Abstract
Gastric bypass surgery is an effective long-term weight loss intervention. Key to its success appears a putative shift in food preference away from high-energy-density foods associated with a reduced appetitive drive and loss of neural reactivity in the reward system of the brain towards food. Post-prandial exaggerated satiety gut hormone responses have been implicated as mediators. Whilst the positive impact of bariatric surgery on both physical and psychological outcomes for many patients is clearly evident, a subset of patients appear to be detrimentally affected by this loss of reward from food and by a lack of alternative strategies for regulating affect after surgery. Mindfulness training has emerged as a potential tool in reducing the need for immediate reward that underpins much of eating behaviour. Further research is needed to help identify patients who may be more vulnerable after gastric bypass and which forms of support may be most beneficial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arguably, the most important challenge in treating obesity on an individual level is not alleviating hunger or increasing satiation but finding a solution for the human association of food with positive affect and reward [1]. The neural networks controlling our experience of reward and goal-directed behaviour are potently and primarily stimulated by food and especially high-energy-density food [2–13]. Therefore, it is ironic that surgical, rather than psychological, interventions for obesity should prove to be the most effective tool that we have to treat obesity [14, 15]. On the other hand, if anatomical manipulations of the gut powerfully alter the hedonic evaluation of food, then the gut-brain axis may prove to be the most important target for the development of future treatments of obesity. There is increasing evidence to suggest that gastric bypass surgery affects food reward at a neural level. Functional magnetic resonance imaging (fMRI) studies have shown distinct patterns of brain activation to food pictures in humans after gastric bypass surgery, compared to before surgery [16, 17] and compared to unoperated obese patients or to gastric banding surgery patients [18]. Gastric bypass surgery also appears to distinctly alter striatal dopamine signalling compared to dieting [19]. Whilst on the face of this, bariatric surgery may therefore address perhaps the most important driver of overeating, there may also be consequences from losing the hedonic appeal of food that are not yet fully appreciated. Understanding the psychological and neurobiological underpinnings and consequences of bariatric surgical procedures will ensure the enduring success of any current or future intervention for obesity. In this paper, we aim to review the existing literature supporting the changes in food reward following gastric bypass surgery, explore whether there is evidence for subsequent reward deficiency in some patients and what the behavioural consequences or potential mediators may be.
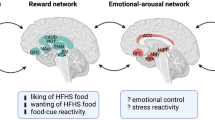
Akin to drug or alcohol addiction, alterations in dopaminergic and opioid pathways involved in the expectancy, appraisal and receipt of food reward appear to be important in the development and maintenance of obesity. Several components of the reward system, including the striatal nucleus accumbens and caudate nucleus (key to dopaminergic reward conditioning, expectancy and motivation), amygdala (which governs emotional responses to rewarding stimuli), anterior insula (integrating gustatory and other sensory information) and orbitofrontal cortex (OFC) (reward value appraisal, cognitive control and attention), have been implicated [20–22].
Activation in these areas to food cues not only predicts food consumption [23] and choice [24, 25], and prospective weight gain [26, 27], but also may be altered in obesity [21], predict the success of weight loss strategies [28], and change with successful weight loss [29, 30]. Activation is also altered by specific eating behaviour psychopathology such as dietary restraint [31–33], binge eating disorder [34, 35] and hyperphagia in genetic obesity [36]. Top-down regulation of these brain regions by the dorsolateral pre-frontal cortex during the exercise of self-control appears to modulate responses to food [28, 37], and higher trait mindfulness is associated with less enduring reward system activation to food cues [38]. Interestingly, modulation of activation of these reward systems both at rest and in response to food stimuli by gut hormones (including the anorexigenic hormones PYY and GLP-1, secretion of which is exaggerated after gastric bypass surgery) has also been described [13, 39–41], particularly when administered in combinations to obtain additive effects [42]. Surgical treatments of obesity alter activation in these areas to food cues and different surgical techniques appear to alter activation differently [16, 18, 43]. The influence that learning processes have on establishing these changes remains to be determined.
In 2012, Ochner et al. reported a study of 14 female patients scanned 1 month before and 1 month after Roux-en-Y gastric bypass (RYGB) in which they found significant reductions in activation to food (high and low energy density) cues (visual and auditory) post-operatively in the dorsal striatum (lentiform nucleus and putamen) and middle and superior frontal gyrus. These reductions were significantly greater for the response to high-energy, compared to low-energy, food pictures and words [16]. Reductions in brain response for high- compared to low-energy food cues in the lentiform nucleus, caudate, middle and superior frontal gyri, ACC, thalamus and inferior parietal lobule significantly predicted reductions in desire to eat particularly high-energy foods.
