Abstract
Larger waist circumference or waist-hip ratio, as crude indicators of visceral fat mass, are associated with adverse metabolic profile, but their role in predicting future coronary heart disease (CHD) events has been less investigated. Recent epidemiologic findings suggest that these simple and inexpensive measures of abdominal fat distribution predict CHD independently of body mass index, and, to a certain extent, cardiovascular disease risk factors. The magnitude and shape of the association between abdominal adiposity and CHD have been shown to vary with age, gender, and ethnicity. Studies have also suggested that lower body fat is associated with reduced CHD risk, although the clinical relevance for this finding needs further elucidation. Assessing body fat distribution may be useful for improving CHD risk assessment, although more studies are needed to assess consistency in CHD risk predictions across populations. A consensus is also needed to define the clinically relevant cut-off points for waist circumference or waist-hip ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is characterised by an excess amount of fat that is deleterious to health [1]. Excess fat in the abdominal region, particularly in the visceral area, has been suggested as being responsible for the myriad of metabolic consequences of obesity [2, 3]. Because measures such as waist circumference and waist-hip ratio are known correlates of visceral fat mass, these simple measures may be used to improve assessment for coronary heart disease (CHD) risk [4]. Indeed, the World Health Organization [1] and the National Institutes of Health in the United States [5] developed clinical guidelines in late 1990s that suggested incorporation of waist circumference assessment in clinical settings to help improve cardiovascular risk assessment. Similar clinical guidelines were developed in the United States [6], the United Kingdom [7], and Canada [8] in the early and mid-2000s. However, the evidence for the clinical relevance of abdominal adiposity was generally linked to their associations with intermediate markers of metabolic disease, such as blood pressure, lipid profile, and insulin sensitivity. Until recently, there were only a handful of studies that looked at important health outcomes such as mortality or development of CHD. It is therefore relevant to re-examine current evidence on the importance of abdominal fat distribution in the etiology of CHD and its relevance to CHD prevention and management. Two recent systematic reviews examined the prospective association of waist circumference and waist-hip ratio with cardiovascular disease (CVD) [9•] and CHD [10•]. Furthermore, since the publication of those reviews, additional findings have been reported from a number of newer studies.
The main purpose of this article is to provide an update on the relationship between fat distribution and CHD, examine current controversies that need to be carefully considered, and describe implications of these findings for physicians and health professionals in the community. This article also focuses on prospective studies examining the association between waist circumference or waist-hip ratio and CHD, although other health end points are discussed as relevant.
Fat Distribution and Metabolic Complications: The Anatomic Basis
Body fat is stored in various depots, with over 85% of fat stored subcutaneously and around 10% stored in the viscera [11]. Other fat depots that may have relevance to atherosclerotic disease include pericardial fat [12], buccal fat [13], and ectopic fat (adipose tissue stored in other organ systems) [14]. Fat distribution can also be crudely categorized either as upper body fat, truncal or abdominal fat (where bigger girth is indicative of increased visceral fat mass), or lower body fat (which is mainly subcutaneous). Adipose tissues in these different compartments also reflect variations in metabolic characteristics. Compared with subcutaneous fat, visceral fat depots have higher lipolytic rates [15], are less responsive to the antilipolytic effect of insulin [16], show increased expression of inflammatory markers and secretion of other adipokines [17], and exhibit an enhanced activity of intravascular coagulation factors [18]. Anatomically, layers of abdominal subcutaneous fat have been recognized, with lower metabolic activity observed in the superficial layer than in the deeper layer [19]. As compared with upper body subcutaneous fat, lower body subcutaneous fat is characterized by lower blood flow rate and lower hormone-sensitive lipase rate of action [20]. This wide variation in metabolic characteristics suggests that preferential storage of excess fat in specific depots may provide the basis for the differences in metabolic risks associated with fat distribution.
