Abstract
The aim of this work was to characterize the air mycobiota of two production plants, one of artisanal chocolate and another of products for special regimes in a food factory in Cuba at different times of the year. The air was sampled during 1 year every 25 days distributed in rainy season and non-rainy. The method proposed by Omeliansky was applied and four bacteriostats were used: chloramphenicol, lactic acid, sodium chloride, and iodized common salt with malt agar extract in Petri dishes exposed for 1 h. The temperature and relative humidity were monitored to determine their influence on the fungal concentration, which was higher in the rainy season. Among the main fungi isolated from the air mycobiota of these production plants, Neurospora crassa was the predominant species along with the genera Aspergillus and Trichoderma. In addition, other less frequent genera as Cladosporium and Fusarium were detected. However, Penicillium and Mucor were predominant and non-frequent in the artisanal chocolate area. The adequate constructive design of the production plants and the correct location of dehumidifiers were identified as important elements to decrease the fungal contamination. Likewise, this study suggests the use of iodized salt as an economic alternative in air microbiological sampling of indoor environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental air of indoor food factories represent a serious problem in industry with potential to affect the comfort and safety of employees and significantly cause losses to the economy (Sun et al. 2017). In addition, the manufacture of some products impose rigorous controls on environmental air quality to decrease the possibility of contamination. Therefore, it is necessary to monitor the air in contact with food products (Lee and Shin 2017). The studies related to control the properties of air, mainly temperature and humidity, offer the possibility of reduce the presence and growth rate of some microorganisms in manufacturing and storage areas (Kim et al. 2015; Ndraha et al. 2018). Several studies recognize that geographical area and the period of the year in which the samples are taken influence the microbial results (Fröhlich-Nowoisky et al. 2012; Rojas and Aira 2012). Hence, in tropical countries, it is recommended covering periods that allow general conclusions, for that reason, should be considered the rainy season and the non-rainy (Rica et al. 2008).
Among the air biota, filamentous fungi (air mycobiota) are the most studied for their biodeteriorant and pathogenic attributes (Górny 2004; Borrego and Perdomo 2012). The heretotropic absorption of fungi involves the secretion of enzymes, the degradation in structural ashlars of organic matter, and its later incorporation into the fungal body. So, this type of nutrition causes the biodeterioration of substrates. This is mainly due to its complex exoenzymatic system and the secretion of mycotoxins by strains of the fungal genera Aspergillus (aflatoxins and ochratoxin), Fusarium (fumonisin, deoxynivalenol, and zearalenone), and Penicillium (patulin and citrinin) (Sánchez-Hervás et al. 2008; Bhat et al. 2010). They are reproduced from propagule whose concentration varies with humidity because they are highly hygroscopic (Reponen et al. 2001).
In this context, international regulatory standards establish the permissible limits of microorganisms to outdoor and indoor air (Nevalainen 2009). In many countries, it is regulated for food processing (Radler et al. 2000; Federación Española de Empresas de Calidad Ambiental Interior. Madrid 2007; Asociación Española de Normalización y Certificación 2010). For example, in the European Union since 1995 to agro–food companies is imposed demonstrate the microbiological quality of air of premises where food is produced according to European standard Pr-EN-1632-4 (Comunidad Europea 1995). It is stated that in the food processing, laboratories can be evaluated by methodologies to study air quality (Hernández and Marín 2013). However, the Cuban Standard NC 1020:2014 (2014) (Air quality Health and hygiene requirements) does not include microbiological contamination. Only in the Cuban fishing industry adapted a methodology proposed by Omeliansky method as a branch standard NRP 201:1987 (1987) applies to monitor air quality microbiology laboratories. In Havana, indoor environment studies were carried out, using the Omeliansky method. These studies always oriented to the preventive conservation of documents with patrimonial character (Borrego and Perdomo 2014; Borrego et al. 2017). So far in Cuba, we have not done such studies to characterize the interior environment of their local food production. The control of the microbiological quality of the air, not only a safe food is guaranteed. With a clean air, in chocolate production premises and food with special regimes, the preservation of the raw material is guaranteed and thus avoid economic losses. It is estimated that large sums of money are lost when raw material in poor condition has to be discarded due to contamination of microorganisms. In Cuba, it is a high cost to buy this raw material for the elaboration of these foods; it is a reason why correct conservation is necessary. Research on the microbiological quality of indoor air has a positive economic and social impact. The subject investigated has great importance and relevance with impact on the food industry. This is the first work carried out in buildings of the food industry in Cuba. Therefore, the objective of this work was characterized by the indoor air mycobiota of two locals in a food industry in Cuba.