In our cross-sectional study, we found that obese patients after RYGB had healthier gut-brain-hedonic responses to food than patients after gastric banding (BAND) surgery, despite the groups being similar in current body mass index and time since surgery [18]. Patients after RYGB had lower activation than patients after BAND in brain reward systems when evaluating food pictures, particularly to high-energy foods, including the OFC, amygdala, caudate, nucleus accumbens and hippocampus. This was associated with lower palatability of ice cream given after scanning compared to patients after BAND (and which correlated with reward system activation when evaluating high-energy foods). Patients after RYGB also had lower appeal ratings of high-energy foods and healthier eating behaviour, including less self-reported fat intake, compared to patients after BAND and/or BMI-matched unoperated controls. These differences were not explicable by differences in hunger or psychological traits between the surgical groups.
Weight loss after gastric BAND surgery can be predicted by increased activity when viewing food pictures, in the medial, middle, and superior frontal gyrus and posterior cingulate cortex (areas associated with cognitive control) [44].
Several animal and human studies have shown not only reduction in hunger but also changes in taste acuity and a shift towards healthy food choices after gastric bypass surgery [45], although the human literature is limited by most studies relying on verbal report [46]. Post-ingestive symptoms (dumping symptoms) after sweet and fatty foods may contribute to this change in hedonic value of certain foods, by conditioned aversion or avoidance. Several animal studies have found that rather than total avoidance of sweet and fatty foods, there continues to be multiple approaches towards these foods after gastric bypass surgery, but a decreased overall intake [47], implying that they may still enjoy these foods but learn to consume small quantities to avoid discomfort, and hence, current observation favours the theory of conditioned avoidance rather than aversion. Food addiction, arguably the behavioural phenotype most closely correlated to alcohol or drug addiction, and therefore the group most likely to benefit from an intervention reducing reward from food, reduces after gastric bypass surgery as measured by the Food Addiction Scale [48].
Post-prandial, and sometime also fasting, anorexigenic gut hormones and bile acids are elevated after gastric bypass surgery and have the potential to influence the taste system and food hedonics. Data from our studies show that acute reversal of the exaggerated post-prandial plasma PYY and GLP-1 concentrations in RYGB patients by administration of the somatostatin analogue octreotide increases the hedonic appeal and reward system activation to food cues suggesting a potential mechanism behind the reduced food reward after gastric bypass surgery [49]. Similarly, the additive role of gut hormone or agonist administration to further increase the effect of gastric bypass surgery (or indeed another bariatric operation such as BAND), as suggested by animal studies [50], warrants further investigation in humans and may have particular relevance for patients where weight regain occurs after RYGB.
It has been hypothesized that a reduction in reward from food after gastric bypass surgery may create a reward deficiency associated with heightened hedonic responses to other emotion-regulating substances, such as alcohol, referred to as ‘switching addiction’. There is strong evidence to suggest that alcohol addiction may be a significant potential complication after gastric bypass surgery in some patients [51]. King’s and other studies have shown increased alcohol and other drug misuse and dependency in obese bariatric surgery patients [51, 52], particularly after gastric bypass surgery [53].
A retrospective study of patients presenting to the Mayo Clinic found that around 5 % of patients between 30 and 60 years of age presenting for treatment of alcohol dependency had undergone bariatric surgery in the past, mostly gastric bypass surgery [54]. Their histories reflected that 39 % had a past history of alcohol misuse, but a surprising 17 % were teetotalers before surgery, suggesting an increased vulnerability initiated by the surgery. There was an increase in alcohol intake around 17 months after surgery, progressing on to dependency levels by year 3, and severe enough to warrant inpatient treatment by year 5. Men progressed to alcohol dependency faster than women. In another study of 141 gastric bypass surgery patients at least 2 years post-surgery, 14 % fulfilled criteria for substance misuse disorder (tobacco, sedative, alcohol, cannabis and cocaine in descending relative frequency of use) [55]. Substance misuse was associated with poor weight loss and a family history of substance misuse as well as pre-operative food addiction, maladaptive eating behaviour, and heightened responsiveness to environmental food cues. In their sample, 70 % of those who met post-surgical probable substance misuse criteria reported developing this problem de novo following their surgery.