Fat Distribution and Metabolic Complications: The Physiologic Basis
In obesity, increased visceral fat releases excess free fatty acids into the portal vein [21]. It has been hypothesized that exposing the liver to elevated fatty acid concentration contributes to peripheral and hepatic insulin resistance, impaired insulin secretion, and development of atherogenic dyslipidemia (“portal theory”) [2]. Although most of the free fatty acids in the portal circulation originate from subcutaneous fat [22•], the proportion of free fatty acids in the portal circulation originating from the viscera increases with larger visceral fat mass [21].
Subcutaneous fat may also be important in modulating metabolism [22•]. Adipocytes in the lower body adipose tissues have the capacity to increase in number or size to store excess fat and may serve as a metabolic “sink” by buffering elevated postprandial fatty acid and lipid fluxes [23]. Impairment of this function could have important metabolic consequences. The importance of subcutaneous fat in metabolism has been suggested by a number of elegant animal studies involving lipoatrophic mice and ob/ob mice, both of which exhibit insulin resistance and dyslipidemia. Transplantation of subcutaneous fat in lipoatrophic mice [24] and expansion of subcutaneous fat in obese mice overexpressing the mutated adiponectin gene [25••] resulted in normalization of lipid levels and glucose homeostasis in these animals. In humans, lipodystrophy is associated with insulin resistance and dyslipidemia [26]. Intriguingly, the removal of subcutaneous fat in morbidly obese patients by liposuction has neither led to marked improvement in their short-term or long-term metabolic profile [27]. In diabetic patients, the use of thiazolidinedione, a peroxisome proliferator–activated receptor γ agonist that induces preadipocyte differentiation and subcutaneous fat mass expansion, improves insulin sensitivity [28]. It has been suggested that impairment of or exceeding the storage capacity of subcutaneous fat depot may lead to an overflow of fatty acids into the circulation that will contribute more free fatty acids in the portal circulation. The excess subcutaneous fat may even be stored ectopically in organs such as skeletal muscle and liver, which in turn could contribute to metabolic dysregulation (ie, the “overflow theory”) [29].
Although similar to the two previous hypotheses, a third paradigm stresses the importance of intrahepatic fat as the underlying mechanism that explains the complications of abdominal obesity. Matched for the same visceral fat mass volume, individuals with higher intrahepatic triglyceride content had lower insulin sensitivity and higher secretion rate of triglyceride-rich very low-density lipoprotein [30••]. In contrast, when matching for intrahepatic triglyceride content, no metabolic differences were observed with visceral fat mass volume, suggesting that the obesity-related complications were more closely dependent on intrahepatic fat than on visceral fat. This study has indeed challenged the dogma that visceral fat mass explains the metabolic consequences of abdominal obesity. However, it is likely that visceral and intrahepatic fat mass will correlate in obese persons. It certainly remains unclear if both represent distinct or related pathologic processes. Alternatively, these mechanisms need not be mutually exclusive; in a persistent condition of positive energy balance, the mechanisms may represent stages of metabolic dysregulation that consequently promote atherosclerosis. The challenge remains to identify the phenotype of adiposity that best captures the disparate effects of fat depot on clinically relevant outcomes.
Anthropometric Correlates of Fat Depots
Total (overall or general) adiposity can be crudely quantified by calculating body mass index (BMI), which is calculated as weight in kilograms divided by height in meters squared [1]. Although persons who are overweight (BMI of 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) are likely to have excess fat, BMI does not give an indication as to how this fat is distributed in the body. Fat mass in specific depots may be estimated with relative accuracy using CT or MRI, but these methods are impractical to use in clinical settings. Waist circumference and waist-hip ratio are simple anthropometric indicators of abdominal adiposity as they correlate reasonably well with visceral fat mass [1, 4]. However, the amount of fat for a given body size is known to vary by age, gender, and ethnicity [31]. This variability in adiposity may contribute to differences in risks associated with a specific level of adiposity across subgroups within and between populations [32, 33]. Other body composition measurement techniques, such as bioelectrical impedance and dual energy x-ray absorptiometry, may also provide estimates for regional (upper and lower body) fat mass, but these measures do not provide information on visceral fat mass.
