, Lawton JS, Maniar HS, Pasque MK, Damiano Jr RJ, Moon MR. Factors affecting survival after mitral valve replacement in patients with prosthesis-patient mismatch. Ann Thorac Surg. 2010;90:1202–11.
Tasca G, Brunelli F, Cirillo M, Dalla Tomba M, Mhagna Z, Troise G, et al. Impact of the improvement of valve area achieved with aortic valve replacement on the regression of left ventricular hypertrophy in patients with pure aortic stenosis. Ann Thorac Surg. 2005;79:1291–6.
Dumesnil JG. Invited commentary. Ann Thorac Surg. 2005;79:1296.
Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–8.
• Pagé A, Dumesnil JG, Clavel MA, Chan KL, Teo K, Tam JW, et al. Metabolic syndrome is associated with more pronounced impairment of LV geometry and function in patients with calcific aortic stenosis: A substudy of the ASTRONOMER trial. (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin). J Am Coll Cardiol 2010;55:1867–74. This paper presents new evidence showing that LVH is polymorphic and not only conditioned by valve hemodynamics
• Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577–84. This paper presents new evidence showing that LVH is polymorphic and not only conditioned by valve hemodynamics.
Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–64.
Bergler-Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, et al. B-type natriuretic peptide in low-flow, low-gradient aortic stenosis: relationship to hemodynamics and clinical outcome. Circulation. 2007;115:2848–55.
Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation. 1979;59:1024–34.
Pibarot P, Dumesnil JG. Longitudinal myocardial shortening in aortic stenosis: ready for prime time after 30 years of research? Heart. 2009;96:95–6.
Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, et al. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS Study. Circulation. 2008;118(14 Suppl):S234–42.
Clavel MA, Webb J, Rodés-Cabau J, Masson JB, Dumont E, De Larochelliere R, et al. Comparison between surgical and transcatheter prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1943–51.
•• Clavel MA, Webb JG, Pibarot P, Altwegg L, Dumont E, Thompson C, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009;5:1883–91. This study is one of the first to show that percutaneous aortic valve implantation provides superior hemodynamic performance compared with the surgical bioprostheses both in terms of transprosthetic gradient and prevention of severe PPM.
•• Jilaihawi H, Chin D, Spyt T, Jeilan M, Vasa-Nicotera M, Bence J, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the Medtronic-Corevalve bioprosthesis. Eur Heart J 2010;31:857–64. This study is one of the first to show that percutaneous aortic valve implantation provides superior hemodynamic performance compared with the surgical bioprostheses both in terms of transprosthetic gradient and prevention of severe PPM.
Rajappan K, Rimoldi O, Camici PG, Pennell DJ, Sheridan DJ. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470–6.
Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–5.
Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, et al. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol. 2009;106:113–21.
Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation. 2003;108:983–8.
Walther T, Rastan A, Falk V, Lehmann S, Garbade J, Funkat AK, et al. Patient prosthesis mismatch affects short- and long-term outcomes after aortic valve replacement. Eur J Cardiothorac Surg. 2006;30:15–9.
Urso S, Sadaba R, Aldamiz-Echevarria G. Is patient-prosthesis mismatch an independent risk factor for early and mid-term overall mortality in adult patients undergoing aortic valve replacement? Interact Cardiovasc Thorac Surg. 2009;9:510–8.
Ruel M, Al-Faleh H, Kulik A, Chan K, Mesana TG, Burwash IG. Prosthesis-patient mismatch after aortic valve replacement primarily affects patients with pre-existing left ventricular dysfunction: impact on survival, freedom from heart failure, and left ventricular mass regression. J Thorac Cardiovasc Surg. 2006;131:1036–44.
Bleiziffer S, Ali A, Hettich IM, Akdere D, Laubender RP, Ruzicka D, et al. Impact of the indexed effective orifice area on mid-term cardiac-related mortality after aortic valve replacement. Heart. 2010;96:865–71.
Mihaljevic T, Nowicki ER, Rajeswaran J, Blackstone EH, Lagazzi L, Thomas J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–8.
•• Mohty D, Dumesnil JG, Echahidi N, Mathieu P, Dagenais F, Voisine P, et al. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol 2009;53:39–47. Many discrepancies between studies examining the influence of PPM on long-term survival can be reconciled based on this paper.
Mascherbauer J, Rosenhek R, Fuchs C, Pernicka E, Klaar U, Scholten C, et al. Moderate patient-prosthesis mismatch after valve replacement for severe aortic stenosis has no impact on short- and long term mortality. Heart. 2008;94:1639–45.
Howell NJ, Keogh BE, Ray D, Bonser RS, Graham TR, Mascaro J, et al. Patient-prosthesis mismatch in patients with aortic stenosis undergoing isolated aortic valve replacement does not affect survival. Ann Thorac Surg. 2010;89:60–4.
Jamieson WR, Ye J, Higgins J, Cheung A, Fradet GJ, Skarsgard P, et al. Effect of prosthesis-patient mismatch on long-term survival with aortic valve replacement: assessment to 15 years. Ann Thorac Surg. 2010;89:51–8.
Moon MR, Lawton JS, Moazami N, Munfakh NA, Pasque MK, Damiano Jr RJ. POINT: Prosthesis-patient mismatch does not affect survival for patients greater than 70 years of age undergoing bioprosthetic aortic valve replacement. J Thorac Cardiovasc Surg. 2009;137:278–83.
Bleiziffer S, Eichinger WB, Hettich I, Ruzicka DJ, Wottke M, Bauernschmitt R, et al. Impact of prosthesis-patient mismatch on exercise capacity in patients after bioprosthetic aortic valve replacement. Heart. 2008;94:637–41.
Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–9.
Mannacio VA, De Amicis V, Di Tommaso L, Iorio F, Vosa C. Influence of prosthesis-patient mismatch on exercise-induced arrhythmias: a further aspect after aortic valve replacement. J Thorac Cardiovasc Surg. 2009;138:632–8.
Unger P, Dedobbeleer C, Van Camp G, Plein D, Cosyns B, Lancellotti P. Mitral regurgitation in patients with aortic stenosis undergoing valve replacement. Heart. 2009;96:1627–32.
Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–9.
Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–48.
• Bach DS. Echo/Doppler evaluation of hemodynamics after aortic valve replacement: Principles of interrogation and evaluation of high gradients. J Am Coll Cardio Img 2010;3:296-304. This paper interestingly complements previous attempts to analyze this problem (see Pibarot and Dumesnil [6], Zoghbi et al. [10••], and Pibarot and Dumesnil [48]) and provides further impetus for the conception of Figure 1.
Garcia D, Pibarot P, Dumesnil JG, Sakr F, Durand LG. Assessment of aortic valve stenosis severity: a new index based on the energy loss concept. Circulation. 2000;101:765–71.
Acknowledgments
This study was supported in part by Canadian Institutes of Health Research grants (MOP-10929, MOP-57745, MOP-67123, and MOP-86666). P. Pibarot is the holder of the Canada Research Chair in Valvular Heart Disease, Canadian Institutes of Health Research, Ottawa, Canada.
Disclosure
Conflicts of interest: J.G. Dumesnil: none; P. Pibarot: has received speakers’ honoraria from Sorin Medical and Edwards Lifesciences and has received research grants from Medtronic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dumesnil, J.G., Pibarot, P. Prosthesis-Patient Mismatch: An Update. Curr Cardiol Rep 13, 250–257 (2011). https://doi.org/10.1007/s11886-011-0172-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-011-0172-7