Proposed mechanisms for the increased rate of alcohol dependency after gastric bypass surgery include the altered metabolism of alcohol, which means that intoxication is achieved more easily and quickly than before surgery [56]. However, the loss of food as a rewarding substance thereby potentially sensitizing brain reward systems to other addictive substances may also contribute and fits with the finding of a higher risk amongst patients who have previous food addiction [57]. These findings may have particular relevance not only to obese patients undergoing RYGB who have a history of drug or alcohol addiction or misuse but also potentially for those who use the rewarding aspects of food as a way to manage stress, anxiety, depression or other difficult emotions.
Whether other impulsive and addictive behaviours such as gambling, sexual promiscuity and overspending also increase after gastric bypass surgery has not been examined.
Psychological problems including depression and alcohol use after gastric bypass surgery are associated with weight regain [58]. Eating in response to emotional distress after surgery has also been linked to poor outcomes after surgery, and its presence pre-operatively may predict poor outcomes [59, 60].
Furthermore, perhaps too much value is placed on weight loss as an outcome. Whereas weight loss is important for the improvement of physical health, and in general, mental health and well-being are improved following weight loss after bariatric surgery, a minority of patients continue to report no psychological benefit from surgery or a return of pre-morbid depression or new emergence of new psychological difficulties [61]. Increasingly, patients report psychological difficulties after bariatric surgery, which are not necessarily captured by traditional means of testing such as the Beck Depression Inventory. Nonetheless, these impact significantly on health expenditure, quality of life and patients’ experience of bariatric surgery. For instance, post-operative development of maladaptive eating (linked to weight regain), psychological distress (depression, anxiety and guilt), and somatization of psychological distress (leading to increased hospital or medical-attention-seeking behaviour for post-prandial pain, nausea, vomiting and dehydration) have been linked to the presence of specific personality traits and styles of coping [62].
There is also the suggestion from several cross-sectional studies that gastric bypass surgery may be associated with increased suicide risk [63]. The Utah Mortality study comparing approximately 9000 patients who had undergone gastric bypass surgery compared to BMI-matched unoperated obese controls found an increased risk of suicide and accidental death [64, 65]. Another study found a rate of 13.7 per 10,000 (men) and 5.2 per 10,000 (women) of completed suicides in over 16,000 bariatric surgery patients, compared to 2.4/10,000 (men) and 0.7/10,000 (women) age and sex-matched suicide rates in the US population [66]. Although it could be argued that bariatric surgery patients have a higher psychiatric co-morbidity to begin with than similarly obese patients not seeking surgery, nonetheless, this finding does call into question whether psychological health is truly restored after gastric bypass surgery in all patients or whether there are more subtle effects from the loss of food from a hedonic point of view that are being missed.
For instance, if food is used to dampen negative affect, removal of the positive affective reward from food may in fact make certain individuals more vulnerable to psychological distress, depression and even suicide. Pre-operative assessment and preparation should therefore arguably include the teaching of strategies to help improve emotional regulation particularly in those found to have a strong hedonic attachment to food. More emphasis is also needed on proactive strategies in postsurgical patients rather than a reactive approach that seeks to address the problem only once it has occurred. This may be particularly relevant in morbidly obese people where access to and the ability to use alternative coping strategies (e.g. such as exercise) may have been and may remain diminished by poor mobility, social isolation, depression and low self-esteem. If on the other hand, the reduction of the hedonic appeal of food is generalized, then this may have implications for pharmacological targets for treatment of other forms of addiction.
The psychological preparation and follow-up of post-bariatric surgery patients need further study [67], as we move away from assessment and screening towards a more holistic understanding of how psychological factors may impact on patients’ ability to use surgical treatments and aim towards targeted and personalized interventions pre- and post-surgery [58, 68]. Studies aimed at improving psychological and dietary preparedness have shown limited effects [69–71], but the more lasting effects of bariatric surgery on psychological well-being are woefully understudied and hampered by an overmedicalized approach to obesity.