Fat Distribution and Coronary Heart Disease
In the 1950s, Vague [34] suggested that body habitus was associated with ill health, but it was not until 1984 when two Swedish reports emerged that showed waist-hip ratio was prospectively associated with incident CHD [35, 36]. These findings indicated for the first time that abdominal adiposity was an important risk factor for CHD independent of BMI. Since the publication of these seminal reports, only a limited number of studies have further examined the nature of the relationship between abdominal adiposity and atherosclerotic disease. Detailed analyses of these various studies are shown in two systematic reviews that focus on slightly different but overlapping disease outcomes (CVD [9•] and CHD [10•]). Since the publication of these reviews, findings from a number of studies have also been published on CHD outcomes [37–41, 42••, 43]. Studies published since 2008 are shown in Table 1. Examining the evidence provided by all these investigations may reveal the importance of abdominal fat distribution in the etiology of CHD and help shape how the condition can best be managed.
General Characteristics of the Prospective Studies
Waist circumference and waist-hip ratio are two commonly used indicators for abdominal adiposity in epidemiologic studies that have reported on CHD outcome [10•, 37–40], but waist-height ratio has also been examined in some reports [41, 44]. Many of these studies covered a wide range of age groups, started at different time periods, and involved mostly Western populations. Although fat distribution is known to vary with ethnicity, few compared the influence of fat distribution on incident CHD between ethnic groups within a single population [45–47]. Although data for men and women were available, few studies have included both men and women to allow comparison of effect sizes between the sexes [45, 48••]. There has been a wide variability in the sample size, number of relevant end points, and duration of follow-up across the studies. Many studies combined fatal and nonfatal outcomes, whereas others solely considered CHD-related deaths. Case ascertainment was made on the basis of adjudication by independent assessors or identified from hospital records or death certificates.
Findings from Prospective Studies
Higher waist-hip ratio is associated with increased risk for CHD independently of BMI in British men and women, even among non-obese individuals (BMI < 30 kg/m2) [48••]. Although similar findings were observed for waist circumference, risk estimates were less consistent and robust. In other studies, there was a lack of consistent significant association between waist circumference and CHD, but generally there were a fewer number of events in the analyses (around 20 to 500 CHD cases) or inappropriate adjustment for mediating factors [10•, 37–39]. However, in a study with over 2000 CHD cases, waist circumference or waist-hip ratio was prospectively associated with CHD independently of BMI as well as other classic cardiovascular disease risk factors [48••]. Studies with a larger number of cases may have the sufficient statistical power to show an independent effect despite the high correlation between abdominal adiposity measures (particularly waist circumference) and BMI.
Shape and Magnitude of Association
The relationship between BMI and mortality, including deaths related to the circulatory system, has been suggested to be J-shaped in that excess risk is noted in the lower end as well as in the upper end of the distribution of BMI [49•]. The shape of the association between fat distribution and CHD is less clear. For waist-hip ratio, the relation with atherosclerotic disease showed a graded, linear association across the whole range of this measure in both men and women [48••]. However, for waist circumference, the shape varies slightly depending on whether or not the association has been adjusted for BMI and/or other covariates [48••, 50]. Because it is highly correlated with BMI, it may closely follow the shape of the association of BMI with CHD.
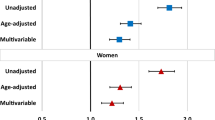
The magnitude of the risk associated with higher abdominal adiposity is rather strong. Comparing the risk of those in the top versus bottom fifth of the distribution of adiposity measure in a British cohort, the age-adjusted hazard ratios for waist-hip ratio and waist circumference were 2.06 (95% CI, 1.75–2.43) and 1.85 (95% CI, 1.57–2.17), respectively, in men, and 2.44 (95% CI, 1.88–3.17) and 2.35 (95% CI, 1.84–3.01), respectively, in women [48••]. To put this into context, the risk estimates for BMI were 1.74 (95% CI, 1.49–2.02) in men and 1.99 (95% CI, 1.60–2.47) in women. Adjustment for other covariates attenuates the associations for waist circumference and waist-hip ratio, but the associations generally remained significant. These effect sizes are comparable in other studies [40, 43, 50].