Materials and methods
Location of local objects of study
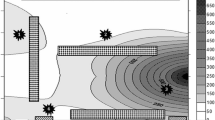
The air samples were collected from two manufacturing plants of a food factory in Havana, Cuba. In a semi-urban area, surrounded by abundant vegetation (trees and shrubs). The buildings have a single floor that implies the direct incidence of solar radiation on the roofs. The first location was an artisanal chocolate plant (ACP) in which are manufactured 44 hand-made products that do not receive heat treatment for their conservation (Fig. 1). The second place was a plant of products for special regimes (SRP), in which dry raw materials are rich in starch and proteins (isolated from soy and dehydrated egg). Therefore, they are easily contaminated microbiologically, mainly by filamentous fungi, and require a very strict control to prevent spoilage by these agents (Fig. 2).
Illustrative diagram of the plant of products for special regimes (SRP). The Roman numbers represent the different areas of the plant (I): Production and (II): Packaging and the Arabic numbers indicate the points where the Petri dishes were placed. The rectangles of dotted lines on the edges and the center represent windows and the stairway to the hopper, respectively
Mycological air sampling
The air samples were taken between 1:00 and 2:00 p.m. (Rojas et al. 2008) every 25 days from March to November 2017, covering the rainy seasons and little rain. Two environmental variables were established within the two selected production plants, temperature, and relative humidity of the air, which were measured with a digital thermohydrometer (Hygro-Thermometer DHT-1, China) with scale of T of 5 to 50 °C (0.1 °C precision) and RH of 10 to 100% (1% precision). The gravimetric method proposed by Omeliansky was used to collect the samples (Bogomolova and Kirtsideli 2009; Awad and Mawla 2012; Anaya et al. 2014). Fungal concentration was determined by the following equation:
Where N is fungal concentration (CFU m3) or (CFU m−3), a: number of colony per Petri dish; b: dish surface (cm2); t: exposure time (min).
Petri plates were 90 mm in diameter, and exposure time was 1 h to follow the methodology proposed by (Pasquarella et al. 2000) for determining the microbiological environmental index (MEI), which has been standardized and evaluated in numerous environmental studies (Pasquarella et al. 2012). These authors propose sample for 1 h, placing the plates at 1 m above the floor and 1 m from the walls (1:1:1). Depending on the size of the premises, the Petri dishes were placed along diagonal lines of 3 or 5 points (Federación Española de Empresas de Calidad Ambiental Interior. Madrid 2007; Rojas and Aira 2012) and then they incubated at 30 °C for 3 to 5 days (Rojas 2012). Four bacteriostats were used during air sampling, because there is no methodology that indicates which is the most appropriate. Four means of cultivation were prepared 1 L Malt Extract Agar (MEA) (BIOCEN, Cuba) to which was added 0.1 g of separately chloramphenicol, 5.0 mL of lactic acid to 10% until pH = 3.5 to 4, 75.0 g of sodium chloride (Borrego and Perdomo 2012) laboratory reagent grade, and 75.0 g of iodized salt for food use in Cuba. The predominant fungal strains in the Petri dishes were isolated. They were identified to genus level using the key mycological (Barnett and Hunter 2003). Fungal concentration was represented by isolines in contour maps with the program Surfer v. 8 (Rodríguez et al. 2005) to analyze their spatial distribution.
Statistic analysis
Statistical analysis of data was performed with program Statgraphics Centurion XV. The probability distribution was analyzed with normality test chi-cuadrado; ANOVA was performed and the method of least significant difference (LSD) was applied Fisher.
Results and discussion
The areas studied in the artisanal chocolate plant are shown in Fig. 1. This sector does not have a defined geometry and its design causes drafts inside it. In addition, the production area is not covered and does not have air-conditioner, and only in the classroom there is a dehumidifier. The warehouse is internal and the customers must enter to receive the products. However, the room dedicated to special regimes (Fig. 2) has a rectangular shape with two access doors that do not create drafts. The two sampled areas are roofed and only the region of packaging has air conditioner. The production area in the zone has two lateral windows towards the outside, at the level of the hopper where the raw materials are mixed. The differences in the design of both plants indicate the possible degrees of fungal contamination. This evidence is in agreement with the studies carried out by (Anaya et al. 2016) inside he archives building of patrimonial documents built in the form of blocks.