Physical exercise is a positive predictor of outcome after bariatric surgery [72, 73]. Mindfulness has also emerged as the equivalent of regular physical exercise for maintaining mental well-being [74] and has been shown to improve health outcomes in a variety of settings [75], most consistently for the prevention of relapse from depression [76], while also improving maladaptive eating behaviours [77, 78]. Mindfulness is best defined as the learned ability to focus mental attention in a sustained manner in a non-judgmental way so as to increase self-awareness. Mindfulness practice cultivates acceptance and tolerance of distress without having to find a solution. Put otherwise, it mediates the need to immediately resolve distress and improve affect with a substance such as food. As such, it is frequently incorporated into programmes aimed at the management of disorders where tolerance of distress is low, such as emotionally unstable personality disorder [79]. Practicing mindfulness increases work satisfaction, reduces adverse experience of stress, increases creativity and personal success, improves relationships and enhances emotional intelligence [80].
Mindfulness training within an 8-week course has been shown to mediate the reward pathways and provide a way of exercising top-down control over the salience of monetary incentives by altering the value signals in ventromedial pre-frontal cortex (vmPFC) coupled with the bilateral posterior insula [81]. The collective results of studies by Kirk et al. suggest that mindfulness training is capable of lowering activation in the caudate nucleus and elevating bilateral posterior insula activation during reward anticipation. Mindfulness also reduces activations in the vmPFC during reward receipt and is able to modulate amygdala responses to threatening stimuli [81–83]. Overall, it appears that mindfulness integrates interoceptive input from the insula in the context of value computations of both primary and secondary rewards and thus offers a new approach to the problem of reward deficit. Testing the efficacy of mindfulness in patients after gastric bypass surgery appears feasible, especially now that this patient group has been identified as vulnerable.
Conclusion
In summary, the idea that surgery on the gut may change food reward responses in the brain is supported by evidence from behavioural and neuroimaging studies. Identification of the alterations in the gut-brain axis and hence food hedonic responses as a result of altered gut anatomy/physiology provides a novel explanation for the more favourable long-term weight loss seen after gastric bypass surgery compared to other surgical and dietary interventions. Interrogation of the differences in these underlying mechanistic pathways between gastric bypass and gastric banding surgery is therefore an important step towards the improved use of current treatments and the development of new treatments, targeting the gut-brain reward systems.
The inference that there exists a void for emotional sustenance and reward after gastric bypass surgery where food previously functioned is suggested by the evidence of increased risk of addiction to alcohol and other substances and other maladaptive behaviours such as somatic expression of psychological distress and suicide. Whilst the positive impact of bariatric surgery on both physical and psychological outcomes for many patients is clearly evident, a subset of patients appears to be detrimentally affected by this loss of reward from food and by a lack of alternative strategies for managing difficult feelings after surgery. Mindfulness training has emerged as a potential tool in reducing the need for immediate reward that underpins much of eating behaviour and may also underpin the emergence of other maladaptive behaviours after gastric bypass surgery. The positive top-down influence on neuronal reward systems of mindfulness has been demonstrated by fMRI studies and offers encouraging empirical support for it to be formally tested in this setting. Further research is needed to help identify patients who may be more vulnerable after gastric bypass and which forms of support may be most beneficial in preparing pre-operative patients and supporting post-operative patients.
References
Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71(4):478–87.
Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One. 2009;4(7):e6310.
Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32(3):1273–80.
Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15(10):1602–8.
Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169(1):111–9.
LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500.
Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19(4):1381–94.
St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135(5):1014–8.
Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lind). 2009;33(6):653–61.
Goldstone AP. The hypothalamus, hormones, and hunger: alterations in human obesity and illness. Prog Brain Res. 2006;153:57–73.
Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–35.
Goldstone AP, Prechtl de Hernandez CG, Scholtz S, Durighel G, Deliran SS, Wong T, et al. Ghrelin mimics fasting in biasing food appeal towards high-calorie foods. Obes Rev. 2010;11 Suppl 1:187.
Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr. 2014;99(6):1319–30.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J, et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience. 2012;209:128–35.
Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253(3):502–7.
Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63(6):891–902.
Hankir MK, Ashrafian H, Hesse S, Horstmann A, Fenske WK. Distinctive striatal dopamine signaling after dieting and gastric bypass. Trends Endocrinol Metab. 2015;26(5):223–30.
Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13(1):43–56.
Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13(4):279–86.
De Silva A, Salem V, Matthews PM, Dhillo WS. The use of functional MRI to study appetite control in the CNS. Exp Diabetes Res. 2012;2012:764017.
Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63(1):415–22.
Grabenhorst F, Schulte FP, Maderwald S, Brand M. Food labels promote healthy choices by a decision bias in the amygdala. Neuroimage. 2013;74:152–63.
