Abstract
Purpose of Review
Atherosclerosis (AS) is a chronic inflammatory disease that contributes to the development of coronary artery disease, which has become a leading health burden worldwide. Though several strategies such as pharmacological treatment, exercise intervention, and surgery have been used in clinical practice, there is still no effective strategy to cure AS. Exosomes are extensively studied both as diagnostic markers as well as for therapeutic purposes due to their role in pathological processes related to AS. To elucidate the role of exosomes in AS and thus provide a new insight into AS therapy, we review recent advances concerning exosome targets and their function in mediating intercellular communication in AS, and expect to provide a reference for novel effective strategies to cure AS.
Recent Findings
Exosomes exert important roles in the diagnosis, development, and potential therapy of AS. For AS development, (1) activation of CD-137 in endothelial cells represses exosomal-TET2 production, causing a phenotypic switch of vascular smooth muscle cells (VSMC) and promoting plaque formation; (2) exosomal-MALTA1 derived from endothelial cells causes neutrophil extracellular traps (NETs) and M2 macrophage polarization, which aggravates AS; and (3) exosomal-miR-21-3p derived from macrophages inhibits PTEN expression and further promotes VSMC migration/proliferation, leading to AS development. For AS diagnosis, plasma exosomal-miR30e and miR-92a are considered to be potential diagnostic markers. For AS therapy, adipose mesenchymal stem cell-derived exosomes protect endothelial cells from AS aggravation, via inhibiting miR-342-5p.
Summary
Exosome-mediated cross-talk between different cells within the vasculature exerts crucial roles in regulating endothelial function, proliferation and differentiation of vascular smooth muscle cells, and platelet activation as well as macrophage activation, collectively leading to the development and progression of AS. Exosomes can potentially be used as diagnostic biomarkers and constitute as a new therapeutic strategy for AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis (AS) is a chronic inflammatory disease caused by lipid accumulation in the arterial vessel, which is considered a leading cause of coronary artery disease (CAD) and peripheral vascular disease [1, 2]. The elevation of cholesterol (low density lipoprotein, LDL) in plasma is considered to be the main risk factor that accelerates the development of AS. Abnormal lipid accumulation can be induced by high-fat diet [3], smoking [4], and alcohol intake [5] etc. By contrast, interventions such as physical exercise or increase in high-density lipoprotein (HDL) can inhibit plaque formation and AS development [6, 7]. Mechanistically, there are four stages for AS development: (1) reversible lipid striation, (2) intimal fibrous plaque formation, (3) atheromatous plaque establishment, and (4) complicated lesion. Various cell types are involved in the development of AS, including endothelial cells, vascular smooth muscle cells (VSMC), macrophages, and platelets [8]. Endothelial cell dysfunction, excessive VSMC proliferation, platelet activation, and macrophage migration all contribute to the acceleration of AS.
Extracellular vesicles (EVs) are membrane-enclosed and nanosized particles. They were originally reported in the 1960s by Bonucci and Anderson [9, 10]. Over the years, basic knowledge of EVs has been extended. EVs (40–1000 nm in diameter) are released by most kinds of cells and exert important roles in several pathological and physiological processes. EVs can be classified into two subgroups: exosomes and microvesicles, based on their size, biogenesis, and properties (differences are summarized in Table 1). Exosomes (40–150 nm in diameter) are produced from endosome (actually, emerging evidence shows that exosomes can also bud from the plasma membrane). Microvesicles (100–1000 nm in diameter) are released from membrane budding. Notably, the minimal information for studies of extracellular vesicles 2018 (MISEV2018) guidelines recommend referring to exosomes as small EVs (< 200 nm in diameter) to describe endosome-origin exosomes of that particular size. Because of the overlap in their sizes, we present exosomes in this review instead of small EVs.
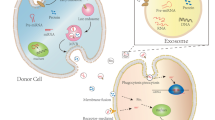
Accumulating evidence shows that exosomes as well as their molecular cargo are closely associated with the occurrence and development of AS [11, 12]. In this review, we take an insight into the role of exosomes in the development of AS. In addition to discussing the complexity of origin of the exosomes, we also summarize the cellular targets of exosomes derived from AS patients or experimental animal models, as well as their mechanistic role in AS diagnosis, development, and therapy (Fig. 1).