The values of temperature, relative humidity, and fungal concentration of the two plants are summarized in Table 1. In general, it was observed that the temperatures in the areas of SR plant were higher than those belonging to the ACP. In the zones without air-conditioner (Production and Processing, respectively), the relative humidity registered were between 70 and 92% for both plants. On the other hand, the region of Packaging of SRP, which has air-conditioner, the entrance of hot air from production area justifies the fluctuation of the relative humidity (RH) that ranged between 51 and 60%. In the same way, this variable showed a similar behavior in the classroom of the ACP (even when there is only one dehumidifier), while in the tunnel, packaging and storage areas respectively, the relative humidity was between 60 and 73%. These are the most important areas in the process of production in both, SRP and ACP sectors, due to they may affect the quality of the final product. This fact is directly related to the variation of the relative humidity which influences crystallization of the chocolate and at the same time allows the microbiological growth in the SRP plant because of the characteristics of the raw materials (dry mixtures) used in this sector.
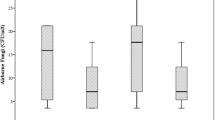
From the relative humidity values of Table 1, wet and dry areas can be identified. The container area of SRP may be considered as a dry zone while all areas of ACP along with the production area of SRP were considered as wet zones. These moist areas are more prone to fungal contamination due to the increased deposition of propagules (Lignell et al. 2007). In this sense, the fungal concentration obtained displayed values (1507 and 1638 CFU m−3 for ACP and SRP, respectively). The data showed a normal distribution (p ≥ 0.05), and the average values of SRP plant with X = 498 CFU m−3 and S = 335 CFU m−3 exceeded those of ACP with X = 288 CFU m−3 and S = 210 CFU m−3 respectively. In the rainy season, the fungal concentrations obtained on the culture media supplemented with lactic acid and chloramphenicol exhibited no significant differences (p ≤ 0.05). A similar fact was observed in the culture media that had the two salts (NaCl and iodized common salt) in their composition (Fig. 3a). However, these results exceeded those of lactic acid and chloramphenicol, showing the suitability of the salts as bacteriostats (Fig. 3b).
This finding represent an advantage over the use of the antibiotic (chloramphenicol) or the organic acid (lactic acid) (Fig. 3b–d). Moreover, the use of the Cuban iodized common salt as added as bacteriostatic for monitoring the fungal air pollution in the conditions studied shows an economical benefit, since the price of the salts is lower when compared with the antibiotic and the organic acid. In addition, it was observed that the employment of the salt increases the sensitivity of the sampling technique when the colonies recover exceed 69 CFU equivalent to 900 CFU m−3 of air (Fig. 3c, d). It is important to mention that this salt is more sensitive than chloramphenicol and lactic acid when the fungal concentration exceeds 107 CFU (1400 CFU m−3 air).
In this study, the possibility of detecting fungal strains in culture media containing the highest values of salts shows that the microorganisms detected could be considered as xerophilous and osmophilic. This evidence indicates that these strains may grow on substrates with low water activity and high salt concentration. Hence, these fungal isolates could cause the biodeterioration of the raw materials and the final products in both plants (Borrego et al. 2012; De Clercq et al. 2014; Micheluz et al. 2015).
The iodized common salt contains minerals in trace amounts that could stimulate the fungal growth along with potassium iodide, present as nutritional supplemented. Differently of sodium chloride, which is a reagent with high level of purity. The phenomenon observed when the fungal concentration is high can be explained because of when increasing the population density in the Petri dish, the availability of sugars and other nutrients decreases (Nayak et al. 2013).
Regarding the environmental variables and the concentration of fungi in the sampled areas, a tendency was observed in the increase of fungal contamination from the area of the lower to the higher temperature (Fig. 4). This is explained by the variation of RH caused by the mixing of hot and cold air due to the physical design of the production plants and the movement of personnel that facilitates the entry of contaminated air. Figure 4 b shows the average values of the sampling in the rainy season.