A quantitative relationship of waist circumference and waist-hip ratio with incident CVD in prospective studies has also been recently reported [9•]. Combining data from 15 publications involving 258,114 participants and 4355 cases of incident CVD (fatal and nonfatal CHD and stroke events), an increase in waist circumference or waist-hip ratio has been significantly associated with increased risk for developing CVD in both men and women. The relative risk for developing CVD increased by 2% (95% CI, 1–3%) for every 1-cm increase in waist circumference and by 5% (95% CI, 4–7%) for every 0.01 increase in waist-hip ratio. The risk estimates were comparable between men and women and persisted after adjusting for potential confounders, including BMI, and potential mediating biological factors.
Consistency Between Populations and Between Subgroups Within a Population
Variations in the associations across important subgroups in the population might be expected considering that adiposity and its distribution are known to vary by age, sex, and ethnicity. Some studies indicate that the association between abdominal adiposity and CHD is stronger in younger than in older adults [42••, 48••, 50]. In the Atherosclerosis Risk in Communities (ARIC) study, risk estimates in men were greater in whites than in blacks, whereas risk estimates in women were greater in blacks than in whites. In the Charleston Heart Study, which included white and black men, abdominal adiposity was not shown to be independently associated with CHD [46]. The lack of association was also evident in Aboriginal Australians [38]. Lack of an association in these studies could be due to lack of statistical power, as the studies involved only relatively small numbers of CHD events. Indeed, in a biracial cohort with over 1000 CHD cases, waist-hip ratio (but not waist circumference) was an independent predictor of incident CHD [40].
Role of Mediating Factors
Hypertension, dyslipidemia, elevated levels of circulating inflammatory markers and other adipokines, impaired glucose homeostasis, and endothelial dysfunction are among the myriad of pathways that may promote atherogenesis. Hence, adjusting for these factors in the analysis to establish the association between fat distribution and CHD may be inappropriate. However, adjusting for such factors could provide information on the importance of specific pathways that link abdominal adiposity with CHD. For example, if the excess risks are mainly mediated by hypertension or hypercholesterolemia, then risk assessment and management may simply relate to controlling such factors to reduce CHD risk in patients with excess abdominal fat. Knowing what these mechanisms are may help identify more relevant ways to prevent, or to reduce the risk of, CHD among abdominally obese persons. Despite the attenuation of CHD risk estimates for abdominal adiposity after adjusting for blood pressure and cholesterol, some studies show that the relation remains significant, confirming that other biological factors may be involved [48••, 50, 51].
Peripheral Adiposity and Metabolic Health
There have been a number of reports suggesting that lower body adiposity is an important component of metabolic health. As mentioned previously, subcutaneous fat may modulate metabolism and could therefore modify metabolic disease risk. There is some evidence in humans that hip or thigh circumference, for a given body or waist size, is related to a better cardiovascular risk profile [52–54]. Studies that used dual energy x-ray absorptiometry (a bone density scan) to estimate lower body adiposity suggested that this protective effect was largely explained by the subcutaneous fat rather than the muscle mass or the intramuscular fat mass [54]. Indeed, after adjusting for BMI and waist circumference, an inverse association between hip or thigh circumference and risk for CHD and diabetes mellitus has been demonstrated in a number of studies [40, 48••, 55]. The magnitude of the effect of hip circumference is substantial. In the European Prospective Investigation into Cancer (EPIC)-Norfolk study, up to 30% to 40% reduction in risk was observed in the top fifth as compared to the bottom fifth of the distribution of hip circumference independently of waist circumference, BMI, biological mediators, and other covariates [48••].