Exosomes as a multifaceted messenger in atherosclerosis. Exosomes and exosomal molecules exert important roles in the diagnosis, development, and therapy for atherosclerosis (AS). Plasmatic exosomal-miR30e and miR-92a are significantly increased in AS patients, which is a potential biomarker for AS diagnosis. Exosome-mediated endothelial cell-vascular smooth muscle cell (VSMC), endothelial cell-macrophage, and macrophage-VSMC cross-talk contribute to AS aggravation. Besides, exosomes derived from adipose tissue mesenchymal stem cell (ADSC) protect endothelial cells against apoptosis and AS development, via inhibiting miR-342-5p. AS, atherosclerosis; TET2, ten-eleven translocation 2; VSMC, vascular smooth muscle cell; MALTA1, metastasis associated in lung adenocarcinoma transcript 1; PTEN, phosphatase and tensin homolog deleted on chromosome 10; ADSC, adipose-derived mesenchymal stem cell; PPP1R12B, protein phosphatase 1 regulatory subunit 12B
Pathophysiological Basis of Atherosclerosis
AS is a systemic chronic disease, which is characterized by abnormal deposition of lipid in the arterial wall. When the hemostatic balance is disturbed, the excess of lipids deposited in the blood vessels will penetrate into the arterial wall, accumulating in the endarterium, leading to plaque formation and arterial thickening [13]. AS results from various causes such as dyslipidemia, hypertension, smoking, and genetic heredity causes. Clinically, pharmacological treatments, exercise interventions, and surgical approaches are used to attenuate AS development. However, there is no therapy that can cure AS [14,15,16]. Exercise improves physical capacity and cardiovascular health [17, 18], yet it is not a perfect method to cure AS. Therefore, there is an unmet need for new effective strategies for AS treatment.
Exosomes
Exosome, 40–150 nm in diameter, is a subtype of EVs and encloses with lipid membrane. Exosomes can be produced by almost all cell types and they can be isolated from a variety of biofluids, such as urine, blood, and saliva. Upon direct fusion of the exosomes to the plasma membrane of the recipient cells, molecular contents of the exosomes are transferred to the recipient cell resulting in modulation of signaling pathways and gene expression. Exosomes can contain a variety of molecular cargo (miRNAs, proteins, lipids), and given that they mediate intercellular cross-talk, exosomes play a crucial role in regulating various physiological or pathological processes such as signal transfer, cell survival, and apoptosis. Notably, a large body of research indicates that miRNAs in the exosomes modulate expression of target genes in the recipient cells.
In recent years, exosomes have received extensive attention as a potential therapeutic strategy for CVDs owing to their high abundance, biocompatibility, non-immunogenicity, and plasticity [19, 20]. Exosomes are considered to be a potential therapeutic tool for many pathologies, including cardiovascular diseases [21]. For example, intravenous administration of mesenchymal stem cell (MSC)-derived exosomes can cause macrophage polarization to M2 phenotype and reduction of their infiltration to the vascular wall, which significantly attenuate plaque area in AS [22].
Biogenesis of Exosomes
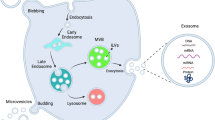
Briefly, the biogenesis of exosomes involves 3 steps: (1) early endosome formation by inward invagination of the plasma membrane; (2) intraluminal vesicles (ILVs) and multivesicular bodies (MVBs) formation by further inward budding of the endosomal membrane; (3) release of exosomes by fusion of MVBs with the plasma membrane. MVB formation is driven by two different mechanisms: endosomal sorting complexes required for transport machinery (ESCRT)-dependent and ESCRT-independent pathway [23•, 24••].
ESCRT-Dependent Pathway
ESCRT, a driver of membrane shaping and scission, constitutes the main sorting machinery during MVB maturation. ESCRT is composed of four complexes (ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III) plus other associated proteins (Alix, VPS4 etc.). ESCRT-0 has ubiquitin-binding domains, which is responsible for ubiquitylated protein recognition, cargo clustering, and initiate the ESCRT machinery. The complex then interacts with ESCRT-I and ESCRT-II, inducing bud formation. The total complex then recruits and combines with ESCRT-III. Subsequently, ESCRT-III drives vesicles abscission with energy supplement by VPS4 ATPase [25]. Besides, several members of ESCRT were required for MVB formation and exosome secretion. For example, Tsg101 (member of ESCRT-I) has been reported to be associated with endosomal membrane budding and cargo clustering. Deletion of Tsg101 can significantly reduce exosome secretion in tumor cells [26]. ARF6 (small GTPase ADP ribosylation factor 6) and PLD2 (phospholipase D2) play important roles in exosome biogenesis via affecting MVB formation. Additionally, the role of syndecan–syntenin–ALIX in regulating exosomes biogenesis was investigated by Maria et al. in the previous research [27]. Inhibitors, such as manumycin A, can block ESCRT-dependent exosome biogenesis and lead to inhibition of exosome release [24••]. Notably, in mammalian cells, ESCRT complex drives not only exosome biogenesis but also cytokinesis release and virus budding [28]. Hence, it is necessary to take the side effects into consideration when we choose ESCRT inhibition as a method of inhibiting exosome secretion [25].