Van der Laan LN, De Ridder DT, Viergever MA, Smeets PA. Appearance matters: neural correlates of food choice and packaging aesthetics. PLoS One. 2012;7(7):e41738.
Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32(16):5549–52.
Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring). 2011;19(9):1775–83.
Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8.
McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90(4):928–34.
Paolini BM, Laurienti PJ, Simpson SL, Burdette JH, Lyday RG, Rejeski WJ. Global integration of the hot-state brain network of appetite predicts short term weight loss in older adult. Front Aging Neurosci. 2015;7:70.
Coletta M, Platek S, Mohamed FB, van Steenburgh JJ, Green D, Lowe MR. Brain activation in restrained and unrestrained eaters: an fMRI study. J Abnorm Psychol. 2009;118(3):598–609.
Demos KE, Kelley WM, Heatherton TF. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. J Cogn Neurosci. 2011;23(8):1952–63.
Schur EA, Kleinhans NM, Goldberg J, Buchwald DS, Polivy J, Del Parigi A, et al. Acquired differences in brain responses among monozygotic twins discordant for restrained eating. Physiol Behav. 2012;105(2):560–7.
Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46(1):31–5.
Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65(8):654–61.
Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355.
Schonberg T, Bakkour A, Hover AM, Mumford JA, Poldrack RA. Influencing food choices by training: evidence for modulation of frontoparietal control signals. J Cogn Neurosci. 2014;26(2):247–68.
Paolini B, Burdette JH, Laurienti PJ, Morgan AR, Williamson DA, Rejeski WJ. Coping with brief periods of food restriction: mindfulness matters. Front Aging Neurosci. 2012;4:13.
van Bloemendaal L, RG IJ, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63(12):4186–96.
Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–9.
Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7(5):400–9.
Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat Rev Endocrinol. 2013;9(7):425–33.
Ochner CN, Laferrere B, Afifi L, Atalayer D, Geliebter A, Teixeira J. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci Res. 2012;74(2):138–43.
Ness A, Bruce J, Bruce A, Aupperle R, Lepping R, Martin L, et al. Pre-surgical cortical activation to food pictures is associated with weight loss following bariatric surgery. Surg Obes Relat Dis. 2014;10(6):1188–95.
Behary P, Miras AD. Food preferences and underlying mechanisms after bariatric surgery. Proc Nutr Soc. 2015:1-7
Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav. 2012;107(4):476–83.
Mathes CM, Bohnenkamp RA, Blonde GD, Letourneau C, Corteville C, Bueter M, et al. Gastric bypass in rats does not decrease appetitive behavior towards sweet or fatty fluids despite blunting preferential intake of sugar and fat. Physiol Behav. 2015;142:179–88.
Pepino MY, Stein RI, Eagon JC, Klein S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity (Silver Spring). 2014;22(8):1792–8.
Miras AD, Scholtz S, Chhina N, Durighel G, Bell JD, le Roux CW, Goldstone AP. Link between satiety gut hormones and reduced food reward after gastric bypass surgery for obesity in humans. Abstract at: The Obesity Society Annual Meeting at Obesity Week 2014; November 2–7, 2014; Boston, MA, USA. 2014; T-3053-OR.
Fenske WK, Bueter M, Miras AD, Ghatei MA, Bloom SR, le Roux CW. Exogenous peptide YY3-36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int J Obes (Lond). 2012;36(3):379–84.
King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–25.
Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148(2):145–50.
Ostlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148(4):374–7.
Cuellar-Barboza AB, Frye MA, Grothe K, Prieto ML, Schneekloth TD, Loukianova LL, et al. Change in consumption patterns for treatment-seeking patients with alcohol use disorder post-bariatric surgery. J Psychosom Res. 2015;78(3):199–204.
Reslan S, Saules KK, Greenwald MK, Schuh LM. Substance misuse following Roux-en-Y gastric bypass surgery. Subst Use Misuse. 2014;49(4):405–17.
Woodard GA, Downey J, Hernandez-Boussard T, Morton JM. Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial. J Am Coll Surg. 2011;212(2):209–14.
Avena NM, Gold MS. Sensitivity to alcohol in obese patients: a possible role for food addiction. J Am Coll Surg. 2011;213(3):451. author reply -2.
Yanos BR, Saules KK, Schuh LM, Sogg S. Predictors of lowest weight and long-term weight regain among roux-en-y gastric bypass patients. Obes Surg. 2014;25(8):1364–70.