Role of Fat Distribution in Coronary Heart Disease Risk Assessment
Despite the evidence that fat distribution is an important determinant of CHD independent of BMI and other classic risk factors of CHD, its role in CHD risk assessment has remained controversial [56]. It is probably not helped by the fact that studies in this research area may have focused, perhaps inadvertently, on controversial issues that could send a mixed or contradictory message outside of the research community. Some of these key issues are described below.
Purpose of Risk Assessment
Abdominal obesity may be considered as an independent risk factor that can potentially be used to improve CHD risk assessment in the clinical setting. From this perspective, various measurements would have been taken (including blood sample collection), and the clinical management is predicated on the overall risk profile. Treatment of specific risk factors, such as prescription of antihypertensive drugs and cholesterol-lowering medications, smoking cessation, and perhaps weight management, may form part of managing the patient [6, 7]. However, as a screening tool, it becomes problematic because hypertension and dyslipidemia, which are part of the biological pathway linking obesity with CHD, are included in clinical assessment of CHD risk [6]. The other perspective focuses on obesity as the condition of interest and its assessment determines the focus of subsequent management, which includes weight management, treatment of CHD risk factors, or both. In this perspective, weight management is viewed as part of reducing blood pressure and cholesterol level and not just to improve nonclassical CHD pathways (eg, reducing low-grade systemic inflammation). These two perspectives overlap in clinical practice, and more so if patients are considered obese. However, the distinction is clearly manifested in those who do not meet the clinical definition for obesity. The importance of adiposity is therefore dependent on the purpose of its measurement—either as a risk factor in a CHD risk score model or as a condition that needs to be clinically managed. Part of this management included addressing the CHD risk. Considering that abdominal adiposity is also associated with other important health outcomes, including all-cause mortality and some types of cancer [42••, 49•], focusing solely on the role of abdominal adiposity in CHD may not be addressing the overall health status of the patient.
Total Versus Regional Adiposity
Whether it is overall fatness or the relative distribution of fat that has a more important clinical relevance remains a controversial issue. Even if findings show independent effects for each of these measures, some measures, particularly BMI and waist circumference, are highly correlated. Few obese individuals are likely to have a small waist circumference. As BMI is widely measured in clinical practice, it offers an advantage over waist circumference. Because obesity is clinically defined using BMI, the issue therefore is less about which measurement is better for CHD assessment in the clinical setting and more about whether or not the addition of another measurement, such as waist circumference, would provide additional and meaningful clinical information, taking into account limitations in patient consultation times. In patients with BMI ≥ 30 kg/m2, there is no evidence that management of excess weight and its co-morbid conditions will have to be modified depending on their waist circumference [57]. However, there is evidence that abdominal adiposity, as measured by waist circumference or waist-hip ratio, could be useful to assess CHD risk in non-obese individuals [48••]. Hence, it is likely that abdominal adiposity assessment may benefit non-obese patients for whom abdominal adiposity assessment may influence subsequent clinical management.
Defining Clinical Cut-off
Although there is no clear agreement as to which anthropometric measure is the single best indicator for abdominal adiposity, clinical guidelines tend to suggest the use of waist circumference because it is generally simple to assess. However, there is no consensus on which cut-off points are best for risk assessment. Defining thresholds is complicated because there are differences in the relation of abdominal adiposity with different risk factors, and the risk estimates for various disease outcomes may differ. Currently, the International Diabetes Federation has recommended waist circumference thresholds for increased risk for cardiovascular disease and diabetes as ≥94 cm in men and ≥80 cm in women. The American Heart Association and National Heart, Lung, and Blood Institute set the thresholds at ≥102 cm in men and ≥88 cm in women, and the World Health Organization uses both thresholds to reflect different levels of risks [58••]. Other groups either adopt or set their own thresholds to reflect the relevance of these cut-off points to their local needs. There is a consensus that more data are needed to assess how robust these thresholds are in relation to gender, age, and ethnicity or country.