ESCRT-Independent Pathway
Susanne Stuffers et al. found that ESCRT-associated protein (Tsg101, Hrs, Vps22, and Vps24) deletion did not affect the multivesicular endosome formation, indicating that MVB formation and exosome biogenesis can be driven by an ESCRT-independent pathway [29]. Three mechanisms were found to drive the MVB formation and exosome biogenesis in an ESCRT-independent way: lipid rafts, the tetraspanin family, and heat shock proteins. (1) Neutral sphingomyelinase (nSMase) inhibition leads to decreased exosome secretion via ceramide reduction in oligodendroglial cells [24••]. Ceramide, a rigid lipid, can induce inward curvature of the MVBs membrane. Hence, GW4869 was used as an inhibitor for exosome release upon its effects on nSMase inhibition [30]. (2) The tetraspanin family (CD9, CD63, and CD81) was shown to be associated with endosomal particle sorting in the absence of ESCRT complex. These four-transmembrane domain proteins play important roles in cargo selection. For example, CD63 takes participate in cargo (such as melanosomes) sorting into ILVs in a ceramide-independent manner [31]. (3) Chaperone hsc70 mediates cargo selection via recruiting the transferrin receptor (TFR) to MVB and interacting with the endosomal membrane [32]. Therefore, both ESCRT-dependent and -independent pathways can mediate sorting and release of exosomes, and the contribution of these two pathways depends on the exosomal machinery and cell type of origin.
Characterization of Exosomes
To characterize exosomes, many aspects should be taken into consideration according to MISEV2018 guidelines [33•]. In brief, transmission electron microscopy is the gold standard for exosome characterization and morphological observation. TEM imaging shows that EVs have cup-shaped or spherical morphology and membrane-enclosed structures. To quantify exosomes or extracellular vesicles, light scattering technologies have been extensively used in experimental practice, including nanoparticle tracking analysis (NTA) [34] and flow cytometry [35,36,37]. Additionally, detection of exosomal protein markers such as tetraspanins (CD9, CD63, CD81) and tumor susceptibility gene 101 (Tsg101) is also suggested to identify exosomes. Notably, when analyzing the function of exosomes, comparing their activity with that of other subtypes (for instance, either large EVs or exosome-depleted fractions) should be kept in mind in functional studies.
Biological Roles of Exosomes
The biological function of exosomes can be summarized in the following three aspects. (I) Cell polarity. Cell polarity is important in organ development, cell migration, and epithelial function. The posterior pole biogenesis can direct cell polarity and migration. Shen et al. reported that posterior pole biogenesis is associated with exosomes biogenesis based on the exosome protein-sorting pathway [38]. (II) EVs affect extracellular matrix (ECM) composition. Exosomes have been found to affect ECM function and composition in neurodegenerative diseases, such as plaques and tangles [39]. (III) Message delivery. The better understood function of exosomes is its role in mediating cell to cell communication and message transfer, which contributes to multiple physiological (such as exercise-induced increase in cardiac function [40]) and pathological processes (such as allergic airway diseases [41] and metabolic disorders [42]).
Dual Roles of Exosomes in AS
Exosomes play central roles in message transfer between different cells and tissues, contributing to systemic communication as well as progression of pathological conditions. In this section, we will review the role of exosomes in AS progression, whereby they are considered multifaceted messengers not only transferring “bad news” to exacerbate atherogenesis but also delivering “positive signal” for AS diagnosis.
Exosomes Exacerbate Inflammation and Lipid Metabolism Dysfunction During AS
Abnormal lipid metabolism and inflammation are considered boosters for the development and progression of AS and “sweet spots” for AS therapeutic target, attracting extensive attention [43].
AS-related inflammatory response is promoted by cytokines, adhesion molecules, inflammatory factors, and inflammation-related pathways such as NF-κB [44]. Exosomes play a critical role in pro-inflammatory response during AS, due to their capacity to deliver pro-inflammatory factors and miRNA to recipient cells [45•]. Exosomes can deliver inflammation-related regulatory molecules to endothelial cells, activating the leukocyte recruitment cascade and promoting inflammatory cell infiltration [46]. Exosomes produced from macrophages, VSMC, and platelets can carry miRNAs (such as miR-155 and miR-223) and trigger activation of the NF-κB pathway, and enhance local inflammation and endothelial cell activation. Subsequently, exosome-induced immune cell infiltration across the endothelial cells exacerbates AS and promotes its progression [47]. In response to inflammatory stimuli, exosomes from endothelial cells transfer miR-92a-3p to VSMC resulting in cell proliferation and migration, which further exacerbates inflammatory response [48].
Besides, exosomes and their cargo are associated with altered lipid metabolism and homeostasis, which contribute to the development of AS [49]. At the early stage of AS, exosomes are considered “scavengers” to remove excess cholesterol from the cell. Macrophages are considered to play a crucial role in maintaining lipid homeostasis via exosome release. However, with the development of inflammation and lipid accumulation, the elevated lipid content in the exosomes may exacerbate the development of AS. Exosomes enriched with lipids such as cholesterol, ceramide, and glycerophospholipids have been shown to be important in causing cell apoptosis and migration during AS [50].