In SRP, the packaging area was cleaner (max 52 CFU m−3 air) than the production area which has the most average values between 750 and 1638 CFU m−3 air. This result may be due to direct communication with the exterior through the windows. Since the hopper is at the same height, this constitutes a risk for the products. In the case of ACP, the warehouse was the cleanest area (max 40 CFU m−3 air) which is explained because of the temperature that is controlled by an air conditioner. Moreover, it has an independent entrance and restricted access which reduces microbiological contamination from other areas. Likewise, the classroom has air conditioner and the fungal concentration was between 500 and 700 CFU m−3 air. The elaboration area was the most polluted (max 1612 CFU m−3 air) in the ACP, which is related to the fact that it is not covered and communicates with the side and main corridors of the plant. Therefore, it directly receives the air that comes from the outside, converting it into a way of entry of microbiological contamination to the process.
The results displayed that in SRP sector, the packaging area was cleaner (max 52 CFU m−3 air) than the production area, the most contaminated in this study (750–1638 CFU m−3 air) which may be due to direct communication with the exterior through the windows. This constitutes a risk for the product since the hopper is at that height. In the case of ACP, the warehouse is the cleanest area (max 40 CFU m−3 air). The presence of air conditioner and the independent entrance with restriction access in this zone reduces microbiological contamination from other areas. However, although the classroom has controlled temperature, fungal concentration was between 500 and 700 CFU m−3 air. In addition, the elaboration area was the most polluted (max 1612 CFU m−3 air), because of it is not covered and communicates with the side and main corridors of the plant. Therefore, it directly receives the air that comes from the outside, converting it into a way of entry of microbiological contamination to the process.
This connection between the processing site and the external environment explains why there was a tendency to decrease the fungal concentration from the low season (March–April) to the rainy season (May–September) and increased again at the beginning of the low rainfall period (October–November) (Fig. 5). According to (Rojas 2012), the rain causes the washed of the atmosphere, and the wind lifts less particles from the soil because it remains humid several days after the precipitation, whereas in the dry season, the wind carries away the dust particles that can move long distances from the ground.
In general, values between 100 to 500 CFU m−3 are acceptable in indoor environments but only 50 CFU m−3 are allowed if they are pathogenic fungi, as is the case of some species of the genus Aspergillus P. micheli ex Haller producing mycotoxins (A. clavatus, A. flavus, A. fumigatus, A. parasiticus and A. ochraceus) (Aquino et al. 2018; Taniwaki et al. 2018). According to (Salustiano et al. 2003) for food processing plants, the American Public Health Association (APHA) proposes 90 CFU m−3 while in other studies cited by this author recommend up to 430 CFU m−3. These values justify those employed by (AK 2010) to study the microbiological quality of the air in Valencia Plant of the Kraft Foods Global Company in Venezuela. This company establishes internal control limits for the production room of mayonnaise and cheeses of 100 and 500 CFU m−3, respectively. Hence, taking into account these previous data, in this study, the production and elaboration areas of SRP and ACP respectively showed high level of contamination which constitutes a microbiological risk. However, the average values of fungal concentration of the remaining areas in both SRP and ACP were between 487 and 464 CFUm−3 respectively; therefore, it can be concluded that in general, their fungal contamination is low (Fig. 5).
The aeromycobiota found in the plants showed Neurospora crassa Shear & B. O. Dodge species together with the predominant fungal genera Aspergillus and Trichoderma Pers. and other less frequent strains of the genera Cladosporium Link and Fusarium Link (Bradford et al. 2018; Garaga et al. 2019; Humbal et al. 2019). These genera were detected in similar studies in the central warehouse of the Instituto de Investigaciones para la Industria Alimentaria, Cuba (IIIA) (Anaya et al. 2014) as well as in food processing plants in other countries (Salustiano et al. 2003; Asefa et al. 2009) which they are considered primary colonizers (Górny 2004). These fungal species are also present in indoor environments of countries with tropical climates due to abiotic factors such as high temperature and relative humidity. It is suggested that the strains of these genera have a high biodeteriorating potentiality (Bogomolova and Kirtsideli 2009) and some can be pathogenic (Nayak et al. 2013). However, Penicillium Link and Mucor P. Michelli ex L. were predominant and not frequent in the AC zone, while this behavior was inverse in SRP.