Canetti L, Berry EM, Elizur Y. Psychosocial predictors of weight loss and psychological adjustment following bariatric surgery and a weight-loss program: the mediating role of emotional eating. Int J Eat Disord. 2009;42(2):109–17.
Castellini G, Godini L, Amedei SG, Faravelli C, Lucchese M, Ricca V. Psychological effects and outcome predictors of three bariatric surgery interventions: a 1-year follow-up study. Eat Weight Disord. 2014;19(2):217–24.
Kubik JF, Gill RS, Laffin M, Karmali S. The impact of bariatric surgery on psychological health. J Obes. 2013;2013:837989.
Marek RJ, Block AR, Ben-Porath YS. The Minnesota Multiphasic Personality Inventory-2-Restructured Form (MMPI-2-RF): incremental validity in predicting early postoperative outcomes in spine surgery candidates. Psychol Assess. 2015;27(1):114–24.
Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961.
Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61.
Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–31.
Tindle HA, Omalu B, Courcoulas A, Marcus M, Hammers J, Kuller LH. Risk of suicide after long-term follow-up from bariatric surgery. Am J Med. 2010;123(11):1036–42.
Herget S, Rudolph A, Hilbert A, Bluher S. Psychosocial status and mental health in adolescents before and after bariatric surgery: a systematic literature review. Obes Facts. 2014;7(4):233–45.
Ratcliffe D, Sogg S, Friedman KE. Letter to the editor: a comparative study of three-year weight loss and outcomes after laparoscopic gastric bypass in patients with “yellow light” psychological clearance. Obes Surg. 2015;25(3):539–40.
Ogden J, Hollywood A, Pring C. The impact of psychological support on weight loss post weight loss surgery: a randomised control trial. Obes Surg. 2015;25(3):500–5.
Ashton K, Drerup M, Windover A, Heinberg L. Brief, four-session group CBT reduces binge eating behaviors among bariatric surgery candidates. Surg Obes Relat Dis. 2009;5(2):257–62.
Ashton K, Heinberg L, Merrell J, Lavery M, Windover A, Alcorn K. Pilot evaluation of a substance abuse prevention group intervention for at-risk bariatric surgery candidates. Surg Obes Relat Dis. 2013;9(3):462–7.
Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Exercise following bariatric surgery: systematic review. Obes Surg. 2010;20(5):657–65.
Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest. 2015;125(1):248–57.
Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin Psychol Rev. 2015;37:1–12.
Hempel S, Taylor SL, Marshall NJ, Miake-Lye IM, Beroes JM, Shanman R, et al. Evidence map of mindfulness. VA evidence-based synthesis program reports. Washington (DC): Department of Veterans Affairs; 2014.
Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2011;31(6):1032–40.
Katterman SN, Kleinman BM, Hood MM, Nackers LM, Corsica JA. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: a systematic review. Eat Behav. 2014;15(2):197–204.
O’Reilly GA, Cook L, Spruijt-Metz D, Black DS. Mindfulness-based interventions for obesity-related eating behaviours: a literature review. Obes Rev. 2014;15(6):453–61.
Chafos VH, Economou P. Beyond borderline personality disorder: the mindful brain. Soc Work. 2014;59(4):297–302.
Paulson S, Davidson R, Jha A, Kabat-Zinn J. Becoming conscious: the science of mindfulness. Ann N Y Acad Sci. 2013;1303:87–104.
Kirk U, Gu X, Harvey AH, Fonagy P, Montague PR. Mindfulness training modulates value signals in ventromedial prefrontal cortex through input from insular cortex. Neuroimage. 2014;100:254–62.
Tang YY, Holzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16(4):213–25.
Kirk U, Brown KW, Downar J. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Soc Cogn Affect Neurosci. 2015;10(5):752–9.
Compliance with Ethics Guidelines
Conflict of Interest
Samantha Scholtz, Anthony P. Goldstone, and Carel W. le Roux declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lipid and Metabolic Effects of Gastrointestinal Surgery
Rights and permissions
About this article
Cite this article
Scholtz, S., Goldstone, A.P. & le Roux, C.W. Changes in Reward after Gastric Bypass: the Advantages and Disadvantages. Curr Atheroscler Rep 17, 61 (2015). https://doi.org/10.1007/s11883-015-0534-5
Published:
DOI: https://doi.org/10.1007/s11883-015-0534-5