Exosomal-miRNAs Are Potential Diagnostic Biomarkers or “Therapy” for AS
Several miRNAs that are potential therapeutic targets have been identified from AS patients or animal models. Though most of the experimental evidence focuses on the role of exosomes as pro-inflammatory, proliferative, and migratory mediators during AS progression, emerging data indicate that several exosomal miRNAs are associated with a lower risk for AS, such as miR-126, miR-199a [48], and miR-30e [51•]. In a clinical study reported by Wang Z. et al. [51•], plasma exosomal miRNA content was analyzed in 42 AS patients. MiR-30e and miR-92a were found to be increased in the plasma exosomes from AS patients compared with healthy subjects. MiR-30e and miR-92a were found to target and inhibit the expression of ATP-binding cassette A1 (ABCA1), subsequently regulating cholesterol metabolism [51•]. The observation led to the conclusion that these miRNAs could be used as novel biomarkers for AS diagnosis [51•]. It was additionally found that exosomes derived from adipose-derived mesenchymal stem cell (ADSC) effectively protected endothelial cell from AS development by inhibiting endothelial miR342-5p and upregulating PPP1R12B (protein phosphatase 1 regulatory subunit 12B) [52••].
Exosome-Mediated Intercellular Communication in AS
AS is a consequence of several cellular pathological changes, including inflammation and endothelial cell dysfunction, proliferation and de-differentiation of VSMC, activation of platelets, and macrophage migration. Briefly, (1) endothelial cell dysfunction contributes to an impairment of vasodilator capacity. During endothelial injury, the elevation of vascular cell adhesion molecule-1 (VCAM-1) promotes recruitment of leukocytes, contributing to plaque formation. Besides, transforming growth factor beta (TGFβ)-induced transition of endothelial cells to mesenchymal cells also exacerbates the progression of AS [53]. (2) Macrophages are considered as “scavengers” for clearance of oxidized low-density lipoprotein (oxLDL) via phagocytosis [54, 55], whose necrosis promotes formation of the necrotic core, leading toplaque instability in AS [16]. Once the excess of lipid is phagocytosed, activated macrophages accelerate intercellular lipid accumulation and foam cell formation, which promote the secretion of cytokines (IL-1, IL-6, TNF-α [56] etc.) and inflammation, causing lesional necrotic core expansion [8, 57]. (3) Under physiological conditions, a moderate activation of VSMC proliferation can improve vascular wall repair, while excessive activation, senescence, secondary necrosis, and differentiation promote inflammation and extracellular matrix formation/fibrosis in AS [58, 59]. Besides, VSMC can communicate with other cells to exacerbate AS progression by producing inflammatory factors. It is therefore crucial for these cells to communicate and transfer information effectively with each other. Under this situation, increasing interest is emerging in the role of exosome-mediated cell-cell communication in AS. It is well accepted that multiple types of cells such as endothelial cells, platelets, VSMC, and macrophages can produce exosomes to exchange information with each other. Regardless of the source of exosomes among these cells, exosomes generally target endothelial cells and VSMC. To shed light into the mechanism mediating information exchange in AS development, studies concerning exosomes as well as exosomal-cargo-dependent cell to cell cross-talk are reviewed next.
Exosome-Mediated VSMC-Endothelial Cell Cross-talk
VSMC are the primary cellular component of arterial blood vessels and they are major promoters of AS plaque progression and extracellular matrix deposition [60]. Endothelial cells constitute the innermost structure of arterial vessels and are considered as the barrier keeping circulating cells separated from the neighboring tissues. Due to their unique physiological characteristics, endothelial cells and the basement membrane exert important roles in regulating blood flow. Functionally, endothelial cells are central in controlling blood flow, vascular endothelial inflammation, and angiogenesis, whose dysfunction is the main reason for AS development [61, 62]. In AS, endothelial dysfunction is characterized by impaired vascular function, loss of anti-thrombotic function, and pro-inflammation [2]. The information exchange between VSMC and endothelial cell is bidirectional, exchanging information with each other.
Endothelial cell-derived exosomes can regulate proliferation and migration of VSMC. Endothelial dysfunction in response to inflammatory stimuli leads to activation of VSMC and AS progression. Under shear stress conditions, the increase in VSMC proliferation and migration is endothelial-cell independent, which is mediated by elevation of fibroblast growth factor-4 (FGF-4) and vascular endothelial growth factor-A (VEGF-A), thereby promoting extracellular signal-regulated kinase (ERK)1/2 phosphorylation in endothelial cells. This endothelial cell-VSMC cross-talk directly affects VSMC proliferation and accelerates AS progression [63]. Ten-eleven translocation 2 (TET2), a member of methylcytosine dioxygenase, is a DNA demethylation that can oxidize 5-methylcytosine to 5-hydroxymethylcytosine [64]. TET2 is considered as a molecular key to switch the phenotype of VSMC, reduction of which causes VSMC de-differentiation. TET2 is also expressed in endothelial cells protecting them against stress stimuli. Co-culture systems were used to investigate the influence of endothelial cells on VSMC cells isolated from mice. In response to pro-inflammatory stimuli, endothelial CD137 pathway was activated and endothelial exosomal-TET2 content was repressed, which upon transfer to VSMC promoted VSMC phenotype switch, eventually leading to plaque formation and AS development [65••].