In the case of the genera Aspergillus, Penicillium and Mucor are part of the mycobiota commonly isolated from cocoa beans (Sánchez-Hervás et al. 2008). However, the presence Trichoderma spp. and N. crassa indicates the possibility of environmental contamination from the outside since these fungi are abundant in soils and vegetation in state of decomposition (Rojas 2012). The propagules of these strains reached the interior environment through natural ventilation. All these strains were isolated with the four bacteriostats used. Since the development of the aerial mycelium of these two genera prevents the growth of other strains, the counting becomes difficult. Thus, the iodized common salt offers a suitable alternative for this kind of studies.
To control the fungic concentration of the air and thus prevent the microbiological contamination of chocolate and foods with special regimes, it is necessary to maintain the premises with a relative humidity and low temperature. This can be achieved with the use of dehumidifiers and the installation of air conditioners.
The implementation of the HACCP system in the manufacture of special regimes food and chocolate can effectively guarantee the safety and quality of food, expand the market, and improve the level of management of the manufacturers. Critical control points were identified, which include processing, packaging, and storage. So, in these places, the cleaning works must be reinforced to eliminate fungal propagules that may alter the harmlessness of the food (Lu et al. 2014).
Conclusions
The sampled locals showed high levels of humidity, which favors fungal development and consequently the contamination although the average detected classified as low in some areas. The importance of the adequate constructive design of the production plants as well as the use and correct location of dehumidifiers was evidenced as a key element to avoid the microbial contamination. The air mycobiota of these places is made up of the species Neurospora crasa together with the fungal genera Aspergillus, Cladosporium, Fusarium, Mucor, Penicillium, and Trichoderma.
References
AK V (2010) Evaluación de la calidad microbiológica del aire de una planta procesadora de alimentos. Tesis presentada en opción al Título de Licenciatura en Biología. Universidad Simón Bolívar. Venezuela
Análisis Ambiental (1987) Método de Omeliansky. Análisis higiénico sanitario y ambiental. Métodos de ensayos microbiológicos.7 pps. Norma Ramal de la Pesca NRP-201. Ciudad de La Habana. Ministerio de la Industria Pesquera.
Anaya M, Borrego S, Cobo H, Valdés O, Molina A (2014) Aeromicobiota de un depósito de alimentos en La Habana, Cuba. AUGMDOMUS 6:95–110
Anaya M, Borrego SF, Gámez E, Castro M, Molina A, Valdés O (2016) Viable fungi in the air of indoor environments of the National Archive of the republic of Cuba. Aerobiologia (Bologna) 32:513–527. https://doi.org/10.1007/s10453-016-9429-3
Aquino S, de Lima JEA, do Nascimento APB, Reis FC (2018) Analysis of fungal contamination in vehicle air filters and their impact as a bioaccumulator on indoor air quality. Air Qual Atmos Health 11:1143–1153. https://doi.org/10.1007/s11869-018-0614-0
Asefa DT, Langsrud S, Gjerde RO, Kure CF, Sidhu MS, Nesbakken T, Skaar I (2009) The performance of SAS-super-180 air sampler and settle plates for assessing viable fungal particles in the air of dry-cured meat production facility. Food Control 20:997–1001. https://doi.org/10.1016/j.foodcont.2008.11.011
Asociación Española de Normalización y Certificación (2010) Hojas Informativas Ambisalud octubre 2010: microbiología del aire. UNE EN ISO 9001. 10
Awad AH, Mawla HA (2012) Sedimentation with the Omeliansky formula as an accepted technique for quantifying airborne fungi. Pol J Environ Stud 21:1539–1541
Barnett HL, Hunter BB (2003) Illustrated genera of imperfect fungi, 4th edn. APS Press The American Phytopathological Society, USA