Implications for Prevention, Treatment, and Management
There are new developments in pharmacotherapy to reduce weight and subsequently modify body fat distribution that look promising and could prove to be useful and effective when used in the clinical setting [59]. However, a pharmacologic approach to improve adiposity phenotype is unlikely to be feasible in the population. Factors such as smoking, physical activity, and diet are known to influence body fat distribution [60, 61], and promoting an overall healthy lifestyle forms part of overall clinical and public health management strategies. Targeting to modify a specific depot is probably less useful because fat mass in all depots are likely to be correlated. For example, individuals with bigger waist girth are likely to have bigger hips, so individuals with bigger hips, which confer lower risk, are likely to have bigger waist girth and therefore benefit from reduction of this excess fat. It has been suggested that a reduction of waist circumference by 5 cm is feasible via dietary restriction and low-intensity exercise three times a week [60]. This reduction can potentially reduce CHD or CVD by 11% to 15% (not to mention the benefits of improving glucose homeostasis) [9•, 48••]. Although this risk reduction may seem small for an individual, it is likely to show a substantial impact on the population as a whole. Considering that about 785,000 new coronary attacks may have occurred in 2009 in the United States [62], even a 10% risk reduction would mean 78,500 new cases could have been prevented this past year.
Conclusions
Body fat distribution is an important determinant of metabolic health. Abdominal obesity could lead to metabolic consequences and increase the risk for CHD as well as other health outcomes. This importance has been underscored by the inclusion of abdominal adiposity in the definition of metabolic syndrome in a recent international consensus [58•]. Nevertheless, more research is needed to examine the relation between various anthropometric indicators of abdominal adiposity and disease outcomes in as wide of a range of age groups, ethnicities, and populations as possible to help inform how best to use and interpret these measures in clinical risk predictions. In the future, it is likely that a more sophisticated and perhaps more personalized algorithm may be needed to improve CHD risk assessment [56]. Increasing our knowledge of the genetic basis of fat distribution and development of pharmacologic drugs targeting specific fat depots may alter clinical practice on CHD risk assessment and management. However, even more importantly, regardless of how body fat is distributed, reduction of obesity prevalence and prevention of excess weight gain are two of the most fundamental public health challenges.
References
Papers of particular interest, published recently, have been highlighted as: • Of Importance •• Of major importance
World Health Organization: Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva: World Health Organization; 2000.
Bjorntorp P: “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990, 10:493–496.
Despres JP, Lemieux I, Bergeron J, et al.: Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008, 28:1039–1049.
Despres JP, Prud’homme D, Pouliot MC, et al.: Estimation of deep abdominal adipose-tissue accumulation from simple anthropometric measurements in men. Am J Clin Nutr 1991, 54:471–477.
National Institutes of Health: Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 1998, 6:51S–209S.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106:3143–3421.
National Insititute for Health and Clinical Effectiveness: Obesity: Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children (Report number CG43). London: National Institute for Health and Clinical Excellence; 2006.
Health Canada: Canadian Guidelines for Body Weight Classification in Adults (Publication ID 4645). Ottawa, Canada: Health Canada Publications Centre; 2003.
• de Koning L, Merchant AT, Pogue J, Anand SS: Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007, 28:850–856. This is the first (and so far only) meta-analysis that quantified the risk for CVD associated with higher waist circumference and higher waist-hip ratio.
• Canoy D: Distribution of body fat and risk of coronary heart disease in men and women. Curr Opin Cardiol 2008, 23:591–598. This is a systematic review of prospective studies examining the association between waist circumference and waist-hip ratio with CHD.
Thomas EL, Saeed N, Hajnal JV, et al.: Magnetic resonance imaging of total body fat. J Appl Physiol 1998, 85:1778–1785.
Fox CS, Gona P, Hoffmann U, et al.: Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation 2009, 119:1586–1591.
Levine JA, Ray A, Jensen MD: Relation between chubby cheeks and visceral fat. N Engl J Med 1998, 339:1946–1947.
Kelley DE: Skeletal muscle triglycerides: an aspect of regional adiposity and insulin resistance. Ann N Y Acad Sci 2002, 967:135–145.
Arner P: Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 2005, 19:471–482.
Fried SK, Russell CD, Grauso NL, Brolin RE: Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest 1993, 92:2191–2198.