Furthermore, VSMCs can influence endothelial function via exosomal cargo transfer. Recent research demonstrated that VSMC-derived exosomes promote the transfer of miR-155 to endothelial cells, resulting in an increase of endothelial permeability and AS development [66].
Exosomes Mediate Macrophage-Endothelial Cell Communication
Endothelial cells mediate inflammatory cell migration the atherosclerotic region, which is associated with macrophage activation. OxLDL is a primary factor that contributes to endothelial injury. It was found that in AS patients and animal models, the long noncoding RNA-growth arrest-specific 5 (lncRNA GAS5) is increased in plaque as well as in THP-1-derived exosomes. Over-expression of GAS5 showed to promote oxLDL-induced THP-1 apoptosis. Furthermore, exosomes isolated from GAS5-knockdown THP-1 cells attenuated endothelial cell apoptosis via inhibiting the expression of pro-apoptotic proteins, such as P53, Caspase 3, Caspase 7, and Caspase 9 [67]. Consistent with that, exosomes derived from oxLDL-treated endothelial cells promoted M2 macrophage polarization via transferring exosomal-MALAT1 (metastasis associated in lung denocarcinoma transcript 1) [68•]. Notably, endothelial cells with ox-LDL treatment produced MALAT1-containing exosomes which contributed to neutrophil extracellular traps and aggravation of AS [69•].
Furthermore, exosomes derived from lipopolysaccharide (LPS)-treated monocytes showed to elevate expression levels of intercellular adhesion molecule 1 (ICAM-1) and interleukin 6 (IL-6) in endothelial cells, which triggered macrophage transmigration across the endothelium. Therefore, increased monocyte/macrophage-derived exosomal transfer to the endothelial cells resulted in endothelial inflammation and AS via activating the nuclear factor kappa-B (NF-κB) signaling pathway [70].
Exosomes Mediate Platelet-Endothelial Cell Message Transfer
Platelet activation is an important process in the progression of AS, leading to pro-inflammatory reaction and AS exacerbation [71]. In a platelet-activated mouse model induced by thrombin, the production of exosomes by platelets was rapidly increased. MiRNA analysis demonstrated that miRNAs such as miR-223, miR-339, and miR-21 were significantly increased in activated platelet-derived exosomes. The elevation of exosomal miR-223 suppressed TNF-α-induced ICAM-1 expression after exosome uptake by endothelial cells. Mechanistically, endothelial cell uptake of platelet-derived exosomes inhibited the phosphorylation of mediators of the MAPK signaling pathways (JNK, ERK) as well as the activation of NF-κB [72].
Exosome-Mediated Communication Between Dendritic Cells and Endothelial Cells
Experimental work based on dendritic cell (DC)-derived exosome intravenous administration into apolipoprotein E knockout (ApoE−/−) mice demonstrated that exosomes isolated from DC significantly aggravate arterial injury and endothelial inflammation, via accelerating tumor necrosis factor alpha (TNFα) production and thereby activating NF-κB pathway [73]. The finding suggests exosomal-TNFα exerts important roles in DC-endothelial cell communication, leading to endothelial dysfunction and AS deterioration.
Exosome-Mediated Macrophage-VSMC Cross-talk
Cigarette smoking is a big threat to cardiovascular health because of nicotine. Nicotine can induce endothelial cells injury and inflammatory reaction, contributing to increase incidence and progression of AS. Nicotine aggravates high-fat diet-induced atherosclerotic lesions in mice and exosome production. In nicotine-treated macrophages, miR-21-3p was found significantly enriched in their exosomes, which upon uptake by VSMC, caused reduction in its target gene phosphate and tension homolog (PTEN) on chromosome ten. This investigation revealed that exosomal-miR-21-mediated macrophage-VSMC cross-talk promotes VSMC proliferation and migration via inhibition of PTEN expression, which exacerbates atherosclerosis progression [74••].
Exosome-Mediated Macrophage Polarization
Of note, exosome-mediated cell communication happened not only in the different types of cells but also in the same types. Purified macrophage-derived exosomes containing miR-146a can be taken up by neighboring macrophages, exacerbating macrophage entrapment and AS development [75]. Additionally, it has also been reported that adipose cell-derived exosomes can also influence macrophage polarization, thereby promoting atherosclerosis development [76].
Conclusion
AS is an intricate process. Mechanistically, secondary inflammation after endothelial dysfunction, macrophage activation, and VSMC differentiation contributes to plaque formation and rupture, promoting AS progression. Several strategies such as surgical, pharmacological, and physical interventions have been extensively used in clinical therapy now. However, there is still no optimal strategy to cure AS. Exosomes are a newly popular small extracellular vesicles subtype with lipid bilayer structure and accessible to most barrier space with non-immunogenic properties. During AS development, the intercellular cross-talk mediated by exosomes as well as exosomal-cargos exerts central roles in intercellular communication. In addition to mediating AS progression in most conditions, exosomes can also have a protective function attenuating AS development, being multifaceted messengers in AS. Altogether, exosomes and exosomal miRNA have the potential to be used as diagnostic markers and as a novel therapeutic strategy for AS.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, et al. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. https://doi.org/10.1016/j.ejphar.2017.05.010.
Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: successes, surprises, and future challenges. Circ Res. 2016;118(4):531–4. https://doi.org/10.1161/CIRCRESAHA.116.308334.
Fan Q, Yin X, Rababa’h A, Diaz Diaz A, Wijaya CS, Singh S, et al. Absence of gravin-mediated signaling inhibits development of high-fat diet-induced hyperlipidemia and atherosclerosis. Am J Physiol Heart Circ Physiol. 2019;317(4):H793–810. https://doi.org/10.1152/ajpheart.00215.2019.
Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, et al. Cigarette smoking, smoking cessation, and long-term risk of 3 major atherosclerotic diseases. J Am Coll Cardiol. 2019;74(4):498–507. https://doi.org/10.1016/j.jacc.2019.05.049.
He X, Rebholz CM, Daya N, Lazo M, Selvin E. Alcohol consumption and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2019;62(5):770–8. https://doi.org/10.1007/s00125-019-4833-1.
Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96(12):1221–32. https://doi.org/10.1161/01.RES.0000170946.56981.5c.
Yang J, Cao RY, Gao R, Mi Q, Dai Q, Zhu F. Physical exercise is a potential “medicine” for atherosclerosis. Adv Exp Med Biol. 2017;999:269–86. https://doi.org/10.1007/978-981-10-4307-9_15.
Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–12. https://doi.org/10.1016/j.jacc.2005.09.068.
Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20(1):33–50. https://doi.org/10.1016/s0022-5320(67)80034-0.
Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41(1):59–72. https://doi.org/10.1083/jcb.41.1.59.
Lu X. The role of exosomes and exosome-derived microRNAs in atherosclerosis. Curr Pharm Des. 2017;23(40):6182–93. https://doi.org/10.2174/1381612823666170413125507.
Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19(2):121–7. https://doi.org/10.1097/MED.0b013e32835057e9.
Aluganti Narasimhulu C, Fernandez-Ruiz I, Selvarajan K, Jiang X, Sengupta B, Riad A, et al. Atherosclerosis--do we know enough already to prevent it? Curr Opin Pharmacol. 2016;27:92–102. https://doi.org/10.1016/j.coph.2016.02.006.
Robinson JG, Davidson MH. Can we cure atherosclerosis? Rev Cardiovasc Med. 2018;19(S1):S20–S4.
Wilson DP, Gidding SS. Atherosclerosis: is a cure in sight? J Clin Lipidol. 2015;9(5 Suppl):S1–4. https://doi.org/10.1016/j.jacl.2015.06.010.
Kavurma MM, Rayner KJ, Karunakaran D. The walking dead: macrophage inflammation and death in atherosclerosis. Curr Opin Lipidol. 2017;28(2):91–8. https://doi.org/10.1097/MOL.0000000000000394.
Ainsworth BE, Macera CA. Promoting physical activity in a public health context. J Sport Health Sci. 2018;7(1):1–2. https://doi.org/10.1016/j.jshs.2017.10.004.
Wang L, Lv Y, Li G, Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci. 2018;7(4):433–41. https://doi.org/10.1016/j.jshs.2018.09.008.
Wang J, Zhao C, Xiao J. Exosomes in cardiovascular diseases and treatment: experimental and clinical aspects. J Cardiovasc Transl Res. 2019;12(1):1–2. https://doi.org/10.1007/s12265-018-9860-7.
Zhang Z, Duan Y, Bei Y. Cardiac progenitor cell-derived extracellular vesicles: a rising star for cardiac repair and regeneration. J Cardiovasc Transl Res. 2019;12(1):3–4. https://doi.org/10.1007/s12265-018-9862-5.
Li N, Rochette L, Wu Y, Rosenblatt-Velin N. New insights into the role of exosomes in the heart after myocardial infarction. J Cardiovasc Transl Res. 2019;12(1):18–27. https://doi.org/10.1007/s12265-018-9831-z.
Li J, Xue H, Li T, Chu X, Xin D, Xiong Y, et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(-/-) mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510(4):565–72. https://doi.org/10.1016/j.bbrc.2019.02.005.
• Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. https://doi.org/10.1038/nrm.2017.125. An important study demonstrating the origin, biogenesis, secretion, targeting and fate of extracellular vesicles.
•• Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2020;9(1):1703244. https://doi.org/10.1080/20013078.2019.1703244. A comprehensive review provides an outline of the compounds that have been most extensively studied for inhibiting extracellular vesicles, and their proposed mechanisms of actions.
Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25. https://doi.org/10.1016/j.ceb.2014.05.004.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–65. https://doi.org/10.1242/jcs.128868.