Bhat R, Rai RV, Karim AA (2010) Mycotoxins in food and feed: present status and future concerns. Compr Rev Food Sci Food Saf 9:57–81. https://doi.org/10.1111/j.1541-4337.2009.00094.x
Bogomolova E, Kirtsideli I (2009) Airborne fungi in four stations of the St. Petersburg underground railway system. Int Biodeterior Biodegradation 63:156–160. https://doi.org/10.1016/j.ibiod.2008.05.008
Borrego S, Molina A, Santana A (2017) Fungi in archive repositories environments and the deterioration of the graphics documents. EC Microbiol 11:205–226
Borrego S, Lavin P, Perdomo I, Gómez de Saravia S, Guiamet P (2012) Determination of indoor air quality in archives and biodeterioration of the documentary heritage. ISRN Microbiol 2012:1–10. https://doi.org/10.5402/2012/680598
Borrego S, Perdomo I (2012) Aerobiological investigations inside repositories of the National Archive of the republic of Cuba. Aerobiologia (Bologna) 28:303–316. https://doi.org/10.1007/s10453-011-9235-x
Borrego S, Perdomo I (2014) Caracterización de la micobiota aérea en dos depósitos del Archivo nacional de la República de Cuba. Rev Iberoam Micol 31:182–187. https://doi.org/10.1016/j.riam.2013.09.004
Bradford KJ, Dahal P, Van Asbrouck J et al (2018) The dry chain: reducing postharvest losses and improving food safety in humid climates. Trends Food Sci Technol 71:84–93. https://doi.org/10.1016/j.tifs.2017.11.002
Comunidad Europea (1995) Pr-EN-1632 Parte 4: Métodos de análisis y de medida de la aerobiocontaminación en zona de riesgos (HACCP) http://www.nen.nl/pdfpreview/preview_4915.pdf. Accessed 15 Jan 2019
De Clercq N, Van Coillie E, Van Pamel E et al (2014) Detection and identification of xerophilic fungi in Belgian chocolate confectionery factories. Food Microbiol 46:322–328. https://doi.org/10.1016/j.fm.2014.08.012
Federación Española de Empresas de Calidad Ambiental Interior. Madrid E (2007) FEDECAI-01 Programa de certificación de calidad ambiental en interiores. Calidad ambiental en interiores: Criterios de muestreo. 4–6 https://www.cresca.upc.edu/congreslegionella/arxius/ponencies/cruceta-calidad-ambiental.pdf. Accessed 06 Jan 2019
Fröhlich-Nowoisky J, Burrows SM, Xie Z, Engling G, Solomon PA, Fraser MP, Mayol-Bracero OL, Artaxo P, Begerow D, Conrad R, Andreae MO, Després VR, Pöschl U (2012) Biogeography in the air: fungal diversity over land and oceans. Biogeosciences 9:1125–1136. https://doi.org/10.5194/bg-9-1125-2012
Garaga R, Avinash CKR, Kota SH (2019) Seasonal variation of airborne allergenic fungal spores in ambient PM 10 —a study in Guwahati, the largest city of north-east India. Air Qual Atmos Health 12:11–20. https://doi.org/10.1007/s11869-018-0624-y
Górny RL (2004) Filamentous microorganisms and their fragments in indoor air--a review. Ann Agric Environ Med 11:185–197
Hernández AM, Marín AF (2013) Elaboración de un protocolo de muestreo que permita evaluar la calidad microbiológica del aire para el laboratorio de análisis de aguas y alimentos de la Universidad Tecnológica de Pereira. Universidad Tecnológica de Pereira, Facultad de Tecnología Química, Colombia
Humbal C, Joshi SK, Trivedi UK, Gautam S (2019) Evaluating the colonization and distribution of fungal and bacterial bio-aerosol in Rajkot , western India using multi-proxy approach. https://doi.org/10.1007/s11869-019-00689-6
Kim WR, Aung MM, Chang YS, Makatsoris C (2015) Freshness gauge based cold storage management: a method for adjusting temperature and humidity levels for food quality. Food Control 47:510–519. https://doi.org/10.1016/j.foodcont.2014.07.051
Lee YS, Shin B (2017) Efficacy of commercial sanitizers against fungi of concern in the food industry. J Alloys Compd 722:474–481. https://doi.org/10.1016/j.jallcom.2017.06.094
Lignell U, Meklin T, Putus T, Rintala H, Vepsäläinen A, Kalliokoski P, Nevalainen A (2007) Effects of moisture damage and renovation on microbial conditions and pupils’ health in two schools - a longitudinal analysis of five years. J Environ Monit 9:225–233. https://doi.org/10.1039/b615459j