Fontana L, Eagon JC, Trujillo ME, et al.: Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56:1010–1013.
Peverill RE, Teede HJ, Malan E, et al.: Relationship of waist and hip circumference with coagulation and fibrinolysis in postmenopausal women. Clin Sci (Lond) 2007, 113:383–391.
Kelley DE, Thaete FL, Troost F, et al.: Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000, 278:E941–E948.
Tan GD, Goossens GH, Humphreys SM, et al.: Upper and lower body adipose tissue function: a direct comparison of fat mobilization in humans. Obes Res 2004, 12:114–118.
Nielsen S, Guo Z, Johnson CM, et al.: Splanchnic lipolysis in human obesity. J Clin Invest 2004, 113:1582–1588.
• Jensen MD: Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008, 93:S57–S63. This is an important review of the role of adipose tissue in regulating fatty acid metabolism. It highlights studies that are associated with direct quantification of fatty acid concentration in portal circulation in vivo.
Frayn KN: Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002, 45:1201–1210.
Gavrilova O, Marcus-Samuels B, Graham D, et al.: Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 2000, 105:271–278.
• Kim JY, van de Wall E, Laplante M, et al.: Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007, 117:2621–2637. This is a landmark study showing that expansion of the subcutaneous fat in obese mice led to an improvement and normalization of the adverse metabolic profile despite weight gain.
Simha V, Garg A: Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol 2006, 17:162–169.
Mohammed BS, Cohen S, Reeds D, et al.: Long-term effects of large-volume liposuction on metabolic risk factors for coronary heart disease. Obesity (Silver Spring) 2008, 16:2648–2651.
Adams M, Montague CT, Prins JB, et al.: Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest 1997, 100:3149–3153.
Danforth E Jr: Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 2000, 26:13.
••Fabbrini E, Magkos F, Mohammed BS, et al.: Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A 2009, 106:15430–15435. This study highlights the importance of intrahepatic fat as the potential underlying pathology associated with abdominal obesity in humans. The results of this study have indeed questioned the centrality of visceral fat as the key player in the metabolic consequences of abdominal obesity.
Carroll JF, Chiapa AL, Rodriquez M, et al.: Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008, 16:600–607.
Herrera VM, Casas JP, Miranda JJ, et al.: Interethnic differences in the accuracy of anthropometric indicators of obesity in screening for high risk of coronary heart disease. Int J Obes (Lond) 2009, 33:568–576.
Ghandehari H, Le V, Kamal-Bahl S, et al.: Abdominal obesity and the spectrum of global cardiometabolic risks in US adults. Int J Obes (Lond) 2009, 33:239–248.
Vague J: The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956, 4:20–34.
Larsson B, Svardsudd K, Welin L, et al.: Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984, 288:1401–1404.
Lapidus L, Bengtsson C, Larsson B, et al.: Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984, 289:1257–1261.
Cameron AJ, Dunstan DW, Owen N, et al.: Health and mortality consequences of abdominal obesity: evidence from the AusDiab study. Med J Aust 2009, 191:202–208.
Bradshaw PJ, Alfonso HS, Finn JC, et al.: Coronary heart disease events in Aboriginal Australians: incidence in an urban population. Med J Aust 2009, 190:583–586.
Dhaliwal SS, Welborn TA: Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores. Am J Cardiol 2009, 103:1403–1407.
Parker ED, Pereira MA, Stevens J, Folsom AR: Association of hip circumference with incident diabetes and coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Epidemiol 2009, 169:837–847.
Page JH, Rexrode KM, Hu F, et al.: Waist-height ratio as a predictor of coronary heart disease among women. Epidemiology 2009, 20:361–366.
••Pischon T, Boeing H, Hoffmann K, et al.: General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008, 359:2105–2120. As part of a collaborative cohort study across Europe, this article shows findings on the importance of fat distribution as a risk factor for all-cause and cause-specific mortality. To date, this study involves the largest number of cohort participants that addressed health consequences of abdominal obesity.