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–85. https://doi.org/10.1038/ncb2502.
Bissig C, Gruenberg J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014;24(1):19–25. https://doi.org/10.1016/j.tcb.2013.10.009.
Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–37. https://doi.org/10.1111/j.1600-0854.2009.00920.x.
Iguchi Y, Eid L, Parent M, Soucy G, Bareil C, Riku Y, et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. 2016;139(Pt 12):3187–201. https://doi.org/10.1093/brain/aww237.
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–21. https://doi.org/10.1016/j.devcel.2011.08.019.
Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131–9. https://doi.org/10.1016/j.devcel.2010.12.003.
• Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750. The most professional guidelines include tables and outlines of suggested protocols and steps to follow to document specific EV-associated functional activities.
Roux Q, Van Deun J, Dedeyne S, Hendrix A. The EV-TRACK summary add-on: integration of experimental information in databases to ensure comprehensive interpretation of biological knowledge on extracellular vesicles. J Extracell Vesicles. 2020;9(1):1699367. https://doi.org/10.1080/20013078.2019.1699367.
Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, et al. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano. 2018;12(1):671–80. https://doi.org/10.1021/acsnano.7b07782.
McVey MJ, Spring CM, Kuebler WM. Improved resolution in extracellular vesicle populations using 405 instead of 488 nm side scatter. J Extracell Vesicles. 2018;7(1):1454776. https://doi.org/10.1080/20013078.2018.1454776.
Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, et al. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles. 2015;4:25530. https://doi.org/10.3402/jev.v4.25530.
Shen B, Fang Y, Wu N, Gould SJ. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. J Biol Chem. 2011;286(51):44162–76. https://doi.org/10.1074/jbc.M111.274803.
Quek C, Hill AF. The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun. 2017;483(4):1178–86. https://doi.org/10.1016/j.bbrc.2016.09.090.
Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol. 2017;112(4):38. https://doi.org/10.1007/s00395-017-0628-z.
Hough KP, Deshane JS. Exosomes in allergic airway diseases. Curr Allergy Asthma Rep. 2019;19(5):26. https://doi.org/10.1007/s11882-019-0857-3.
Yao ZY, Chen WB, Shao SS, Ma SZ, Yang CB, Li MZ, et al. Role of exosome-associated microRNA in diagnostic and therapeutic applications to metabolic disorders. J Zhejiang Univ Sci B. 2018;19(3):183–98. https://doi.org/10.1631/jzus.B1600490.
Nasonov EL, Popkova TV. Atherosclerosis: perspectives of anti-inflammatory therapy. Ter Arkh. 2018;90(5):4–12. https://doi.org/10.26442/terarkh201890514-12.
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. 2018;8(3). https://doi.org/10.3390/biom8030080.
• Khalyfa A, Kheirandish-Gozal L, Gozal D. Exosome and macrophage crosstalk in sleep-disordered breathing-induced metabolic Dysfunction. Int J Mol Sci. 2018;19(11). https://doi.org/10.3390/ijms19113383. The study discussed the effect of exosomes in metabolic dysfunction, and reviewed the major biological functions of macrophages and exosomes as pathophysiological effectors.
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110(9):3234–44. https://doi.org/10.1182/blood-2007-03-079152.
Lu M, Yuan S, Li S, Li L, Liu M, Wan S. The exosome-derived biomarker in atherosclerosis and its clinical application. J Cardiovasc Transl Res. 2019;12(1):68–74. https://doi.org/10.1007/s12265-018-9796-y.
Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3(6):e001249. https://doi.org/10.1161/JAHA.114.001249.
Reiss AB, Vernice NA, Siegart NM, De Leon J, Kasselman LJ. Exosomes in cholesterol metabolism and atherosclerosis. Cardiovasc Hematol Disord Drug Targets. 2017;17(3):185–94. https://doi.org/10.2174/1871529X18666180103124443.
Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol. 2007;212(1):174–81. https://doi.org/10.1002/jcp.21013.
• Wang Z, Zhang J, Zhang S, Yan S, Wang Z, Wang C, et al. MiR30e and miR92a are related to atherosclerosis by targeting ABCA1. Mol Med Rep. 2019;19(4):3298–304. https://doi.org/10.3892/mmr.2019.9983. The study identified two plasma exosomal miRNAs (miR-30e and miR-92a) upon AS patients, which may be a new biomarker for clinical diagnosis and treatment of coronary atherosclerosis.
•• Xing X, Li Z, Yang X, Li M, Liu C, Pang Y, et al. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging (Albany NY). 2020;12(4):3880–98. https://doi.org/10.18632/aging.102857. This work found a new atherosclerosis-associated miR-342-5p and revealed a possible mechanism in which miR-342-5p mediated by ADSCs-derived exosomes protects endothelial cells against atherosclerosis.
Chen PY, Simons M. Fibroblast growth factor-transforming growth factor beta dialogues, endothelial cell to mesenchymal transition, and atherosclerosis. Curr Opin Lipidol. 2018;29(5):397–403. https://doi.org/10.1097/MOL.0000000000000542.