Lu J, Pua XH, Te LC et al (2014) The implementation of HACCP management system in a chocolate ice cream plant. J Food Drug Anal 22:391–398. https://doi.org/10.1016/j.jfda.2013.09.049
Micheluz A, Manente S, Tigini V, Prigione V, Pinzari F, Ravagnan G, Varese GC (2015) The extreme environment of a library: Xerophilic fungi inhabiting indoor niches. Int Biodeterior Biodegrad 99:1–7. https://doi.org/10.1016/j.ibiod.2014.12.012
Nayak AP, Green BJ, Beezhold DH (2013) Fungal hemolysins. Med Mycol 51:1–16. https://doi.org/10.3109/13693786.2012.698025
NC 1020 (2014) Calidad del aire. Reglas para la vigilancia de la calidad del aire en asentamientos humanos. http://www.nc.cubaindustria.cu. Accessed 01 Feb 2019
Ndraha N, Hsiao HI, Vlajic J, Yang MF, Lin HTV (2018) Time-temperature abuse in the food cold chain: review of issues, challenges, and recommendations. Food Control 89:12–21. https://doi.org/10.1016/j.foodcont.2018.01.027
Nevalainen A ML (2009) WHO biological agents in indoor environments. Assessment of health risks. https://www.aspergillus.org.uk/content/biological-agents-indoor-environments-assessment-health-risks-work-conducted-who-expert. Accessed 13 Dec 2018
Pasquarella C, Pitzurra O, Savino A (2000) The index of microbial air contamination. J Hosp Infect 46:241–256. https://doi.org/10.1053/jhin.2000.0820
Pasquarella C, Saccani E, Sansebastiano GE, Ugolotti M, Pasquariello G, Albertini R (2012) Proposal for a biological environmental monitoring approach to be used in libraries and archives. Ann Agric Environ Med 19:209–212
Radler F, Neto DA, Fernando L, Siqueira DG (2000) Guidelines for indoor air quality in offices in Brazil. Proc Heal Build 4:549–554
Reponen T, Grinshpun SA, Conwell KL, Wiest J, Anderson M (2001) Aerodynamic versus physical size of spores: measurement and implication for respiratory deposition. Grana 40:119–125. https://doi.org/10.1080/00173130152625851
Rica UDC, Para M, Estudios R et al (2008) Metodología para realizar estudios de evidencia microbiológica en plantas procesadoras de alimentos. Agron Mesoam 19:131–137
Rodríguez S, Sauri M, Peniche I, Pacheco J, Ramírez J (2005) Aerotransportables viables en el área de tratamiento y disposición final de residuos sólidos municipales de Mérida. Yucatán Ingeniería 9:19–29
Rojas TI, Martinez E, Maria Aire MA (2008) Aeromicota de ambientes internos: comparacion de metodos de muestreo. Boletìn Micológico 23:67–73
Rojas TI (2012) Diversidad fúngica en ambientes interiores y exteriores en áreas urbanas de Ciudad de La Habana. Tesis de Doctorado en Ciencias Biológicas. Universidad de La Habana, Cuba
Rojas TI, Aira MJ (2012) Fungal biodiversity in indoor environments in Havana, Cuba. Aerobiologia (Bologna) 28:367–374. https://doi.org/10.1007/s10453-011-9241-z
Salustiano VC, Andrade NJ, Cardoso Brandão SC et al (2003) Microbiological air quality of processing areas in a dairy plant as evaluated by the sedimentation technique and a one-stage air sampler. Braz J Microbiol 34:255–259. https://doi.org/10.1590/S1517-83822003000300015
Sánchez-Hervás M, Gil JV, Bisbal F, Ramón D, Martínez-Culebras PV (2008) Mycobiota and mycotoxin producing fungi from cocoa beans. Int J Food Microbiol 125:336–340. https://doi.org/10.1016/j.ijfoodmicro.2008.04.021
Sun F, DAI Y, Yu X (2017) Air pollution, food production and food security: a review from the perspective of food system. J Integr Agric 16:2945–2962. https://doi.org/10.1016/S2095-3119(17)61814-8
Taniwaki MH, Pitt JI, Magan N (2018) Aspergillus species and mycotoxins: occurrence and importance in major food commodities. Curr Opin Food Sci 23:38–43. https://doi.org/10.1016/j.cofs.2018.05.008
Funding
This study was financially supported by the Instituto de Investigaciones para la Industria Alimentaria, Cuba.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anaya, M., Gámez-Espinosa, E., Falco, A.S. et al. Characterization of indoor air mycobiota of two locals in a food industry, Cuba. Air Qual Atmos Health 12, 797–805 (2019). https://doi.org/10.1007/s11869-019-00707-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-019-00707-7