Yang L, Kuper H, Weiderpass E: Anthropometric characteristics as predictors of coronary heart disease in women. J Intern Med 2008, 264:39–49.
Gelber RP, Gaziano JM, Orav EJ, et al.: Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol 2008, 52:605–615.
Folsom AR, Stevens J, Schreiner PJ, McGovern PG: Body mass index, waist/hip ratio, and coronary heart disease incidence in African Americans and whites. Atherosclerosis Risk in Communities Study Investigators. Am J Epidemiol 1998, 148:1187–1194.
Stevens J, Keil JE, Rust PF, et al.: Body mass index and body girths as predictors of mortality in black and white men. Am J Epidemiol 1992, 135:1137–1146.
Stevens J, Keil JE, Rust PF, et al.: Body mass index and body girths as predictors of mortality in black and white women. Arch Intern Med 1992, 152:1257–1262.
••Canoy D, Boekholdt SM, Wareham N, et al.: Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation 2007, 116:2933–2943. This population-based prospective study examines the nature of the association between fat distribution and CHD risk in both men and women in more detail. The investigators are also the first to report the importance of hip circumference as an independent predictor of lower CHD risk.
• Whitlock G, Lewington S, Sherliker P, et al.: Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009, 373:1083–1096. Using data from various prospective cohort studies involving almost 1 million participants, the investigators were able to examine risk for all-cause and cause-specific mortality across the whole range of distribution of BMI.
Rexrode KM, Carey VJ, Hennekens CH, et al.: Abdominal adiposity and coronary heart disease in women. JAMA 1998, 280:1843–1848.
Aekplakorn W, Pakpeankitwatana V, Lee CM, et al.: Abdominal obesity and coronary heart disease in Thai men. Obesity (Silver Spring) 2007, 15:1036–1042.
Canoy D, Luben R, Welch A, et al.: Fat distribution, body mass index and blood pressure in 22,090 men and women in the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk) study. J Hypertens 2004, 22:2067–2074.
Canoy D, Wareham N, Luben R, et al.: Serum lipid concentration in relation to anthropometric indices of central and peripheral fat distribution in 20,021 British men and women: results from the EPIC-Norfolk population-based cohort study. Atherosclerosis 2006, 189:420–427.
Wiklund P, Toss F, Weinehall L, et al.: Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J Clin Endocrinol Metab 2008, 93:4360–4366.
Heitmann BL, Frederiksen P: Thigh circumference and risk of heart disease and premature death: prospective cohort study. BMJ 2009, 339:b3292.
Despres JP: CVD risk assessment: do we need the metabolic syndrome or better global cardiometabolic risk calculators? Int J Obes (Lond) 2008, 32:S1–S4.
Klein S, Allison DB, Heymsfield SB, et al.: Waist Circumference and Cardiometabolic Risk: a Consensus Statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring) 2007, 15:1061–1067.
•• Alberti KG, Eckel RH, Grundy SM, et al.: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120:1640–1645. The use of different definitions for metabolic syndrome espoused by different professional societies may have confused researchers and clinicians alike. In this report, a consensus has finally been achieved to use a standarized definition of metabolic syndrome. More importantly, abdominal adiposity is considered in this consensus to be one of the main features of the syndrome.
Drolet B, Simard C, Poirier P: Impact of weight-loss medications on the cardiovascular system: focus on current and future anti-obesity drugs. Am J Cardiovasc Drugs 2007, 7:273–288.
Slentz CA, Duscha BD, Johnson JL, et al.: Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE–a randomized controlled study. Arch Intern Med 2004, 164:31–39.
Canoy D, Wareham N, Luben R, et al.: Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res 2005, 13:1466–1475.
Lloyd-Jones D, Adams R, Carnethon M, et al.: Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009, 119:480–486.
Disclosure
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canoy, D. Coronary Heart Disease and Body Fat Distribution. Curr Atheroscler Rep 12, 125–133 (2010). https://doi.org/10.1007/s11883-010-0092-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-010-0092-9