Gordon S. The macrophage. Bioessays. 1995;17(11):977–86. https://doi.org/10.1002/bies.950171111.
Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19(6):526–37. https://doi.org/10.1038/s41590-018-0113-3.
Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27:165–97. https://doi.org/10.1146/annurev.immunol.021908.132620.
Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118(4):653–67. https://doi.org/10.1161/CIRCRESAHA.115.306256.
Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). 2015;214(1):33–50. https://doi.org/10.1111/apha.12466.
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114(4):622–34. https://doi.org/10.1093/cvr/cvy007.
Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–44. https://doi.org/10.1038/s41569-019-0227-9.
Kruger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. 2019;20(18):1. https://doi.org/10.3390/ijms20184411.
Gimbrone MA Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–36. https://doi.org/10.1161/CIRCRESAHA.115.306301.
Jia L, Wang L, Wei F, Li C, Wang Z, Yu H, et al. Effects of Caveolin-1-ERK1/2 pathway on endothelial cells and smooth muscle cells under shear stress. Exp Biol Med (Maywood). 2020;245(1):21–33. https://doi.org/10.1177/1535370219892574.
He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333(6047):1303–7. https://doi.org/10.1126/science.1210944.
•• Li B, Zang G, Zhong W, Chen R, Zhang Y, Yang P, et al. Activation of CD137 signaling promotes neointimal formation by attenuating TET2 and transferrring from endothelial cell-derived exosomes to vascular smooth muscle cells. Biomed Pharmacother. 2020;121:109593. https://doi.org/10.1016/j.biopha.2019.109593. The study of CD137-TET2 signaling demonstrated that exosomes mediated cross-talk between endothelial cells and VSMC and the mechanism of the neointima formation in AS.
Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, et al. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol Ther. 2017;25(6):1279–94. https://doi.org/10.1016/j.ymthe.2017.03.031.
Chen L, Yang W, Guo Y, Chen W, Zheng P, Zeng J, et al. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS One. 2017;12(9):e0185406. https://doi.org/10.1371/journal.pone.0185406.
• Huang C, Han J, Wu Y, Li S, Wang Q, Lin W, et al. Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization. Mol Med Rep. 2018;18(1):509–15. https://doi.org/10.3892/mmr.2018.8982. The study revealed a novel mechanism underlying the onset of atherogenesis: exosomal MALAT1 mediated crosstalk between endothelial cells and macrophage, providing a novel scientific basis for the understanding of atherosclerosis progression.
• Gao H, Wang X, Lin C, An Z, Yu J, Cao H, et al. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol Chem. 2020;401(3):367–76. https://doi.org/10.1515/hsz-2019-0219. The study strengthened the evidence that exosomal MALAT1 played important roles in AS progression, which may provide a novel scientific basis for understanding AS progression.
Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-kappaB in endothelial cells. FASEB J. 2016;30(9):3097–106. https://doi.org/10.1096/fj.201600368RR.
Khodadi E. Platelet function in cardiovascular disease: activation of molecules and activation by molecules. Cardiovasc Toxicol. 2020;20(1):1–10. https://doi.org/10.1007/s12012-019-09555-4.
Li J, Tan M, Xiang Q, Zhou Z, Yan H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res. 2017;154:96–105. https://doi.org/10.1016/j.thromres.2017.04.016.
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappaB pathway. J Cell Mol Med. 2016;20(12):2318–27. https://doi.org/10.1111/jcmm.12923.
•• Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L, et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9(23):6901–19. https://doi.org/10.7150/thno.37357. New cross-talk between macrophage and VSMC mediated by exosomal miR-21-3p was identified, whose increase accelerated the development of AS.
Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, et al. Extracellular vesicles secreted by atherogenic macrophages transfer MicroRNA to inhibit cell migration. Arterioscler Thromb Vasc Biol. 2018;38(1):49–63. https://doi.org/10.1161/ATVBAHA.117.309795.
Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc. 2018;7(5). https://doi.org/10.1161/JAHA.117.007442.
Funding
This work was financially supported by the grants from the National Natural Science Foundation of China (81722008 and 81911540486 to JJ Xiao), Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-09-E00042 to JJ Xiao), the grant from Science and Technology Commission of Shanghai Municipality (18410722200 and 17010500100 to JJ Xiao), National Key Research and Development Project (2018YFE0113500 to JJ Xiao), and the “Dawn” Program of Shanghai Education Commission (19SG34 to JJ Xiao).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This review does not contain any studies with human or animal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genetics and Genomics
Rights and permissions
About this article
Cite this article
Wang, H., Xie, Y., Salvador, A.M. et al. Exosomes: Multifaceted Messengers in Atherosclerosis. Curr Atheroscler Rep 22, 57 (2020). https://doi.org/10.1007/s11883-020-00871-7
Published:
DOI: https://doi.org/10.1007/s11883-020-00871-7